
Exhibit 99.01

Fibromyalgia Reimbursement Perspective July 2012 OTC/QB: TNXP

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Disclosures Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as "anticipate," "believe," "forecast," "estimated" and "intend," among others . These forward - looking statements are based on TONIX’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our ability to continue as a going concern ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payer reimbursement ; limited sales and marketing efforts and dependence upon third parties ; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . TONIX does not undertake an obligation to update or revise any forward - looking statement . Investors should read the risk factors set forth in the Annual Report on Form 10 - K filed with the SEC on March 30 , 2012 and future periodic reports filed with the Securities and Exchange Commission . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements . 2

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Pain: Cost to Society • Pain management is one of society’s major challenges - Abuse and addiction to prescription opiates is a fast growing national crisis - Top priority of multiple governmental agencies - Highlighted by Professor Derek Bok (former President of Harvard), “The Politics of Happiness” (2010, Princeton University Press) • Fibromyalgia is a significant part of the growing prescription drug misuse problem - No medical justification for several drug categories widely used in fibromyalgia - Fibromyalgia pain originates in the brain and is not alleviated by opiates, and the sleep disturbance is not resolved by sleep drugs 3

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia: Cost to Society • Fibromyalgia presents a significant economic burden to the healthcare system* - “Mean annual expenditures for fibromyalgia patients were … similar to rheumatoid arthritis … ” - “A greater proportion of patients with fibromyalgia had any short - term disability days than those with rheumatoid arthritis . ” - “Mean costs for absence from work and short - term disability in the fibromyalgia and rheumatoid arthritis groups were substantial and similar . ” • Additional references: 4 * Silverman et al. Cur Med Res Opin , 2009;25:829. - Berger et al . Int J Clin Pract , 2007 ; 61 : 1498 . - Carville et al . Ann Rheum Dis, 2008 ; 67 : 536 . - Howard et al . JOEM, 2010 ; 52 : 1186 . - McNett et al . Cur Med Res Opin , 2011 ; 27 : 673 . - Robinson et al . J Rheum, 2003 ; 30 : 1318 . - Sanchez et al . Cur Med Res Opin , 2011 ; 27 : 663 .
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia Reimbursement: Generics Not Approved by FDA • Some formularies list off - label generics in Tier 1 for fibromyalgia - However, as these drugs are not FDA approved, they cannot be marketed for fibromyalgia - Managed care and Medicaid can rely on compendia, like USP, which list products that have some peer reviewed studies • Tier 1 status of generic off - label drugs is driven only by lower cost 5

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia Reimbursement: FDA - Approved Products • FDA - approved fibromyalgia products have strong sales and growth* - Cymbalta®: ~$560 million in fibromyalgia in the US in 2011 and growing - Lyrica®: ~$450 million in fibromyalgia in the US in 2011 and growing - Growth continues despite gabapentin, venlafaxine, bupropion, etc. generics in Tier 1 • Physicians generally start fibromyalgia patients on samples when prescribing products FDA approved for the indication - If a branded product is effective, doctors and patients are motivated to overcome reimbursement hurdles - Physicians and patients respond to managed care hurdles such as prior authorization and Tier 2 or Tier 3 status 6 * Decision Resources Pain Management Study: Fibromyalgia, 2011
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia Reimbursement: FDA - Approved Products (2) • Many patients continue to be unsatisfied by current therapies - Efficacy, durability and tolerability motivate patients to seek alternatives - Managed care hurdles not expected for new FDA - approved products • Fibromyalgia patients tend to be proactive in obtaining medications that work for them - Large opportunity for new products that reduce therapeutic failure, need for medication switching, and non - pharmaceutical care • Managed care push - back on physicians in fibromyalgia typically limited to requesting validation of diagnosis - Managed care companies sometimes request physicians to document treatment failure 7
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia Reimbursement: TNX - 102 Anticipated Launch Year* • Duloxetine (Cymbalta®) will be generic and likely in Tier 1 - Potential for duloxetine to replace off - label generics in Tier 1 • “Managed Care” study on TNX - 102 prior to launch to document improved adverse event profile versus generic cyclobenzaprine - Modified PK curve as a result of sublingual formulation expected to result in reduced next day somnolence • Patient dissatisfaction and familiarity with cyclobenzaprine safety and efficacy will drive patients to adopt TNX - 102 • Few options in fibromyalgia pipeline will be vying for future reimbursement - None in development with “sleep quality” mechanism of action 8 * Anticipated launch year is 2017
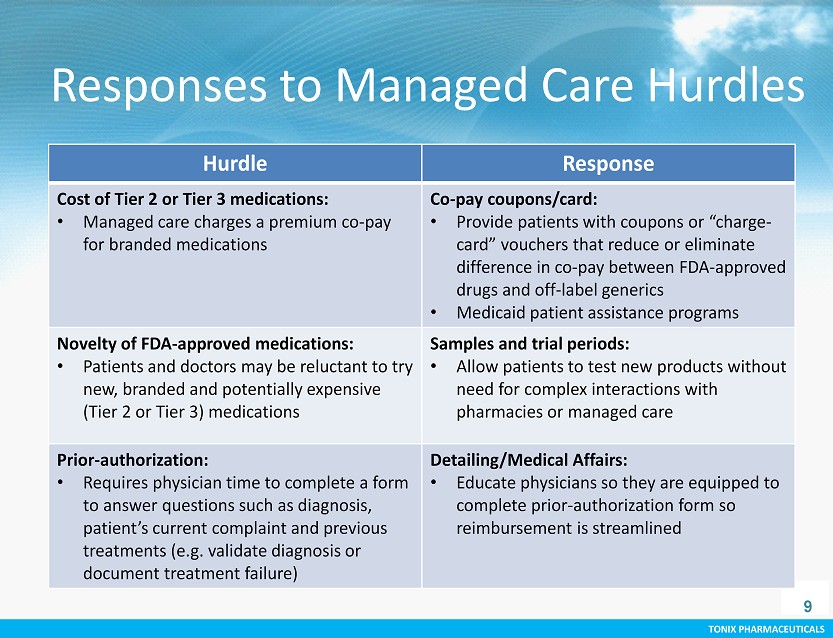
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Responses to Managed Care Hurdles 9 Hurdle Response Cost of Tier 2 or Tier 3 medications: • Managed care charges a premium co - pay for branded medications Co - pay coupons/card: • Provide patients with coupons or “charge - card” vouchers that reduce or eliminate difference in co - pay between FDA - approved drugs and off - label generics • Medicaid patient assistance programs Novelty of FDA - approved medications: • Patients and doctors may be reluctant to try new, branded and potentially expensive (Tier 2 or Tier 3) medications Samples and trial periods : • Allow patients to test new products without need for complex interactions with pharmacies or managed care Prior - authorization: • Requires physician time to complete a form to answer questions such as diagnosis, patient’s current complaint and previous treatments (e.g. validate diagnosis or document treatment failure) Detailing/Medical Affairs: • Educate physicians so they are equipped to complete prior - authorization form so reimbursement is streamlined

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102 Reimbursement Precedents • Novel formulations with novel PK profiles that address unmet needs are reimbursed 10 Generic / OTC Drug Labeled Indication Reformulated Rx Product Labeled Indication US Annual Sales* Comment Oxycodone Pain OxyContin ® Pain >$3bn • Novel PK profile protected by PK patents Omega - 3 ethyl esters None (supplement) Lovaza ® Hypertriglyceridemia >$1bn • Received Rx indication for OTC supplement Niacin None (vitamin) Niaspan® Dyslipidemia >$1bn • Novel PK to reduce flushing & new indication Minocycline General antibiotic Solodyn ® Acne ~$900mm • Lower dose, novel PK & new indication Methylphenidate ADHD Concerta ® ADHD ~300mm** • Novel PK for improved efficacy 4 - Aminopyridine None (compounded) Ampyra ® Walking in multiple sclerosis ~$250mm • Fixed dose of available compounded agent * Current run rate as of May 2012 ** Authorized generic launched in 2011; twelve months sales ending 2/28/11 sales were ~$1.5bn
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Supreme Court Upholds ACA • The Patient Protection and Affordable Care Act (ACA) requires that medications like TNX - 102 will be covered by formularies - ACA Section 3307 expands the concept of “class” and “category” from the M edicare Modernization Act and extends it to prescription drug plans - TNX - 102 is expected to be first and only FDA - approved “sleep quality” medication for fibromyalgia - TNX - 102 is expected to be first and only FDA - approved cyclobenzaprine - containing and “muscle relaxant” - category product for fibromyalgia - Medicaid may be expanded state - by - state to cover the uninsured • Few options in fibromyalgia pipeline will be vying for future reimbursement; none with novel mechanism of action (sleep) 11

OTC/QB: TNXP