

Corporate Presentation May 2012 Ticker: TNXP.OB

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Disclosures Forward - looking Statements The statements and discussions contained in this presentation that are not historical facts constitute forward - looking statement s. These can be identified by the use of forward - looking words, such as “believes”, “expects”, “may”, “intends”, “anticipates”, “plans”, “es timates”, or any other analogous or similar expressions intended to identify forward - looking statements. These forward - looking statements and es timates as to future performance, estimates as to future valuations, and other statements contained herein regarding matters that are no t h istorical facts, are only predictions and actual events or results may differ materially. We cannot assure or guarantee that any futur e r esults described in this presentation will be achieved, and actual results could vary materially from those reflected in such forward - looking sta tements. Information contained in this presentation has been complied from sources believed to be credible and reliable. However, we ca nnot guarantee such credibility and reliability. The forecasts and projections of events contained herein are based upon subjecti ve valuations, analyses, and personal opinions. Information Regarding Disclosures The Common Stock and Warrants have not and will not be registered under the Securities Act of 1933, as amended (the “Act”), o r u nder any state securities laws, nor has the Securities and Exchange Commission (the “Commission”) or any state regulatory authority en dor sed the Offering. Any representation to the contrary is a criminal offense. In making an investment decision, investors must rely upon their own examination of the company and the terms of the Offering , i ncluding the merits and risks involved. The acquisition of the Stock, if offered, should be considered only by persons who can bear t he economic risk of their investment of ran indefinite period of time and can afford a total loss of their investment. Each prospective inves tor in the Offering should, prior to purchasing any Stock, consult his own attorney and business advisor as to the legal, business, tax, and rel ate d matters concerning its investment and is urged to ask questions of, and receive answers from, the Company concerning the terms and co ndi tions of the Offering and request any additional information they may consider Necessary in making an informed investment decision. This presentation does not constitute an offer to sell or a solicitation of an offer to purchase any securities of any nature wh atsoever, nor do the contents of the presentation constitute legal, tax, or business advice. This presentation and the offering of the Company's Stock shall be kept confidential. The recipient agrees not to disclose to any third party any information contained herein, or any terms, conditions, or other facts with respect to he Offering, including, without li mit ation, that the Company is or may be contemplating the Offering. Information included herewith has been obtained from the Company and other sources believed to be reliable, but the accuracy or completeness of such information is not guaranteed by, and should not be construed as a representation by the Company. Any representations and warranties will be contained only in a definitive agreement signed by the investor and the Company. 2
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TONIX Summary • Specialty pharmaceutical company - Non - addictive treatments for chronic pain syndromes - Capital - efficient development strategy • Innovative products for high - value central nervous system (CNS) indications: - Fibromyalgia Syndrome (FM) • Three FDA approvals validate condition • Expect to follow successes of Lyrica® and Cymbalta® in FM - Post T raumatic S tress D isorder (PTSD) • Significant interest from Department of Defense • FM Phase 2a study demonstrated statistically significant improvement in core symptoms 3

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Experienced Leadership Management Team Seth Lederman, MD Founder, CEO, Chairman Vela, Targent, Validus, Fontus Benjamin Selzer Chief Operating Officer Aton, Reliant, investment banking (Lehman Brothers & Banc of America Securities) Leland Gershell, MD, PhD Chief Financial Officer Cowen, Apothecary Capital, Favus Institutional Research, Madison Williams Bruce Daugherty, PhD, MBA Senior Director, Drug Development Merck, Roche Institute 4

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Accomplished Independent Board Board of Directors Seth Lederman, MD Founder, CEO, Chairman Stuart Davidson Former CEO of Alkermes & Combion Patrick Grace WR Grace, Chemed, Grace Institute Donald W. Landry, MD, PhD Columbia Chair of Medicine Ernest Mario, PhD Former CEO of Glaxo, Alza & Reliant Charles Mather Janney Montgomery Scott Securities, Cowen, Smith Barney John Rhodes Former Partner at Booz Allen Hamilton 5

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS Fibromyalgia Market Opportunity • Approximately 5 million U.S. patients* • U.S. drug market estimated at $1.2 billion** - 2007 - 2010 CAGR of 18.4 %** • Until 2007, there were no FDA approved drugs - Lyrica (Pfizer) approved 2007: replacing off - label generic analgesics - Cymbalta (Lilly) approved 2008, Savella® (Forest) approved 2009: replacing off - label generic anti - depressants • TNX - 102 is seeking FDA approval as a first - in - class drug - Expect to replace off - label generic muscle relaxants in FM * National Institutes of Health, U.S. Department of Health and Human Services ** Source: Frost & Sullivan Fibromyalgia Market Study, December 2010 6
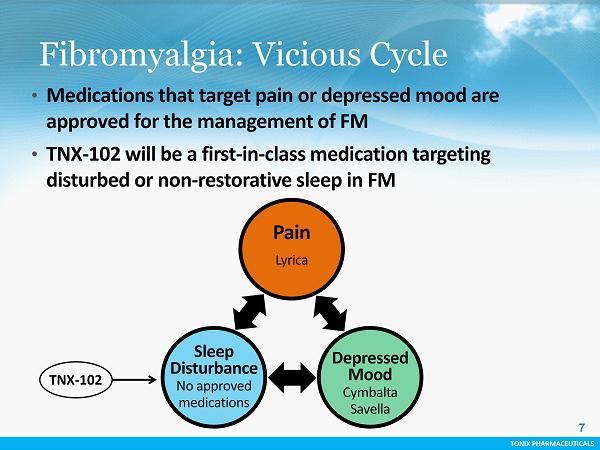
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia: Vicious Cycle 7 • Medications that target pain or depressed mood are approved for the management of FM • TNX - 102 will be a first - in - class medication targeting disturbed or non - restorative sleep in FM TNX - 102 Pain Lyrica Sleep Disturbance No approved medications Depressed Mood Cymbalta Savella
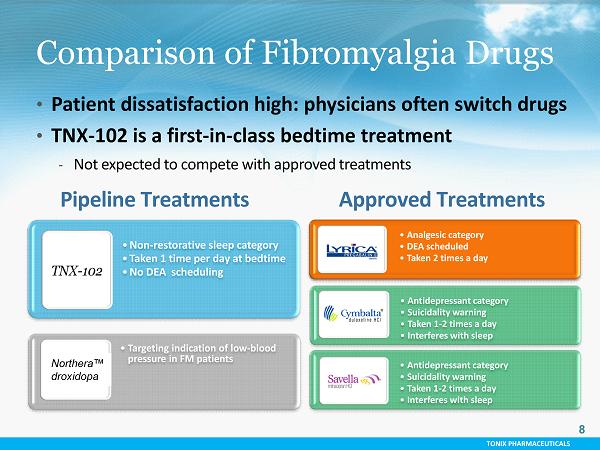
TONIX PHARMACEUTICALS Comparison of Fibromyalgia Drugs Approved Treatments Pipeline Treatments • Antidepressant category • Suicidality warning • Taken 1 - 2 times a day • Interferes with sleep • Analgesic category • DEA scheduled • Taken 2 times a day • Antidepressant category • Suicidality warning • Taken 1 - 2 times a day • Interferes with sleep TONIX PHARMACEUTICALS Comparison of Fibromyalgia Drugs Approved Treatments Pipeline Treatments • Antidepressant category • Suicidality warning • Taken 1 - 2 times a day • Interferes with sleep • Analgesic category • DEA scheduled • Taken 2 times a day • Antidepressant category • Suicidality warning • Taken 1 - 2 times a day • Interferes with sleep • Targeting indication of low - blood pressure in FM patients 8 • Patient dissatisfaction high: physicians often switch drugs • TNX - 102 is a first - in - class bedtime treatment - Not expected to compete with approved treatments • Non - restorative sleep category • Taken 1 time per day at bedtime • No DEA scheduling Northera ™ droxidopa

TONIX PHARMACEUTICALS Drugs Used to Treat Fibromyalgia 9 • Off - label drugs dominate the sleep quality market - Approved as “muscle relaxants” • TNX - 102 to be first - in - class sleep quality treatment Non - restorative Sleep Analgesic / P ain Killers Anti - depressant Muscle Relaxants FDA Approved • Lyrica ( pregabalin ) • Cymbalta (duloxetine) • Savella ( milnacipran ) In Development • TNX - 102 (VLD - cyclobenzaprine) Development Abandoned • Rekinla (sodium oxybate ) Off L abel • Xyrem (sodium oxybate ) • Neurontin (gabapentin) • Opiates • (venlafaxine) • (bupropion) • (cyclobenzaprine) • ( tizanidine ) • (baclofen) • ( carisoprodol ) • ( metaxalone )

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Cyclobenzaprine Has an Impressive Safety Record and is Widely Used • Off - label Cyclobenzaprine: third most widely prescribed medication for FM* • 1977: FDA approved Flexeril® (Merck) • 1990’s: Extensive safety & efficacy studies (Merck) • 2007: FDA approved controlled release formulations (15/30 mg) • 2010: > One billion tablets prescribed • No DEA scheduling, no recognized addictive potential 10 * Source: Frost & Sullivan Fibromyalgia Market Study, December 2010
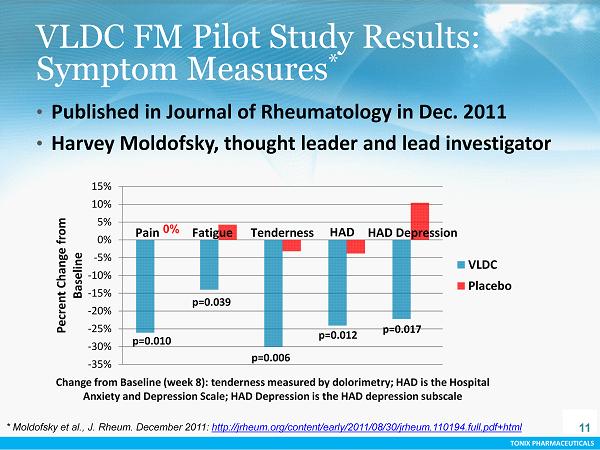
TONIX PHARMACEUTICALS VLDC FM Pilot Study Results: Symptom Measures * Change from Baseline (week 8): tenderness measured by dolorimetry; HAD is the Hospital Anxiety and Depression Scale; HAD Depression is the HAD depression subscale -35% -30% -25% -20% -15% -10% -5% 0% 5% 10% 15% Pecrent Change from Baseline VLDC Placebo p=0.010 p=0.039 p=0.006 p=0.012 p=0.017 Fatigue HAD HAD Depression Pain Tenderness 0% 11 * Moldofsky et al., J. Rheum. December 2011: http://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html • P ublished in Journal of Rheumatology in Dec . 2011 • Harvey Moldofsky , thought leader and lead investigator

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102: VLDC New Formulation • Specifically designed for the treatment of FM - Fast absorption - Elevated blood levels during the night to improve sleep quality - Low next - day blood levels to minimize morning somnolence • Differentiated from, but not competitive with, other FM therapies - First - in - class sleep quality treatment - Indicated for bedtime treatment - Patient dissatisfaction high: physicians often switch drugs • With a unique formulation and new indication, reimbursement coverage is expected 12

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102: Development Plan • Formulation and PK trials - PK Study on first version completed: 30 subjects; three week study - PK Study on second version: completion expected in September 2012 - Pharmacodynamic study contemplated by year - end • Pivotal Efficacy Trial - 2 - arm, 12 - week study; 150 patients per arm - Study design, endpoints to mirror those used by Lyrica and Cymbalta • Pain and a composite endpoint of other FM symptoms - Results expected early 2014 • Partnership for second pivotal and commercialization 13

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TNX - 105: VLDC for PTSD • 3.5% of U.S. adult population will have suffered from PTSD in past 12 months* - Any trauma can lead to PTSD • Unsatisfied market - Only Zoloft® and Paxil® have FDA approval • Widespread painkiller abuse and addiction • Leverage formulation/clinical work of TNX - 102 to advance TNX - 105 14 * National Institutes of Mental Health & National Institutes of Health

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS FM and PTSD - Related Conditions • Overlap of PTSD and FM symptoms - PTSD is thought to be exacerbated by non - restorative sleep - Some are believed to suffer from both conditions simultaneously - Some patients with FM meet PTSD criteria, and vice versa • PTSD has both combat and civilian forms - Zoloft and Paxil are approved for PTSD; market unsatisfied - Brand prescriptions filled by generic sertraline and paroxetine - DOD has a strong interest in promoting research on therapeutics 15

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TONIX Pharmaceuticals Pipeline • TONIX has a comprehensive pipeline of CNS products 16 Product Indication Status TNX - 102 Fibromyalgia • Very low dose cyclobenzaprine in novel formulation • Phase 2a successfully completed • Completing formulation to take into first pivotal trial • T rial expected to begin Q1 2013 TNX - 105 Post - Traumatic Stress Disorder • Low dose cyclobenzaprine in novel formulation • Will leverage data from TNX - 102 PK trial • Pivotal trials anticipated to start 2013 • Applied for Department of Defense funding TNX - 201 Headache • NDA process for existing grandfathered, or DESI, product • Potentially shortened process for FDA approval • DESI to NDA switch products enjoy mandated exclusivity TNX - 301 Alcoholism • US patent allowed • Potential for government funding
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Intellectual Property • Active patenting strategy to extend exclusivity - Plan to file patents around TONIX products’ unique PK profiles, which are a difficult patent class to circumvent • TNX - 102 - Methods of Use patents for use of VLDC in treatment of FM issued; expiration mid - 2020 - Two formulation patents issued; expiration in mid - 2021 - PK patents expected to be filed near term • TNX - 105 - Methods of Use patent for use of VLDC in treatment of PTSD filed - Two formulation patents issued; expiration in mid - 2021 17

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS Upcoming Milestones 18 • Short and intermediate term value inflection milestones Timing Milestones Related to TNX - 102 Q2 2012 • PK study on second formulation Q3 2012 • PK/PD on “commercial” formulation and dose Q1 2013 • Commencement of initial pivotal trial Q3 2013 • Interim look at initial pivotal trial data Q1 - Q2 2014 • Completion of initial pivotal trial • Partnering

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Why Invest in TONIX? • Capital efficient drug development strategy focused on high - value, first - in - class products • FM and PTSD are significant unmet needs with large market opportunities • TNX - 102 is expected to be a first - in - class treatment for FM and differentiated from generic cyclobenzaprine • Low risk, low - cost development pathway • Near - term value inflection point • Experienced management and board 19

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Financial Information 20 Ticker TNXP:OTCQB Shares Outstanding (in millions) 34.3 Cash Balance at 12/31/11 (in millions)* $4.0 52 Week Trading Range (as of 4/30/12)** $0.99 - $2.06 * Pro - forma for the closing of a PIPE financing with net proceeds of $3.9 million in Q1 2012 ** Stock first traded in February 2012