

Corporate Presentation January 2013 OTC/QB: TNXP

TONIX PHARMACEUTICALS 2 CONFIDENTIAL TONIX PHARMACEUTICALS Disclosures Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as "anticipate," "believe," "forecast," " estimate" and "intend," among others . These forward - looking statements are based on TONIX’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our ability to continue as a going concern ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payer reimbursement ; limited sales and marketing efforts and dependence upon third parties ; and risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . TONIX does not undertake an obligation to update or revise any forward - looking statement . Investors should read the risk factors set forth in the Annual Report on Form 10 - K filed with the SEC on March 30 , 2012 and future periodic reports filed with the Securities and Exchange Commission . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
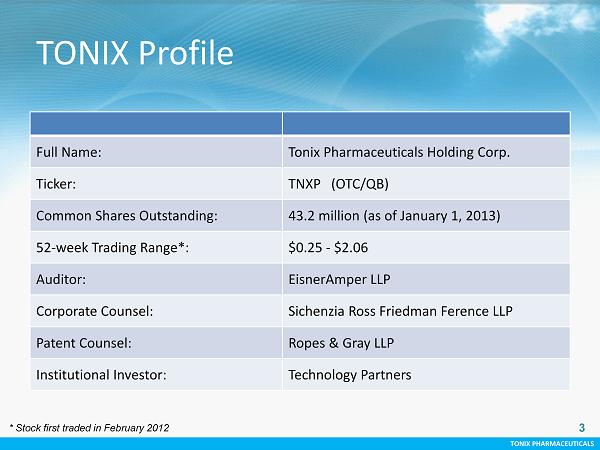
TONIX PHARMACEUTICALS 3 CONFIDENTIAL TONIX PHARMACEUTICALS 3 Full Name: Tonix Pharmaceuticals Holding Corp. Ticker: TNXP (OTC/QB) Common Shares Outstanding: 43.2 million (as of January 1, 2013) 52 - week Trading Range*: $0.25 - $2.06 Auditor: EisnerAmper LLP Corporate Counsel: Sichenzia Ross Friedman Ference LLP Patent Counsel: Ropes & Gray LLP Institutional Investor: Technology Partners TONIX Profile * Stock first traded in February 2012

TONIX PHARMACEUTICALS 4 Company Overview • Developing novel medications for challenging disorders of the central nervous system (CNS) - Large and underserved indications • Pivotal trial in fibromyalgia (FM) to report in 2013 - Phase 2 data demonstrated efficacy - Unique, non - addictive treatment approach – targeting sleep quality • Capital - efficient strategy mitigates risk and cost - 505(b)(2) leverages established safety database • Strong market exclusivity on lead product candidates - Protection expected to 2033 on proprietary sublingual tablet • Experienced management and board 4

TONIX PHARMACEUTICALS 5 CONFIDENTIAL TONIX PHARMACEUTICALS Experienced Leadership Selected Previous Corporate Affiliations Selected Previous Product Affiliations Seth Lederman, MD CEO & Chairman • Vela • Targent • Validus • Fontus Leland Gershell, MD, PhD CFO • Cowen • Apothecary Capital • Favus Research • Madison Williams Bruce Daugherty, PhD, MBA Senior Director of Drug Development • Merck • Roche Institute 5
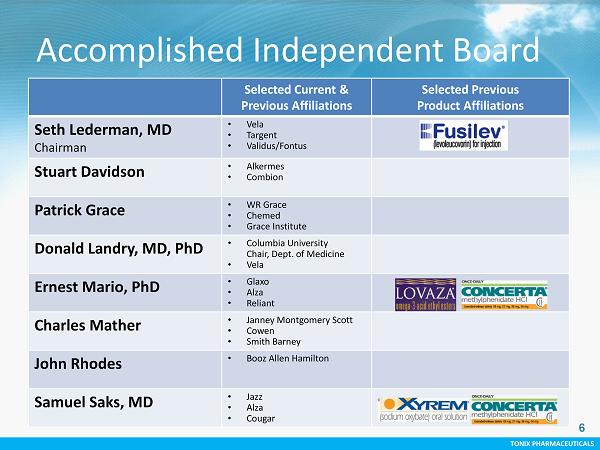
TONIX PHARMACEUTICALS 6 CONFIDENTIAL TONIX PHARMACEUTICALS Accomplished Independent Board Selected Current & Previous Affiliations Selected Previous Product Affiliations Seth Lederman, MD Chairman • Vela • Targent • Validus/Fontus Stuart Davidson • Alkermes • Combion Patrick Grace • WR Grace • Chemed • Grace Institute Donald Landry, MD, PhD • Columbia University Chair, Dept. of Medicine • Vela Ernest Mario, PhD • Glaxo • Alza • Reliant Charles Mather • Janney Montgomery Scott • Cowen • Smith Barney John Rhodes • Booz Allen Hamilton Samuel Saks, MD • Jazz • Alza • Cougar 6
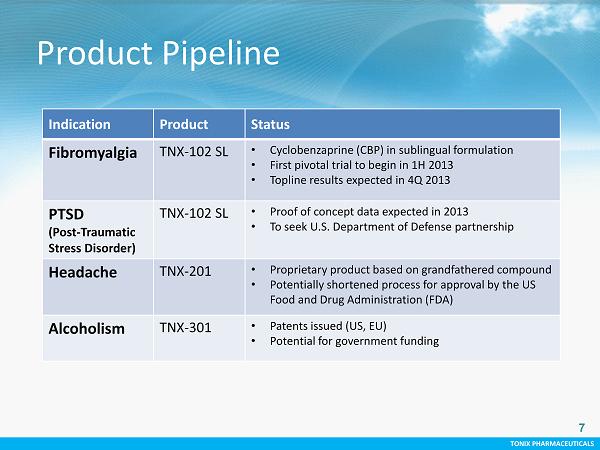
TONIX PHARMACEUTICALS 7 CONFIDENTIAL TONIX PHARMACEUTICALS Product Pipeline 7 Indication Product Status Fibromyalgia TNX - 102 SL • C yclobenzaprine (CBP) in sublingual formulation • First pivotal t rial to begin in 1H 2013 • Topline results expected in 4Q 2013 PTSD (Post - Traumatic Stress Disorder) TNX - 102 SL • Proof of concept data expected in 2013 • To seek U.S. Department of Defense partnership Headache TNX - 201 • Proprietary product based on grandfathered compound • Potentially shortened process for approval by the US Food and Drug Administration (FDA) Alcoholism TNX - 301 • Patents issued (US, EU) • Potential for government funding

TONIX PHARMACEUTICALS 8 TONIX PHARMACEUTICALS 8 Fibromyalgia TNX - 102 SL Program:

TONIX PHARMACEUTICALS 9 • Complex syndrome - Despite three FDA - approved medications, patients are dissatisfied - FDA primary endpoint is pain • Complaint: “Hurt all over, can’t sleep” - Central pain – originates in the CNS - Restorative sleep can improve pain and other symptoms - No benefit from opiates or prescription sleep drugs • ~90% of diagnosed patients are female - Management believes affected men may self - medicate Fibromyalgia 9 Pain Sleep Mood

TONIX PHARMACEUTICALS 10 CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia Market Opportunity • ~5 million U.S. patients* • U.S. prescription drug market in 2011 ~$1.4 billion** - 2007 – 2011 CAGR of 17%** • First approved drug for fibromyalgia in 2007 • Market growth driven by on - label drugs replacing legacy off - label generics*** * National Institutes of Health, U.S. Department of Health and Human Services ** Decision Resources Pain Management Study: Fibromyalgia, January 2012 *** Frost & Sullivan Fibromyalgia Market Study, December 2010 **** EU only Product Company Prior Indication Approval Year 2011 U.S. S ales in FM** Lyrica® Pfizer Pain (neuropathic) 2007 $450 million Cymbalta® Eli Lilly Depression 2008 $560 million Savella® Forest Depression**** 2009 $101 million 10

TONIX PHARMACEUTICALS 11 Managed Care Perspective on FM • Fibromyalgia presents a significant economic burden - Studies show high cost in overall care, lost productivity, and disability • Physicians and payors are aware of high unmet need in pharmacological treatment of fibromyalgia - Patients take many products without evidence of efficacy • All FDA - approved fibromyalgia products are branded and on - patent - Reimbursed at Tier 2 and enjoy growing sales in fibromyalgia - Growth continues despite presence of legacy off - label generics in Tier 1 11
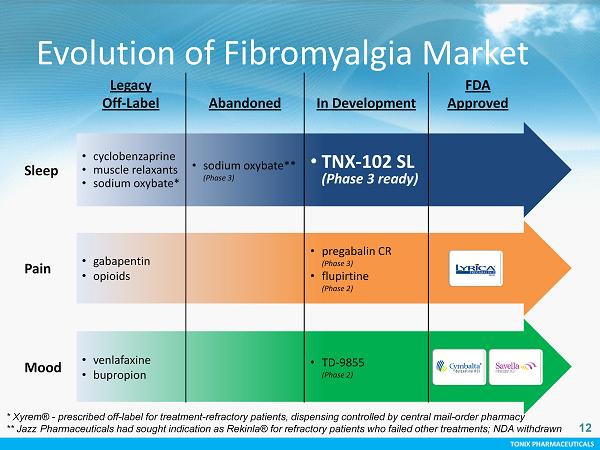
TONIX PHARMACEUTICALS 12 Legacy Off - Label Abandoned In Development FDA Approved Sleep • cyclobenzaprine • muscle relaxants • sodium oxybate * • sodium oxybate ** (Phase 3) • TNX - 102 SL (Phase 3 ready) Pain • gabapentin • opioids • pregabalin CR (Phase 3) • flupirtine (Phase 2) Mood • venlafaxine • bupropion • TD - 9855 (Phase 2) Evolution of Fibromyalgia Market * Xyrem ® - prescribed off - label for treatment - refractory patients, dispensing controlled by central mail - order pharmacy ** Jazz Pharmaceuticals had sought indication as Rekinla ® for refractory patients who failed other treatments; NDA withdrawn 12

TONIX PHARMACEUTICALS 13 CONFIDENTIAL TONIX PHARMACEUTICALS Sleep Quality: Validated Target in FM • TNX - 102 SL expected to be first - in - class FDA - approved product targeting sleep quality for the “management of FM ” • Cyclobenzaprine is FDA approved as a muscle relaxant but has off - label use as a slow - acting sleep aid in fibromyalgia TNX - 102 SL Mood Other cyclobenzaprine formulations approved as muscle relaxants Previously approved for treating neuropathic pain Previously approved for treating depression Pain Sleep

TONIX PHARMACEUTICALS 14 CONFIDENTIAL TONIX PHARMACEUTICALS Cyclobenzaprine: Impressive Safety, Widely Used • Not a controlled substance • No recognized addictive potential • FDA approved as Flexeril ® since 1977 for muscle spasm • Used off - label as slow - acting sleep aid in fibromyalgia • Not approved for treating sleep • Alza bought Flexeril from Merck in 2001 • High - dose controlled - release product for muscle spasm ( Amrix ®) approved by FDA in 2007 14
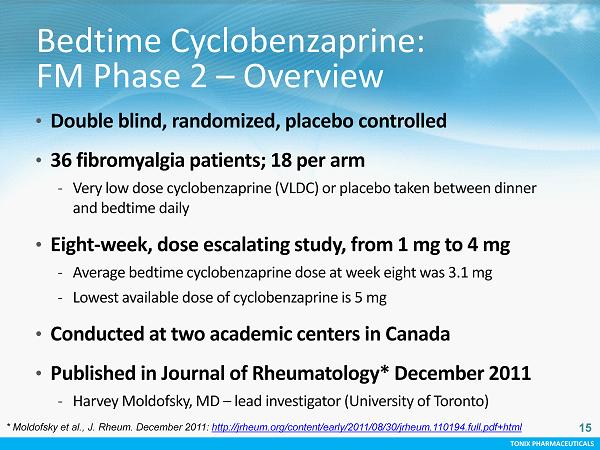
TONIX PHARMACEUTICALS 15 CONFIDENTIAL TONIX PHARMACEUTICALS Bedtime Cyclobenzaprine: FM Phase 2 – Overview • Double blind, randomized, placebo controlled • 36 fibromyalgia patients; 18 per arm - Very low dose cyclobenzaprine (VLDC) or placebo taken between dinner and bedtime daily • Eight - week, dose escalating study, from 1 mg to 4 mg - Average bedtime cyclobenzaprine dose at week eight was 3.1 mg - Lowest available dose of cyclobenzaprine is 5 mg • Conducted at two academic centers in Canada • Published in Journal of Rheumatology* December 2011 - Harvey Moldofsky, MD – lead investigator (University of Toronto) * Moldofsky et al., J. Rheum. December 2011: http://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html 15
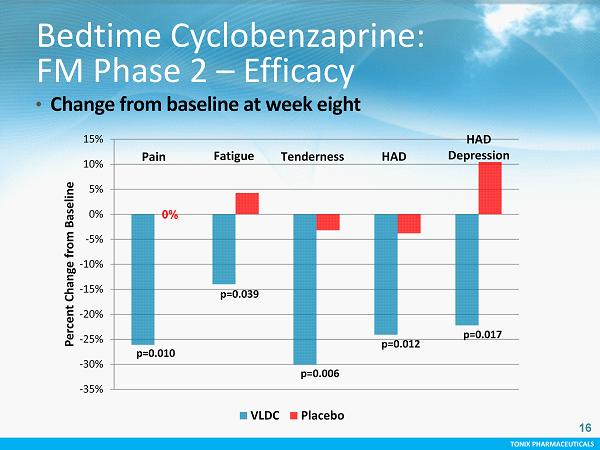
TONIX PHARMACEUTICALS 16 -35% -30% -25% -20% -15% -10% -5% 0% 5% 10% 15% Percent Change from Baseline VLDC Placebo p=0.010 p=0.039 p=0.006 p=0.012 p=0.017 Fatigue HAD HAD Depression Pain Tenderness 0% Bedtime Cyclobenzaprine: FM Phase 2 – Efficacy • Change from baseline at week eight 16

TONIX PHARMACEUTICALS 17 Current CBP Products Not Optimal for Chronic Fibromyalgia Treatment • Current daytime regimens poorly suited for FM - Chronic daytime cyclobenzaprine may confound long - term efficacy • Current doses poorly suited for bedtime use - Off - label use of available doses of cyclobenzaprine at bedtime associated with next - morning grogginess • Current formulations poorly suited for bedtime use - Slow systemic absorption via oral route • Despite shortcomings, legacy off - label cyclobenzaprine is widely used in the management of fibromyalgia 17

TONIX PHARMACEUTICALS 18 CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102 SL : First - in - Class Fibromyalgia Medicine • Chronic bedtime dosing - Drug exposure during the night to target non - restorative sleep • Lower dose than available CBP tablets - Tailored to reduce next - morning grogginess • Proprietary sublingual formulation - Transmucosal delivery - Rapid systemic absorption demonstrated in humans - Avoids “first - pass” metabolism which produces psychoactive metabolite 18
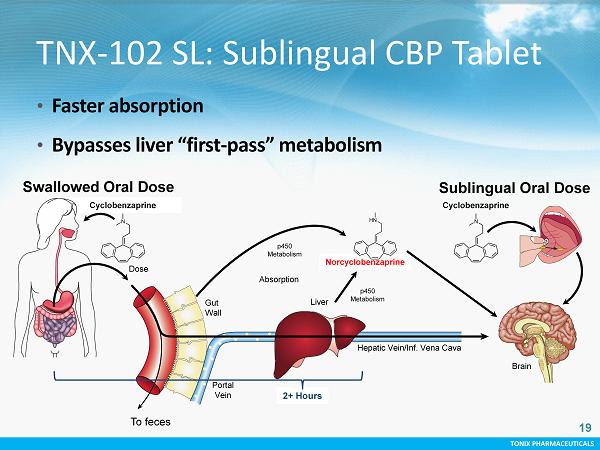
TONIX PHARMACEUTICALS 19 CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102 SL: Sublingual CBP Tablet • Faster absorption • Bypasses liver “first - pass” metabolism Cyclobenzaprine p450 Metabolism To feces Liver Dose Absorption Norcyclobenzaprine Sublingual Oral Dose Swallowed Oral Dose Cyclobenzaprine p450 Metabolism Brain Portal Vein Gut Wall Hepatic Vein/Inf. Vena Cava 2+ Hours 19
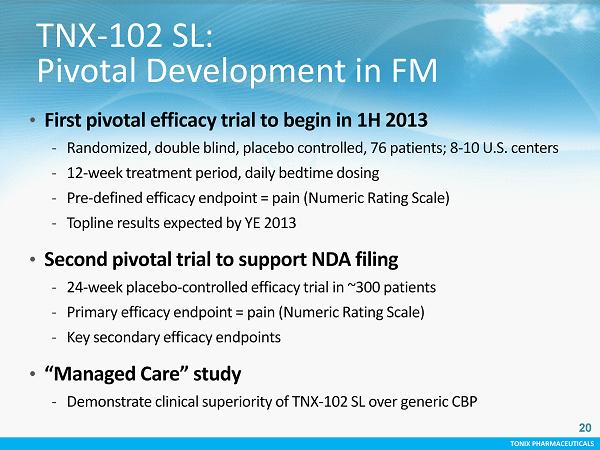
TONIX PHARMACEUTICALS 20 CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102 SL: Pivotal Development in FM • First pivotal efficacy trial to begin in 1H 2013 - Randomized, double blind, placebo controlled, 76 patients; 8 - 10 U.S. centers - 12 - week treatment period, daily bedtime dosing - Pre - defined efficacy endpoint = pain (Numeric Rating Scale) - Topline results expected by YE 2013 • Second pivotal trial to support NDA filing - 24 - week placebo - controlled efficacy trial in ~300 patients - Primary efficacy endpoint = pain (Numeric Rating Scale) - Key secondary efficacy endpoints • “ Managed Care” study - Demonstrate clinical superiority of TNX - 102 SL over generic CBP 20

TONIX PHARMACEUTICALS 21 TONIX PHARMACEUTICALS 21 Post - Traumatic Stress Disorder TNX - 102 SL Program:

TONIX PHARMACEUTICALS 22 TONIX PHARMACEUTICALS TNX - 102 SL: Sublingual CBP for PTSD • Patients experience disturbed sleep and widespread pain - Painkiller abuse and addiction is common • 3.5% of U.S. adult population has suffered from PTSD in past 12 months* - Experiencing any trauma can lead to PTSD • Unsatisfied market - Zoloft ® and Paxil® are the only FDA approved products for PTSD • Phase 2 proof - of - concept study to be conducted in 2013 - Pre - IND meeting held October 2012 encourages further development - Leverage fibromyalgia formulation and clinical work * National Institutes of Mental Health & National Institutes of Health 2010 22

TONIX PHARMACEUTICALS 23 CONFIDENTIAL TONIX PHARMACEUTICALS Timing Milestones - Fibromyalgia 1H 2013 • Commence first pivotal trial 4Q 2013 • Topline results from first pivotal trial • Evaluate partnership opportunities Timing Milestones - PTSD 1H 2013 • Commence proof of concept study in PTSD patients Upcoming Milestones 23

TONIX PHARMACEUTICALS 24 CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 102 SL: Intellectual Property • Pharmacokinetics (PK) - Patent filed around unique PK profile • Surprising and unexpected observations • Protection expected through 2033 - Difficult patent class to circumvent • Method of Use - FM: issued patent, expiration mid - 2021 - PTSD: patent filed in 2010 • Composition of Matter - Patent in preparation 24

TONIX PHARMACEUTICALS 25 Investment Highlights • Developing novel medications for challenging disorders of the central nervous system - Large and underserved indications • Pivotal trial in fibromyalgia to report in 2013 - Phase 2 data demonstrated efficacy - Unique, non - addictive treatment approach – targeting sleep quality • Capital - efficient strategy mitigates risk and cost - 505(b)(2) leverages established safety database • Strong market exclusivity on lead product candidates - Protection expected to 2033 on proprietary sublingual tablet • Experienced management and board 25

OTC/QB: TNXP