
Exhibit 99.01

Corporate Presentation August 2012 OTC/QB: TNXP

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS 2 Disclosures Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as "anticipate," "believe," "forecast," " estimate" and "intend," among others . These forward - looking statements are based on TONIX’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our ability to continue as a going concern ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payer reimbursement ; limited sales and marketing efforts and dependence upon third parties ; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . TONIX does not undertake an obligation to update or revise any forward - looking statement . Investors should read the risk factors set forth in the Annual Report on Form 10 - K filed with the SEC on March 30 , 2012 and future periodic reports filed with the Securities and Exchange Commission . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

TONIX PHARMACEUTICALS • Specialty pharmaceutical company developing innovative non - addictive products for chronic pain syndromes - Fibromyalgia syndrome (FM ) - Post - traumatic stress disorder (PTSD) • Unmet medical needs and large commercial opportunities - Targets sleep pathology - Central pain syndromes poorly addressed by opiate pain drugs or prescription sleep drugs • Capital efficient, risk - mitigated development pathway - Near - term, value - creating milestones • Experienced management and board Company Overview 3

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS 4 Experienced Leadership Selected Previous Corporate Affiliations Selected Previous Product Affiliations Seth Lederman, MD CEO & Chairman • Vela • Targent • Validus • Fontus Benjamin Selzer COO • Reliant • Aton • Investment Banking (Lehman, BofA) Leland Gershell, MD, PhD CFO • Cowen • Apothecary • Favus • Madison Williams Bruce Daugherty, PhD, MBA Senior Director of Drug Development • Merck • Roche Institute
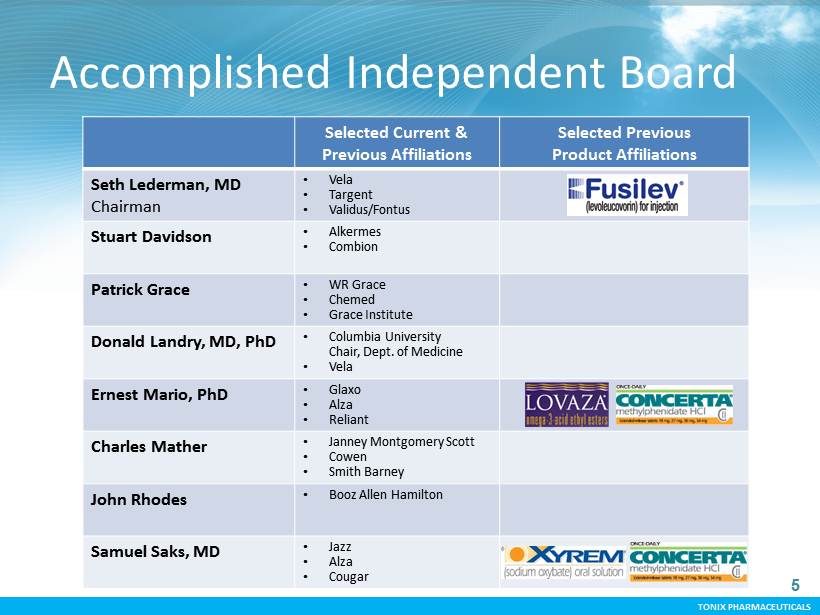
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS 5 Accomplished Independent Board Selected Current & Previous Affiliations Selected Previous Product Affiliations Seth Lederman, MD Chairman • Vela • Targent • Validus/Fontus Stuart Davidson • Alkermes • Combion Patrick Grace • WR Grace • Chemed • Grace Institute Donald Landry, MD, PhD • Columbia University Chair, Dept. of Medicine • Vela Ernest Mario, PhD • Glaxo • Alza • Reliant Charles Mather • Janney Montgomery Scott • Cowen • Smith Barney John Rhodes • Booz Allen Hamilton Samuel Saks, MD • Jazz • Alza • Cougar
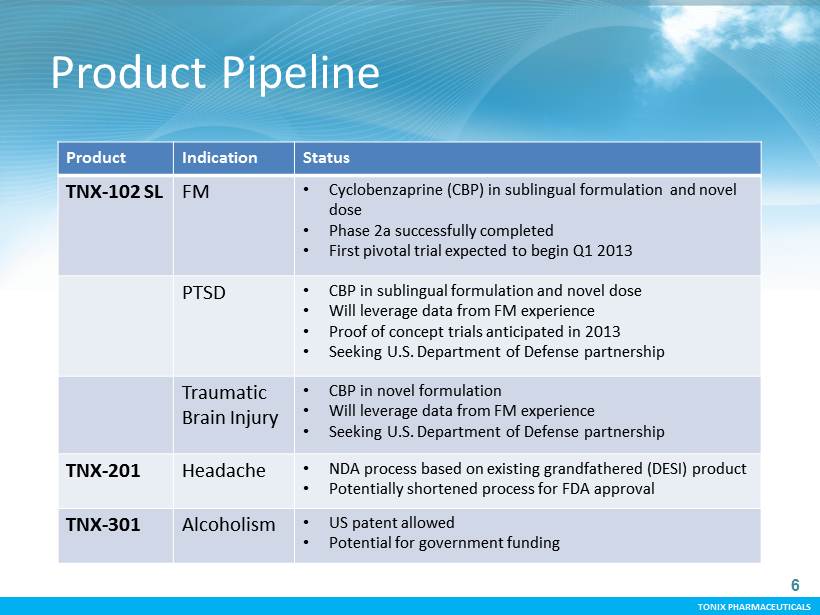
TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS Product Pipeline 6 Product Indication Status TNX - 102 SL FM • C yclobenzaprine (CBP) in sublingual formulation and novel dose • Phase 2a successfully completed • First pivotal t rial expected to begin Q1 2013 PTSD • CBP in sublingual formulation and novel dose • Will leverage data from FM experience • Proof of concept trials anticipated in 2013 • Seeking U.S. Department of Defense partnership Traumatic Brain Injury • CBP in novel formulation • Will leverage data from FM experience • Seeking U.S. Department of Defense partnership TNX - 201 Headache • NDA process based on existing grandfathered (DESI) product • Potentially shortened process for FDA approval TNX - 301 Alcoholism • US patent allowed • Potential for government funding

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS FM Market Opportunity • ~5 million U.S. patients* • U.S. prescription drug market estimated at $1.4 billion** - 2007 - 2010 CAGR of 18.4%*** • First approved drug for FM in 2007 - Lyrica® (Pfizer) approved 2007: $450 million in FM sales in 2011** - Cymbalta® (Eli Lilly) approved 2008: $560 million in FM sales in 2011** - Savella® (Forest) approved 2009: $137 million in FM sales in 2011** * National Institutes of Health, U.S. Department of Health and Human Services ** Decision Resources Pain Management Study: Fibromyalgia, January 2012 *** Frost & Sullivan Fibromyalgia Market Study, December 2010 7

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS FM Market Dynamics • No FDA approved drugs until 2007 • Market growth driven by on - label drugs replacing off - label generics* - Lyrica replacing off - label generic analgesics - Cymbalta and Savella replacing off - label generic anti - depressants • Drugs for pain and mood approved, yet none for disturbed or non - restorative sleep - TNX - 102 to replace off - label generic muscle relaxants currently being used to address this problem 8 * Frost & Sullivan Fibromyalgia Market Study, December 2010
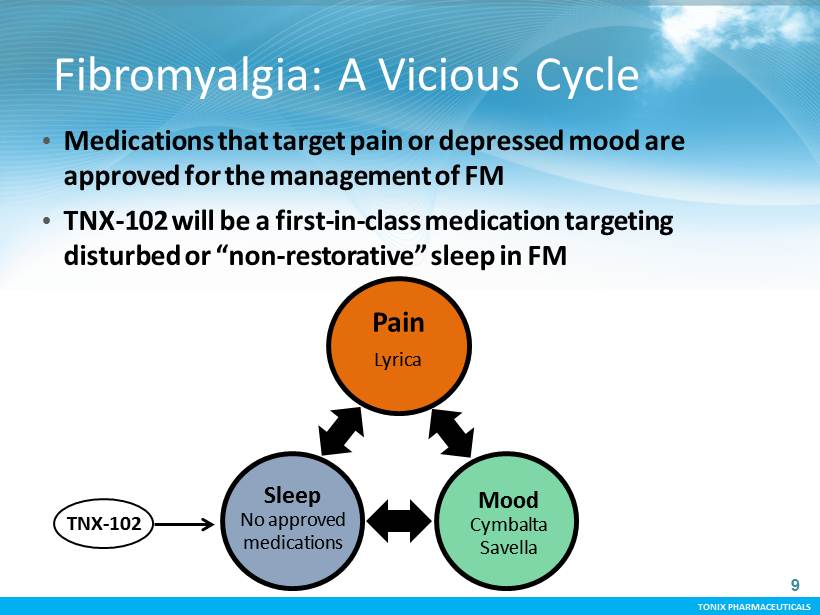
TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS Fibromyalgia: A Vicious Cycle 9 • Medications that target pain or depressed mood are approved for the management of FM • TNX - 102 will be a first - in - class medication targeting disturbed or “non - restorative” sleep in FM TNX - 102 Pain Lyrica Sleep No approved medications Mood Cymbalta Savella
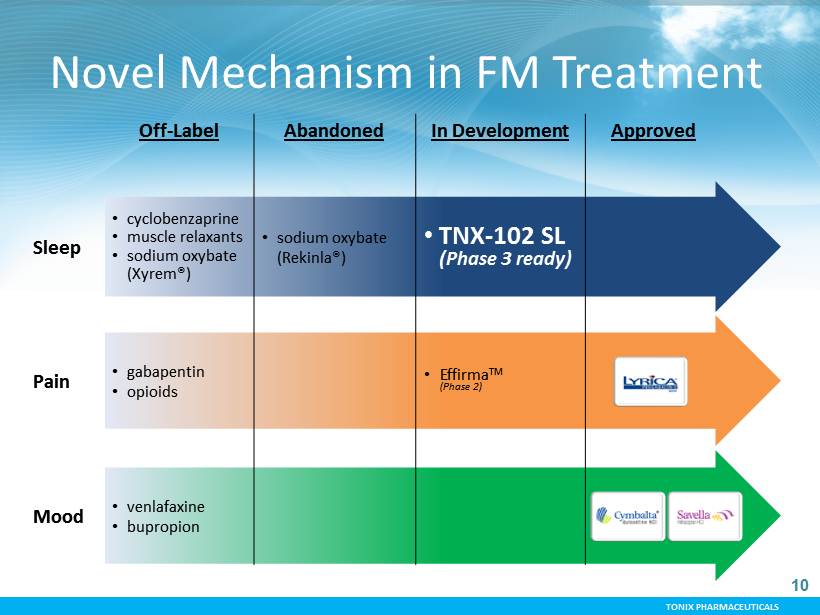
TONIX PHARMACEUTICALS 10 Off - Label Abandoned In Development Approved Sleep • cyclobenzaprine • muscle relaxants • sodium oxybate (Xyrem®) • sodium oxybate (Rekinla®) • TNX - 102 SL (Phase 3 ready) Pain • gabapentin • opioids • Effirma TM (Phase 2) Mood • venlafaxine • bupropion Novel Mechanism in FM Treatment

TONIX PHARMACEUTICALS TNX - 102: Optimizing CBP for FM 11 • CBP widely used off - label in FM • Current doses and formulations poorly suited for FM - Long half - life contributes to somnolence and accumulation - Lowest approved daily dose is 15 mg • Phase 2a trial of bedtime VLD cyclobenzaprine demonstrated improvement in core FM symptoms • TNX - 102 is designed specifically for FM management - Rapid absorption - Minimize next day somnolence - Suitable for chronic use - Appropriate dose

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS CBP: Impressive Safety, Widely Used • Off - label CBP is the third most widely prescribed medication for FM* • 1977: FDA approved Flexeril® (Merck) • 1990s: Extensive safety and efficacy studies (Merck) • 2007: FDA approved controlled - release formulation (15, 30 mg) • 2010: >1 billion tablets prescribed annually • Not a controlled substance, no recognized addictive potential 12 * Frost & Sullivan Fibromyalgia Market Study, December 2010

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Relationship between Sleep and FM • FM patients complain of poor sleep - Non - restorative sleep exacerbates FM symptoms • Cyclic alternating pattern (CAP) is an objective physiological measure of the quality of sleep - A2, A3 patterns = indices of sleep instability (poor sleep quality) - A1 pattern = index of sleep stability • FM patients demonstrate increased A2+A3 13

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Sodium Oxybate Data in FM & Sleep • Jazz Pharmaceuticals was developing sodium oxybate for FM • Phase 3 trials demonstrated highly significant improvements in fibromyalgia symptoms Study Treatment n Responders* n (%) p - value (chi - square) Placebo 183 50 (27) - 06 - 008 oxybate 4.5 g 182 84 (46) <0.001 oxybate 6.0 g 182 72 (40) 0.01 Placebo 188 38 (20) - 06 - 009 oxybate 4.5 g 194 69 (36) <0.001 oxybate 6.0 g 189 68 (36) <0.001 • Sodium oxybate also caused decrease in A2+A3 CAP Rate Placebo ( n = 20) Oxybate 4.5 g (n = 14) p vs. placebo O xybate 6.0 g (n = 13) p vs. placebo A2+A3, % - 0.4 - 2.1 0.18 - 3.9 0.007 A1, % - 0.1 +5.8 0.172 +7.0 0.108 14 Source: Moldofsky et al., J. Rheum. October 2010: http://jrheum.org/content/37/10/2156.full.pdf+html?sid=74107235 - af7a - 478a - 8ed3 - ebf7e2abe3aa Source: FDA briefing documents. * Subjects that reported a 30% or more reduction in overall pain in week 14 as compared to baseline

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Very Low Dose CBP (VLDC) FM Phase 2 a – Overview • Published in Journal of Rheumatology * December 2011 • Harvey Moldofsky, MD – lead investigator (University of Toronto) • Patients with documented FM • Double blind, randomized, placebo controlled • 36 patients; 18 per arm • VLDC or placebo taken between dinner and bedtime daily • Eight - week, dose escalating study, from 1mg to 4mg • Average dose at week eight was 3.1mg • Conducted at two academic centers in Canada 15 * Moldofsky et al., J. Rheum. December 2011: http://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS VLDC FM Phase 2a – Endpoints • Endpoints consistent with ACR* and OMERACT** guidelines • Pain (Visual Analog Scale) and fatigue assessed ̴ 24 hours following each dose • Tenderness assessed via dolorimetry • Mood assessed via Hospital Anxiety and Depression (HAD) scale and HAD depression subscale • Fatigue also measured via clinical and patient global impression of change (CGIC/PGIC) * American College of Rheumatology **Outcome Measures in Rheumatology Clinical Trials 16
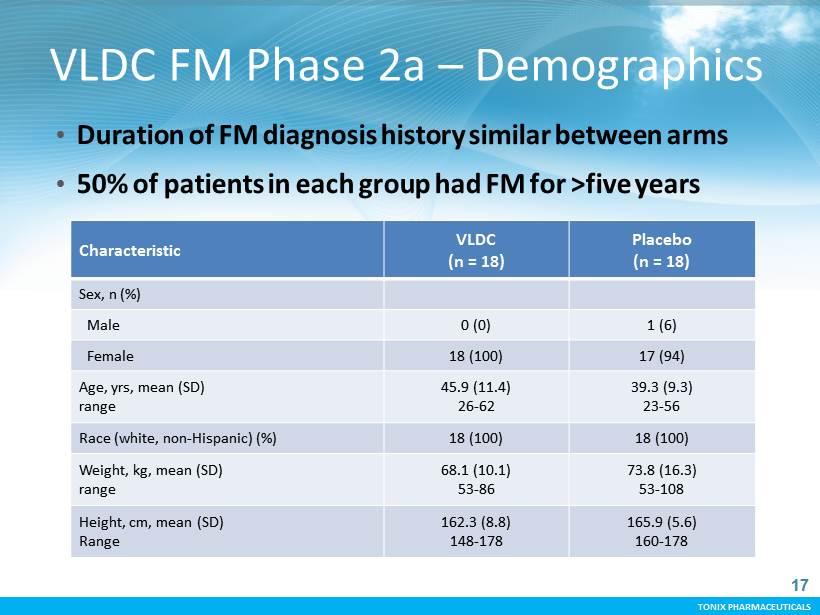
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Characteristic VLD C (n = 18) Placebo (n = 18) Sex, n (%) Male 0 (0) 1 (6) Female 18 (100) 17 (94) Age, yrs, mean (SD) range 45.9 (11.4) 26 - 62 39.3 (9.3) 23 - 56 Race (white, non - Hispanic) (%) 18 (100) 18 (100) Weight, kg, mean (SD) range 68.1 (10.1) 53 - 86 73.8 (16.3) 53 - 108 Height, cm, mean (SD) Range 162.3 (8.8) 148 - 178 165.9 (5.6) 160 - 178 VLDC FM Phase 2a – Demographics • Duration of FM diagnosis history similar between arms • 50% of patients in each group had FM for >five years 17
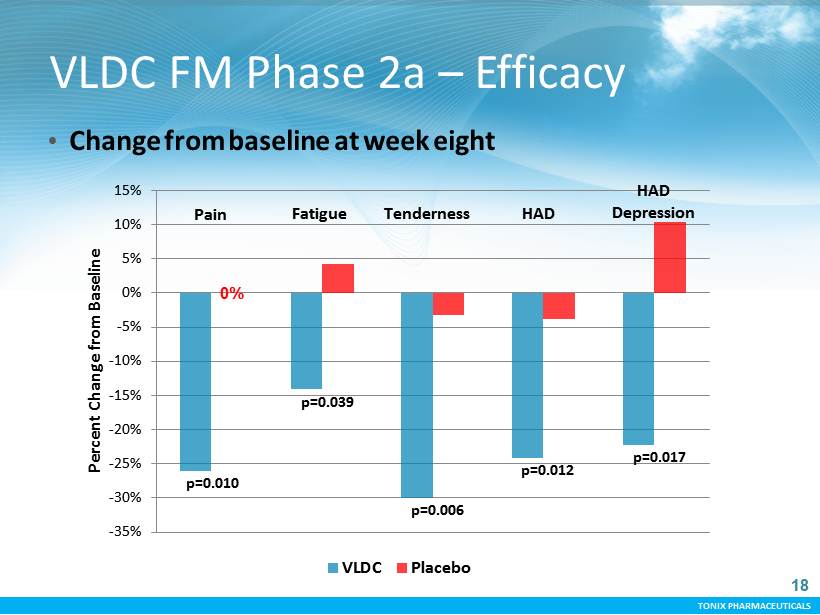
TONIX PHARMACEUTICALS -35% -30% -25% -20% -15% -10% -5% 0% 5% 10% 15% Percent Change from Baseline VLDC Placebo p=0.010 p=0.039 p=0.006 p=0.012 p=0.017 Fatigue HAD HAD Depression Pain Tenderness 0% 18 VLDC FM Phase 2a – Efficacy • Change from baseline at week eight
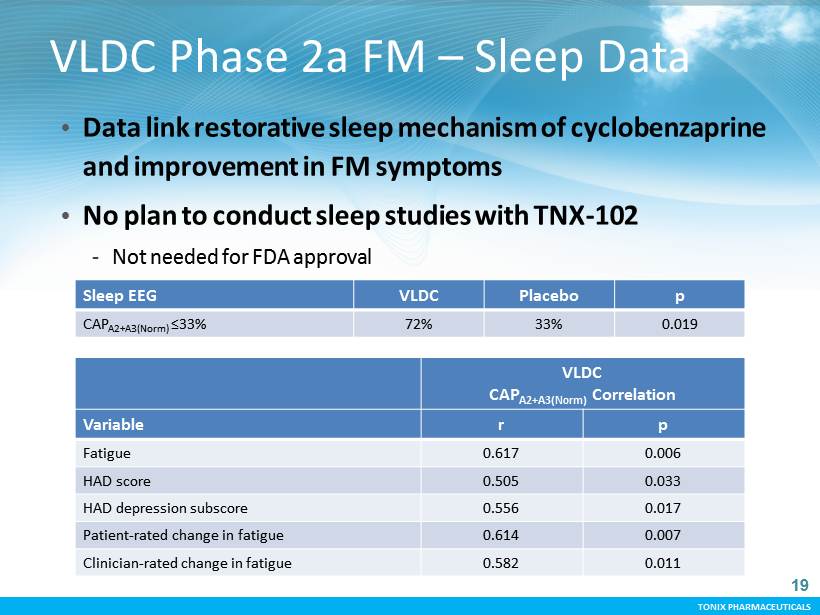
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS VLDC Phase 2a FM – Sleep Data VLDC CAP A2+A3(Norm) Correlation Variable r p Fatigue 0.617 0.006 HAD score 0.505 0.033 HAD depression subscore 0.556 0.017 Patient - rated change in fatigue 0.614 0.007 Clinician - rated change in fatigue 0.582 0.011 • Data link restorative sleep mechanism of cyclobenzaprine and improvement in FM symptoms • No plan to conduct sleep studies with TNX - 102 - Not needed for FDA approval Sleep EEG VLDC Placebo p CAP A2+A3(Norm) ≤33% 72% 33% 0.019 19

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TNX - 102 SL: Sublingual CBP • Faster and more efficient transmucosal absorption - First - in - class sleep quality treatment indicated for chronic, bedtime dosing - Targeting rapid onset and decreased next - morning hang - over • Avoids “first pass” liver production of persistent metabolite - Norcyclobenzaprine is a psychoactive metabolite that interacts with similar brain receptors and accumulates over time decreasing CBP effects • Proprietary formulation - Human PK study of sublingual solutions compared TONIX’s proprietary formulation technology with cyclobenzaprine alone: PK characteristics are not replicated by crushing Flexeril® tablets 20
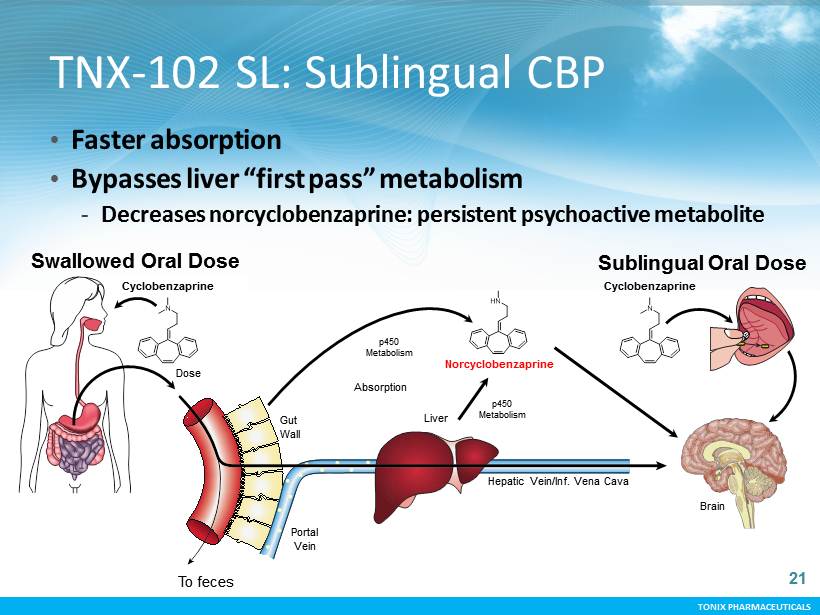
TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TNX - 102 SL: Sublingual CBP • Faster absorption • Bypasses liver “first pass” metabolism - Decreases norcyclobenzaprine: persistent psychoactive metabolite Cyclobenzaprine p450 Metabolism To feces Liver Dose Absorption Norcyclobenzaprine Sublingual Oral Dose Swallowed Oral Dose Cyclobenzaprine p450 Metabolism Brain Portal Vein Gut Wall Hepatic Vein/Inf. Vena Cava 21

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TNX - 102 SL: Pivotal Development in FM • First Phase 3 efficacy trial in FM to begin in Q 1 2013 - Randomized, double - blind, placebo controlled - 12 - week treatment period - Pre - defined efficacy endpoint of pain • Streamlined first pivotal focused on pain - 76 patients; 8 - 10 U.S. centers - Topline results expected by YE 2013 - Cost and time efficient - Other FM endpoints will be studied to inform second Phase 3 trial design • Subsequent requirements for FDA approval - 24 week double - blind, randomized, pivotal in 300 patients - Open - label safety exposure study per ICH guidelines (≥ 100 patients x one year) 22

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102 SL: Unique Market Position in FM • Specifically designed for the treatment of FM • Differentiated from / not competitive with other therapies - First - in - class sleep quality treatment indicated for bedtime dosing - Restorative sleep shown to improve key symptoms - High patient dissatisfaction, physicians frequently switch drugs • With a unique formulation and new indication, reimbursement coverage of TNX - 102 is expected - Initial payor market research indicates strong likelihood of reimbursement 23

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS TNX - 102 SL: Sublingual CBP for PTSD • 3.5 % of U.S. adult population has suffered from PTSD in past 12 months* - Any trauma can lead to PTSD • Unsatisfied market - Only Zoloft® and Paxil® have FDA approval • Widespread painkiller abuse and addiction • Leverage formulation and clinical work in FM for PTSD • Phase 2 proof of concept study to be conducted in 2013 24 * National Institutes of Mental Health & National Institutes of Health

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS FM & PTSD are Related Conditions • Symptom overlap - PTSD is thought to be exacerbated by non - restorative sleep - Some are believed to suffer from both conditions simultaneously - Some patients with FM meet PTSD criteria, and vice versa • PTSD has both combat and civilian forms - Zoloft and Paxil are approved for PTSD - Brand prescriptions filled by generic sertraline and paroxetine - Department of Defense has a strong interest in promoting research on therapeutics 25
TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Intellectual Property • Active patenting strategy to extend TNX - 102 SL exclusivity • Pharmacokinetics (PK) - Patent filed around unique PK profile with sublingual (June 2012) • Surprising and unexpected observations • Protection expected through 2033 - Difficult patent class to circumvent • Method of Use - FM : issued patent , expiration mid - 2021 - PTSD : patent filed in 2010 26

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS 27 Timing Milestones Related to TNX - 102 SL in Fibromyalgia Q4 2012 • Completion of human PK on commercial formulation, dose Q1 2013 • Commencement of first pivotal trial Q4 2013 • Topline results from first pivotal trial • Evaluate partnership opportunities Timing Milestones Related to TNX - 102 SL in PTSD H1 2013 • Commencement of proof of concept study in PTSD patients Upcoming Milestones

TONIX PHARMACEUTICALS CONFIDENTIAL TONIX PHARMACEUTICALS Investment Summary • Significant unmet needs and large market opportunities • First - in - class products; not competitive with existing therapies • Capital efficient, low risk drug development strategy • Near - term value inflection points • Experienced management and board 28

TONIX PHARMACEUTICALS TONIX PHARMACEUTICALS 29 Full Name: Tonix Pharmaceuticals Holding Corp. Ticker: TNXP Exchange: OTC/QB Common Shares Outstanding: 34.3 million 52 - week Trading Range*: $0.83 - $2.06 Auditor: EisnerAmper LLP Corporate Counsel: Sichenzia Ross Friedman Ference LLP Transfer Agent: Vstock Transfer, LLC TONIX Public Profile * Stock first traded in February 2012

OTC/QB: TNXP