

Investor Presentation August 2013 NASDAQ: TNXP

TONIX PHARMACEUTICALS 2 CONFIDENTIAL TONIX PHARMACEUTICALS Safe Harbor Statement Certainstatementsinthispresentationregardingstrategicplans,expectationsandobjectives forfutureoperationsorresultsare“forward-lookingstatements”asdefinedbythePrivate SecuritiesLitigationReformActof1995.Thesestatementsmaybeidentifiedbytheuseof forward-lookingwordssuchas"anticipate,""believe,""forecast,""estimate"and"intend," amongothers.Theseforward-lookingstatementsarebasedonTONIX’scurrentexpectations andactualresultscoulddiffermaterially.Thereareanumberoffactorsthatcouldcauseactual eventstodiffermateriallyfromthoseindicatedbysuchforward-lookingstatements.These factorsinclude,butarenotlimitedto,substantialcompetition;ourabilitytocontinueasa goingconcern;ourneedforadditionalfinancing;uncertaintiesofpatentprotectionand litigation;uncertaintiesofgovernmentorthirdpartypayerreimbursement;limitedsalesand marketingeffortsanddependenceuponthirdparties;andrisksrelatedtofailuretoobtainU.S. FoodandDrugAdministrationclearancesorapprovalsandnoncompliancewithitsregulations. Aswithanypharmaceuticalunderdevelopment,therearesignificantrisksinthedevelopment, regulatoryapprovalandcommercializationofnewproducts.Theforward-lookingstatements inthispresentationaremadeasofthedateofthispresentation,evenifsubsequentlymade availablebytheCompanyonitswebsiteorotherwise.TONIXdoesnotundertakeanobligation toupdateorreviseanyforward-lookingstatement,exceptasrequiredbylaw.Investorsshould readtheriskfactorssetforthintheAnnualReportonForm10-KfortheyearendedDecember 31,2012,asfiledwiththeSecuritiesandExchangeCommission(the“SEC”)onMarch11,2013 andfutureperiodicreportsfiledwiththeSEConorafterthedatehereof.AlloftheCompany's forward-lookingstatementsareexpresslyqualifiedbyallsuchriskfactorsandothercautionary statements.

TONIX PHARMACEUTICALS 3 CONFIDENTIAL TONIX PHARMACEUTICALS Investment Highlights Developing novel medications for central pain disorders Addressing large and unmet needs in the central neuropathic pain market Fibromyalgia –lead indication Phase 2b/3 trial of TNX-102 SL to report in 2H 2014 Significant efficacy on core symptoms demonstrated in Phase 2a Targets pain-sleep ‘vicious cycle’ –unique, non-addictive treatment approach Additional market opportunities Post-traumatic stress disorder (PTSD) Headache, alcoholism Capital-and time-efficient FDA strategy 505(b)(2) pathway: faster timeline and reduced risk Strong market exclusivity Patent protection on lead candidate expected to extend to 2033
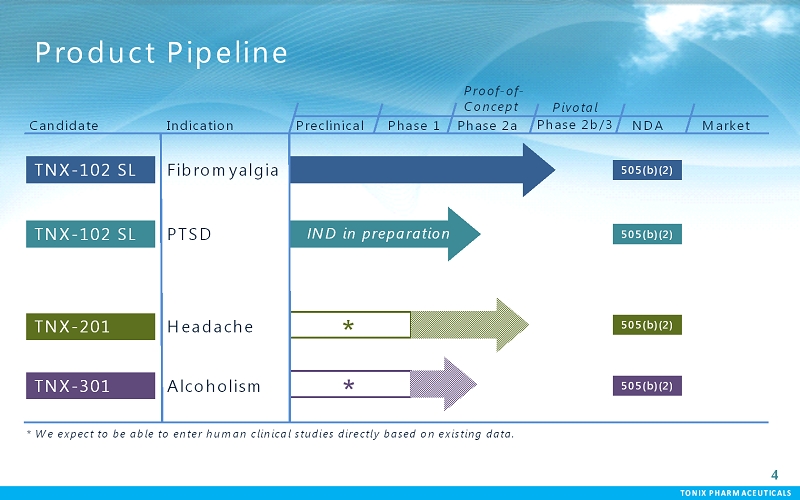
TONIX PHARMACEUTICALS 4 CONFIDENTIAL TONIX PHARMACEUTICALS Product Pipeline TNX-102 SL PTSD Fibromyalgia Preclinical Phase 1 Proof-of- Concept Pivotal NDA Market TNX-102 SL Candidate Indication TNX-201 Headache TNX-301 Alcoholism * * We expect to be able to enter human clinical studies directly based on existing data. * 505(b)(2) 505(b)(2) 505(b)(2) 505(b)(2) Phase 2a Phase 2b/3 IND in preparation

TONIX PHARMACEUTICALS 5 CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia (FM) –Lead Program Patients feel pain all over the body, but it originates in the brain Chronic, widespread pain with sleep, fatigue, mood, and memory problems Impairs daily function and productivity: poor quality of life Typical onset age 20-60; predominantly female Recognized by health authorities in U.S., Canada, and Japan Patients desperate for new therapies despite three approved products Patients often take multiple medications (“polypharmacy”) ‘Off-label’ use of opioids and sedative-hypnotics provide no sustained benefit Expensive, burdensome condition for healthcare system Health utilization and medication costs are substantial Managed care / payorsrecognize need for new therapies Large opportunity for an effective, well-tolerated, differentiated product

TONIX PHARMACEUTICALS 6 CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia Market Opportunity *National Institutes of Health, U.S. Department of Health and Human Services **Robinson et al, Pain 2012; 13: 1366-76. *** Estimates based on information from publicly-available sources ‡ EU only †Frost & Sullivan Fibromyalgia Market Assessment, December 2010 6 5 million U.S. patients* 2.6 million diagnosed; 2.4 million receiving treatment** $1.5 billion U.S. prescription drug market in 2012*** 14% CAGR 2007-12 First FDA approval granted only six years ago Revenue growth of market driven by converting patients from off-label generics to branded drugs approved specifically for FM† Product Company Prior Indication Approval Year in FM 2012 U.S.Sales in FM*** Lyrica ® Pfizer Pain (neuropathic) 2007 $475 million Cymbalta ® Eli Lilly Depression 2008 $600 million Savella ® Forest Depression ‡ 2009 $100 million
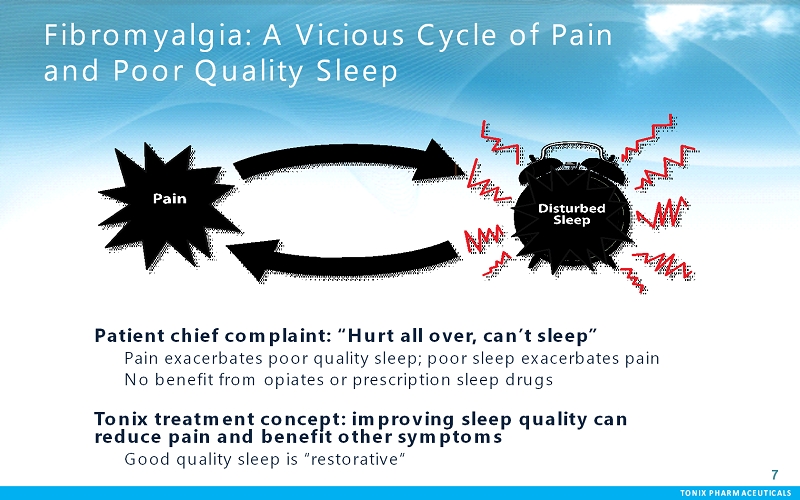
TONIX PHARMACEUTICALS 77 Fibromyalgia: A Vicious Cycle of Pain and Poor Quality Sleep Patient chief complaint: “Hurt all over, can’t sleep” Pain exacerbates poor quality sleep; poor sleep exacerbates pain No benefit from opiates or prescription sleep drugs Tonixtreatment concept: improving sleep quality can reduce pain and benefit other symptoms Good quality sleep is “restorative“
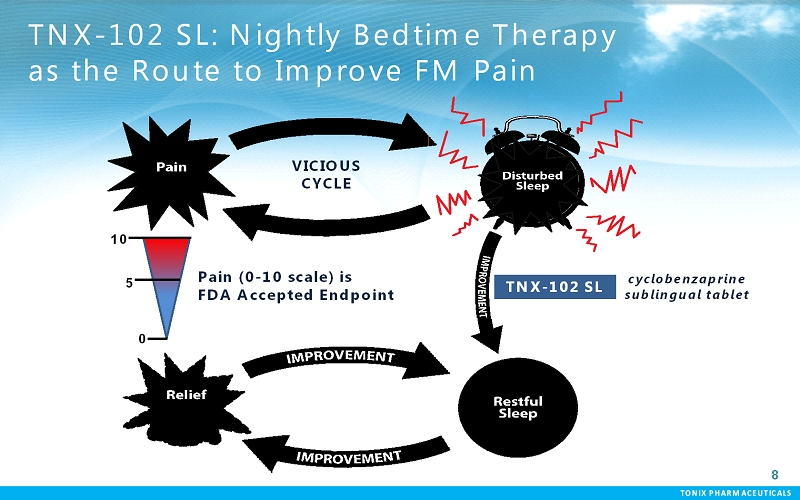
TONIX PHARMACEUTICALS 8 CONFIDENTIAL TONIX PHARMACEUTICALS TNX-102 SL: Nightly Bedtime Therapy as the Route to Improve FM Pain Pain (0-10 scale) is FDA Accepted Endpoint TNX-102 SL 10 5 0 VICIOUS CYCLE cyclobenzaprine sublingual tablet
TONIX PHARMACEUTICALS 9 CONFIDENTIAL TONIX PHARMACEUTICALS Phase 2a Study –Proof-of-Concept Results published in Journal of Rheumatology* Harvey Moldofsky, MD –lead investigator (University of Toronto) Double blind, randomized, placebo controlled study Conducted at two academic centers in Canada •under Canadian Clinical Trial Application 36 fibromyalgia patients; 18 per arm Very low dose cyclobenzaprine (VLD CBP) or placebo Taken between dinner and bedtime daily Eight weeks, dose-escalating 1 –4 mg oral capsules Average dose at week eight = 3.1 mg * Moldofskyet al., J. Rheum. December 2011: http://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html 9

TONIX PHARMACEUTICALS 10 CONFIDENTIAL TONIX PHARMACEUTICALS Positive Results from Phase 2a VLD CBP in FM -30 -20 -10 0 10 20 VLD CBP Placebo HAD = Hospital Anxiety and Depression Scale * p < 0.05, VLD CBP vs. placebo P e r c e n t C h a n g e f r o m B a s e l i n e a t W e e k 8 Pain* HAD Depression* No serious adverse events No discontinuations due to adverse eventsin treatment arm Types of adverse events consistent with approved CBP products (e.g., Flexeril ® ) VLD CBP: •26% reduction in pain vs. 0% with placebo •22% reduction in depressed mood vs. 10% increase with placebo
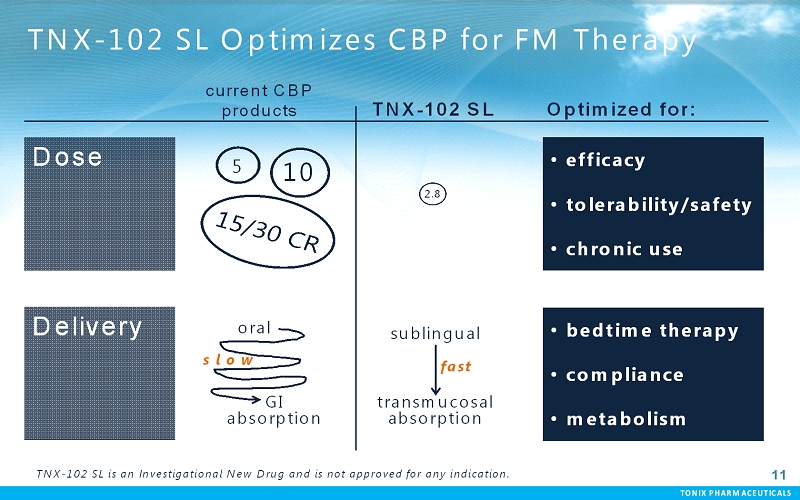
TONIX PHARMACEUTICALS 11 CONFIDENTIAL TONIX PHARMACEUTICALS 11 TNX-102 SL Optimizes CBP for FM Therapy Dose Delivery current CBP products 5 10 oral GI absorption TNX-102 SL 2.8 sublingual transmucosal absorption fast s l o w •efficacy •tolerability/safety •chronic use •bedtime therapy •compliance •metabolism Optimized for: TNX-102 SL is an Investigational New Drug and is not approved for any indication.
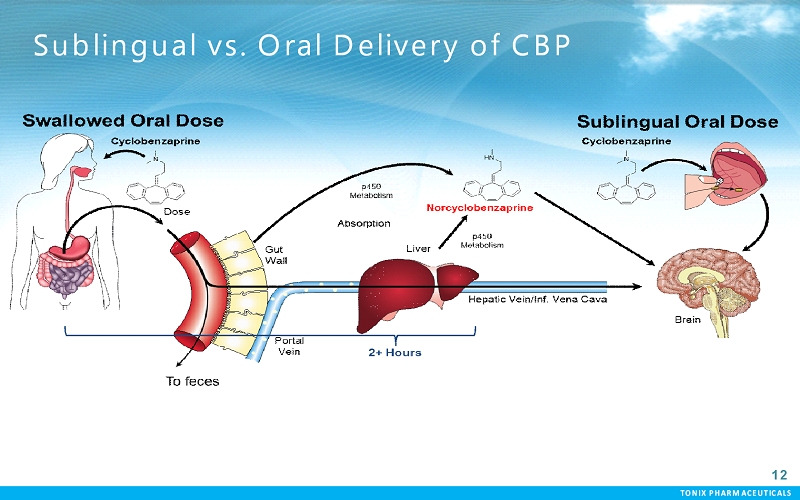
TONIX PHARMACEUTICALS 12 CONFIDENTIAL TONIX PHARMACEUTICALS Sublingual vs. Oral Delivery of CBP 12
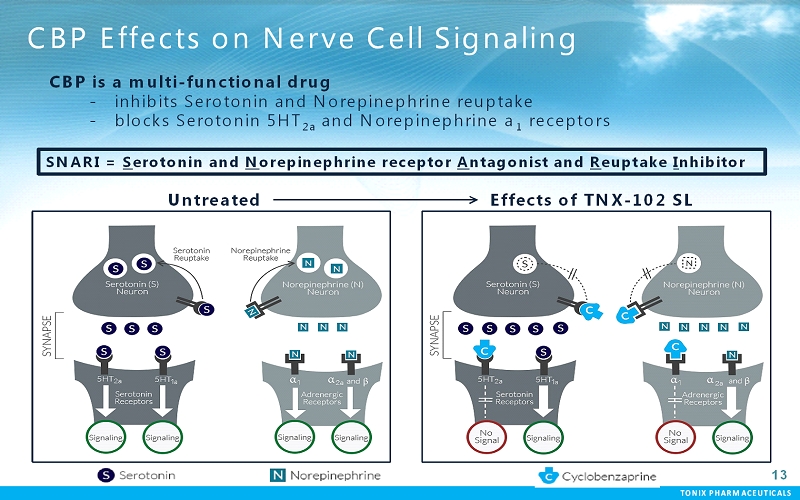
TONIX PHARMACEUTICALS 13 CBP Effects on Nerve Cell Signaling Untreated Effects of TNX-102 SL CBP is a multi-functional drug - inhibits Serotonin and Norepinephrine reuptake - blocks Serotonin 5HT 2a and Norepinephrine a 1 receptors SNARI = Serotonin and Norepinephrine receptor Antagonist and Reuptake Inhibitor
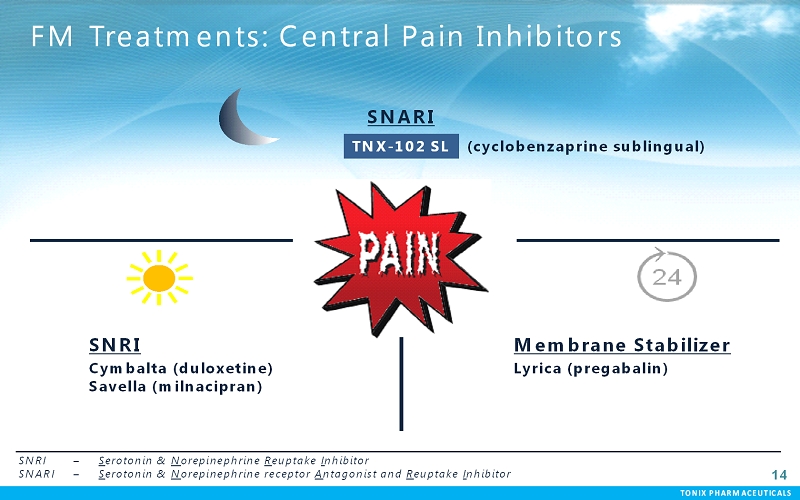
TONIX PHARMACEUTICALS 14 CONFIDENTIAL TONIX PHARMACEUTICALS FM Treatments: Central Pain Inhibitors SNRI Cymbalta (duloxetine) Savella(milnacipran) Membrane Stabilizer Lyrica (pregabalin) SNRI – Serotonin & Norepinephrine Reuptake Inhibitor SNARI – Serotonin & Norepinephrine receptor Antagonist and Reuptake Inhibitor TNX-102 SL SNARI (cyclobenzaprine sublingual)
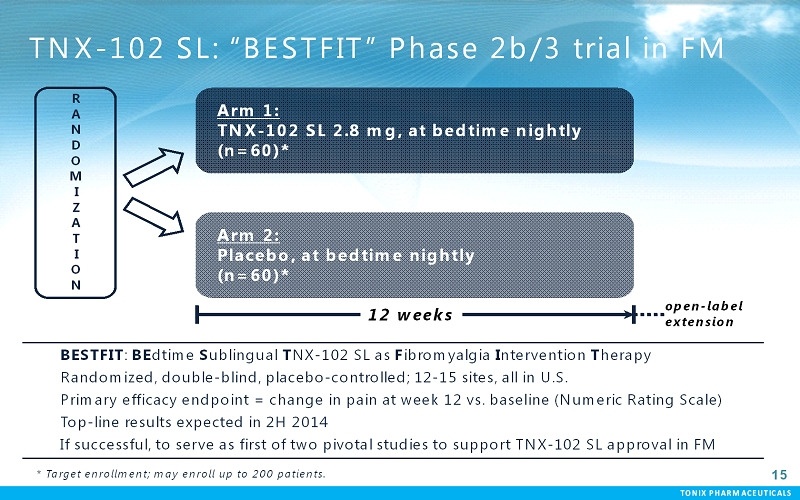
TONIX PHARMACEUTICALS 15 CONFIDENTIAL TONIX PHARMACEUTICALS TNX-102 SL: “BESTFIT” Phase 2b/3 trial in FM BESTFIT: BEdtimeSublingual TNX-102 SL as Fibromyalgia Intervention Therapy Randomized, double-blind, placebo-controlled; 12-15 sites, all in U.S. Primary efficacy endpoint = change in pain at week 12 vs. baseline (Numeric Rating Scale) Top-line results expected in 2H 2014 If successful, to serve as first of two pivotal studies to support TNX-102 SL approval in FM 15 R A N D O M I Z A T I O N Arm 1: TNX-102 SL 2.8 mg, at bedtime nightly (n=60)* Arm 2: Placebo, at bedtime nightly (n=60)* 12 weeks open-label extension * Target enrollment; may enroll up to 200 patients.

TONIX PHARMACEUTICALS 16 CONFIDENTIAL TONIX PHARMACEUTICALS TNX-102 SL: First-in-Class Fibromyalgia Medicine Targets pain and poor sleep SNARI –unique mechanism of action among marketed FM products Sublingual tablet at bedtime Fast onset aligns exposure with sleeping period Designed to optimize ease-of-use, compliance Very low dose –2.8 mg Daytime tolerability Developed for long-term use Significant treatment effect Positive clinical experience with VLD CBP Starting potential registration study in 3Q 2013 16 Brain

TONIX PHARMACEUTICALS 17 TONIX PHARMACEUTICALS TNX-102 SL: Post-Traumatic Stress Disorder Overlap between PTSD and FM ~50% of FM orPTSD patients meet criteria for the otherdisorder Patients experience disturbed sleep and widespread pain Core defining feature is night terrors, a form of sleep disturbance Painkiller abuse and addiction are common Patients desperate despite two FDA approved drugs 3.5% of U.S. adult population has suffered from PTSD in past 12 months* Experiencing any trauma can lead to PTSD High incidence among U.S. soldiers and veterans Associated with suicide and unpredictable violent behaviors Phase 2a proof-of-concept study expected to commence in 1Q 2014 Pre-IND meeting held with FDA Leverage fibromyalgia formulation, clinical experience, manufacturing know-how * National Institutes of Mental Health & National Institutes of Health 2010 17
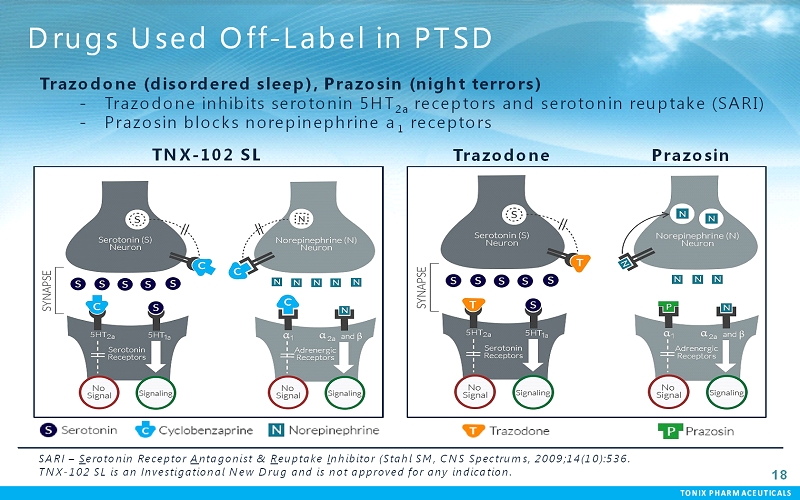
TONIX PHARMACEUTICALS 18 Drugs Used Off-Label in PTSD TNX-102 SL Trazodone Trazodone(disordered sleep), Prazosin(night terrors) - Trazodoneinhibits serotonin 5HT 2a receptors and serotonin reuptake (SARI) - Prazosinblocks norepinephrine a 1 receptors Prazosin SARI –Serotonin Receptor Antagonist & Reuptake Inhibitor (Stahl SM, CNS Spectrums, 2009;14(10):536. TNX-102 SL is an Investigational New Drug and is not approved for any indication.
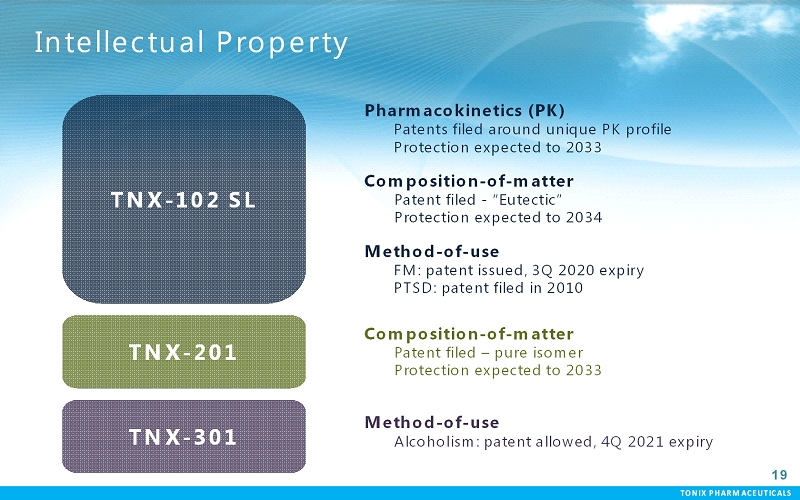
TONIX PHARMACEUTICALS 19 CONFIDENTIAL TONIX PHARMACEUTICALS Intellectual Property 19 Pharmacokinetics (PK) Patents filed around unique PK profile Protection expected to 2033 Composition-of-matter Patent filed -“Eutectic” Protection expected to 2034 Method-of-use FM: patent issued, 3Q 2020 expiry PTSD: patent filed in 2010 TNX-102 SL TNX-201 TNX-301 Composition-of-matter Patent filed –pure isomer Protection expected to 2033 Method-of-use Alcoholism: patent allowed, 4Q 2021 expiry

TONIX PHARMACEUTICALS 20 CONFIDENTIAL TONIX PHARMACEUTICALS Milestones –Recent and Upcoming □ 8/9/13 –TNXP stock uplistedto NASDAQ □ 8/14/13 –gross proceeds of $11.4 million from underwritten offering □ 3Q 2013 –Begin Phase 2b/3 trial of TNX-102 SL in FM □ 4Q 2013 –File IND for TNX-102 SL in PTSD □ 1Q 2014 –Begin Phase 2a trial of TNX-102 SL in PTSD □ 2H 2014 –Top line results of Phase 2b/3 trial of TNX-102 SL in FM x x
TONIX PHARMACEUTICALS 21 CONFIDENTIAL TONIX PHARMACEUTICALS Management Team Selected Previous Corporate Affiliations Selected Previous ProductAffiliations Seth Lederman, MD CEO & Chairman • Vela • Targent • Validus • Fontus Leland Gershell, MD, PhD CFO • Cowen • Apothecary Capital • MadisonWilliams BruceDaugherty, PhD, MBA CSO • Merck • Roche Institute 21

TONIX PHARMACEUTICALS 22 CONFIDENTIAL TONIX PHARMACEUTICALS Board of Directors Selected Current & PreviousAffiliations Selected Previous ProductAffiliations Seth Lederman, MD Chairman • Vela • Targent • Validus/Fontus Stuart Davidson • Alkermes • Combion Patrick Grace • WR Grace • Chemed • Grace Institute Donald Landry, MD, PhD • Columbia University Chair, Dept. of Medicine • Vela Ernest Mario, PhD • Glaxo • Alza • Reliant Charles Mather • Janney MontgomeryScott • Cowen • Smith Barney John Rhodes • Booz Allen Hamilton • NRDC Samuel Saks, MD • Jazz • Alza • Cougar 22

TONIX PHARMACEUTICALS 23 CONFIDENTIAL TONIX PHARMACEUTICALS Why Invest in Tonix 23 •Late stage clinical program in large market indication •Strong evidence of desired treatment effect in Phase 2a •Active ingredient has established safety profile at higher doses •Team distinguished by track record of drug development success •Well-capitalized to execute on key near-term milestones

NASDAQ: TNXP