

Investor Presentation November 2013 NASDAQ: TNXP

TONIX PHARMACEUTICALS 2 CONFIDENTIAL TONIX PHARMACEUTICALS Safe Harbor Statement Certainstatementsinthispresentationregardingstrategicplans,expectationsandobjectivesforfuture operationsorresultsare“forward-lookingstatements”asdefinedbythePrivateSecuritiesLitigationReform Actof1995.Thesestatementsmaybeidentifiedbytheuseofforward-lookingwordssuchas"anticipate," "believe,""forecast,""estimate"and"intend,"amongothers.Theseforward-lookingstatementsarebasedon TONIX’scurrentexpectationsandactualresultscoulddiffermaterially.Thereareanumberoffactorsthat couldcauseactualeventstodiffermateriallyfromthoseindicatedbysuchforward-lookingstatements. Thesefactorsinclude,butarenotlimitedto,substantialcompetition;ourabilitytocontinueasagoing concern;ourneedforadditionalfinancing;uncertaintiesofpatentprotectionandlitigation;uncertaintiesof governmentorthirdpartypayerreimbursement;limitedsalesandmarketingeffortsanddependenceupon thirdparties;andrisksrelatedtofailuretoobtainU.S.FoodandDrugAdministrationclearancesorapprovals andnoncompliancewithitsregulations. As withanypharmaceuticalunderdevelopment,thereare significantrisksinthedevelopment,regulatoryapprovalandcommercializationofnewproducts.The forward-lookingstatementsinthispresentationaremadeasofthedateofthispresentation,evenif subsequentlymadeavailablebytheCompanyonitswebsiteorotherwise.TONIXdoesnotundertakean obligationtoupdateorreviseanyforward-lookingstatement,exceptasrequiredbylaw.Investorsshould readtheriskfactorssetforthintheAnnualReportonForm10-KfortheyearendedDecember31,2012,as filedwiththeSecuritiesandExchangeCommission(the“SEC”)onMarch11,2013andfutureperiodic reportsfiledwiththeSEConorafterthedatehereof.AlloftheCompany'sforward-lookingstatementsare expresslyqualifiedbyallsuchriskfactorsandothercautionarystatements.
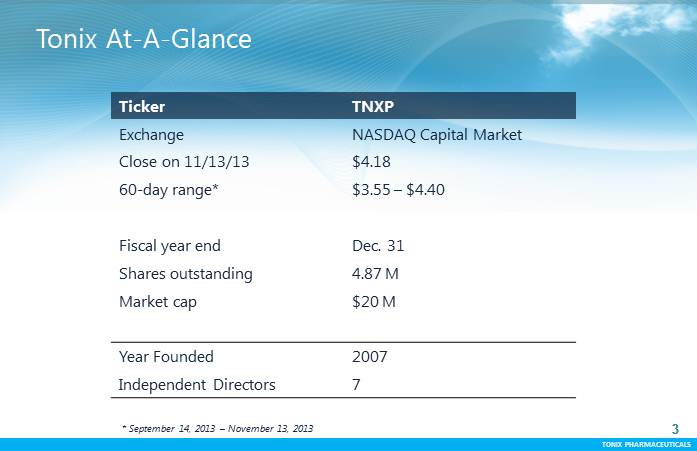
TONIX PHARMACEUTICALS 3 CONFIDENTIAL TONIX PHARMACEUTICALS TonixAt-A-Glance 3 Ticker TNXP Exchange NASDAQ Capital Market Close on 11/13/13 $4.18 60-dayrange* $3.55 –$4.40 Fiscal year end Dec. 31 Shares outstanding 4.87 M Market cap $20 M Year Founded 2007 Independent Directors 7 * September 14, 2013 –November 13, 2013

TONIX PHARMACEUTICALS 4 CONFIDENTIAL TONIX PHARMACEUTICALS Investment Thesis Fibromyalgia (FM) –first anticipated pivotal trial enrolling $1.5B U.S. market; widely recognized; large unmet need Evidence of clinical benefit, well-tolerated in Phase 2 Top line results of Phase 2b/3 trial expected to be reported in 2H 2014 Post-traumatic stress disorder (PTSD) 7 million U.S. patients, rates are on the rise Phase 2 trial expected to begin in 2Q 2014 Repurposing/Reformulating known drugs Capital-and time-efficient FDA strategy Reduced development risk Experienced management and board of directors Track record in drug approvals and value creation All intellectual property owned by Tonixoutright –no royalties Value proposition August 2013 -first underwritten institutional financing, NASDAQ listing Public via reverse merger
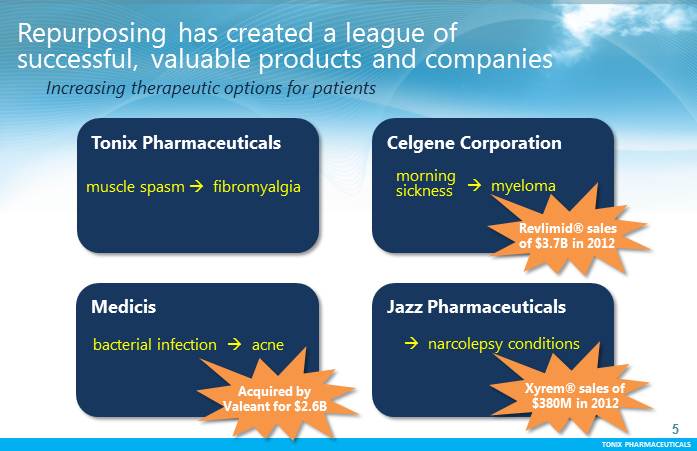
TONIX PHARMACEUTICALS 5 CONFIDENTIAL TONIX PHARMACEUTICALS Repurposing has created a league of successful, valuable products and companies Acquired by Allergan for $958M Revlimid® annual sales of $3.7B, as of year-end 2012 Out-licensed to Purdue Pharmafor up to $145M Medicis CelgeneCorporation Jazz Pharmaceuticals myeloma bacterial infection acne morning sickness narcolepsy conditions Revlimid® sales of $3.7B in 2012 Acquired by Valeant for $2.6B Xyrem® sales of $380M in 2012 TonixPharmaceuticals muscle spasm fibromyalgia Increasing therapeutic options for patients
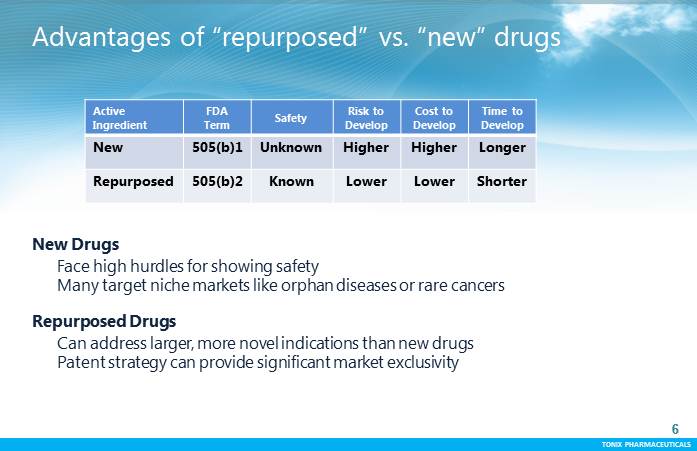
TONIX PHARMACEUTICALS 6 CONFIDENTIAL TONIX PHARMACEUTICALS Advantages of “repurposed” vs. “new” drugs 6 Active Ingredient FDA Term Safety Risk to Develop Costto Develop Time to Develop New 505(b)1 Unknown Higher Higher Longer Repurposed 505(b)2 Known Lower Lower Shorter New Drugs Face high hurdles for showing safety Many target niche markets like orphan diseases or rare cancers Repurposed Drugs Can address larger, more novel indications than new drugs Patent strategy can provide significant market exclusivity
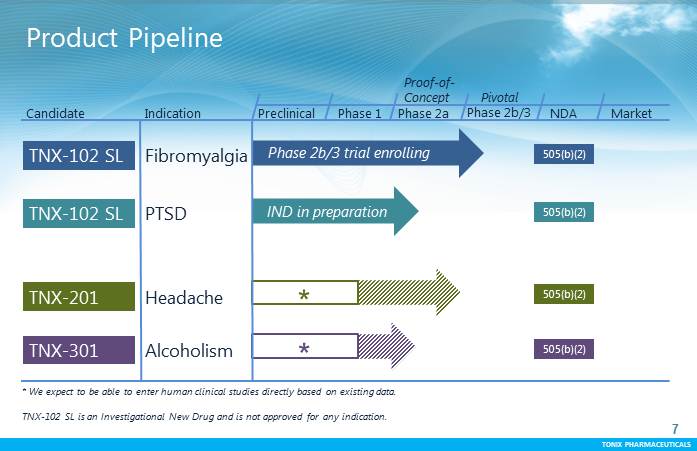
TONIX PHARMACEUTICALS 7 CONFIDENTIAL TONIX PHARMACEUTICALS Product Pipeline TNX-102 SL PTSD Fibromyalgia Preclinical Phase 1 Proof-of- Concept Pivotal NDA Market TNX-102 SL Candidate Indication TNX-201 Headache TNX-301 Alcoholism * * We expect to be able to enter human clinical studies directly based on existing data. TNX-102 SL is an Investigational New Drug and is not approved for any indication. * 505(b)(2) 505(b)(2) 505(b)(2) 505(b)(2) Phase 2a Phase 2b/3 IND in preparation Phase 2b/3 trial enrolling

TONIX PHARMACEUTICALS 8 CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia –a significant market opportunity *National Institutes of Health, U.S. Department of Health and Human Services **Robinson et al, Pain 2012; 13: 1366-76. *** Estimates based on information from publicly-available sources ‡ EU only 8 5 million U.S. patients* 2.6 million diagnosed; 2.4 million receiving treatment** $1.5 billion U.S. prescription drug market in 2012*** 14% CAGR 2007-12 Category Product Company Prior Indication Approval Year in FM 2012 U.S.Sales in FM*** Membrane Stabilizer Lyrica ® Pfizer Pain (neuropathic) 2007 $475 million SNRI Cymbalta ® Eli Lilly Depression 2008 $600 million Savella ® Forest Depression ‡ 2009 $100 million SleepQuality TNX-102SL Tonix Muscle Spasm 2017E

TONIX PHARMACEUTICALS 9 CONFIDENTIAL TONIX PHARMACEUTICALS Fibromyalgia: a large opportunity for an effective, well-tolerated, differentiated product Patients feel pain all over the body, but it originates in the brain Chronic, widespread pain with sleep, fatigue, mood, and memory problems Non-restorative sleep linked to hyper-vigilance Impairs daily function and productivity: poor quality of life Predominantly female Prescription drugs approved in U.S., Canada, Japan and Israel Patients remain unsatisfied despite approved products Patients often take multiple medications (“polypharmacy”) ‘Off-label’ use of opioids and sedative-hypnotics no sustained benefit, addiction Expensive, burdensome condition for healthcare system Health utilization and medication costs are substantial Managed care / payorsrecognize need for new therapies
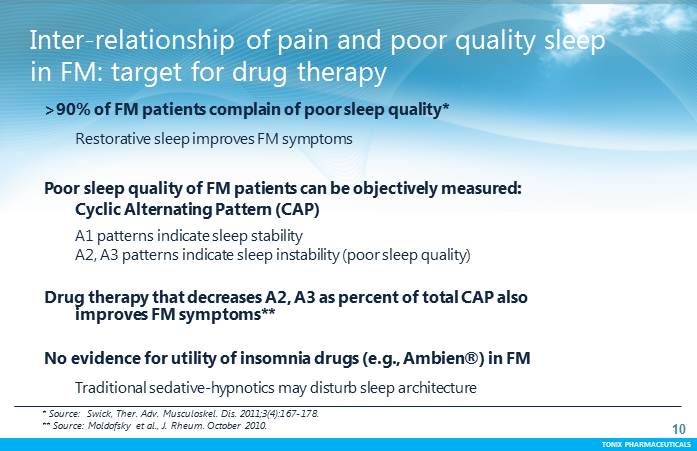
TONIX PHARMACEUTICALS 10 CONFIDENTIAL TONIX PHARMACEUTICALS >90% of FM patients complain of poor sleep quality* Restorative sleep improves FM symptoms Poor sleep quality of FM patients can be objectively measured: Cyclic Alternating Pattern (CAP) A1 patterns indicate sleep stability A2, A3 patterns indicate sleep instability (poor sleep quality) Drug therapy that decreases A2, A3 as percent of total CAP also improves FM symptoms** No evidence for utility of insomnia drugs (e.g., Ambien®) in FM Traditional sedative-hypnotics may disturb sleep architecture * Source: Swick, Ther. Adv. Musculoskel. Dis. 2011;3(4):167-178. **Source: Moldofsky et al., J. Rheum. October 2010. Inter-relationship of pain and poor quality sleep in FM: target for drug therapy
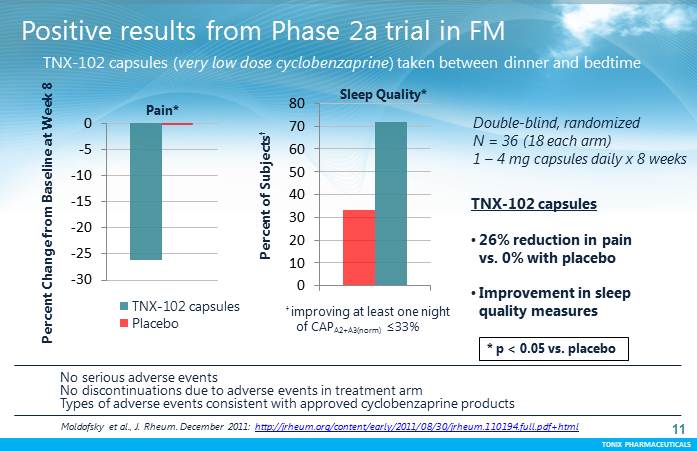
TONIX PHARMACEUTICALS 11 CONFIDENTIAL TONIX PHARMACEUTICALS -30 -25 -20 -15 -10 -5 0 TNX-102 capsules Placebo * p < 0.05 vs. placebo P e r c e n t C h a n g e f r o m B a s e l i n e a t W e e k 8 Pain* P e r c e n t o f S u b j e c t s † Sleep Quality* 0 10 20 30 40 50 60 70 80 Positive results from Phase 2a trial in FM No serious adverse events No discontinuations due to adverse events in treatment arm Types of adverse events consistent with approved cyclobenzaprine products TNX-102 capsules •26% reduction in pain vs. 0% with placebo •Improvement in sleep quality measures TNX-102 capsules (very low dose cyclobenzaprine) taken between dinner and bedtime Double-blind, randomized N = 36 (18 each arm) 1 –4 mg capsules daily x 8 weeks Moldofskyet al., J. Rheum. December 2011: http://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html † improving at least one night of CAP A2+A3(norm) =33%
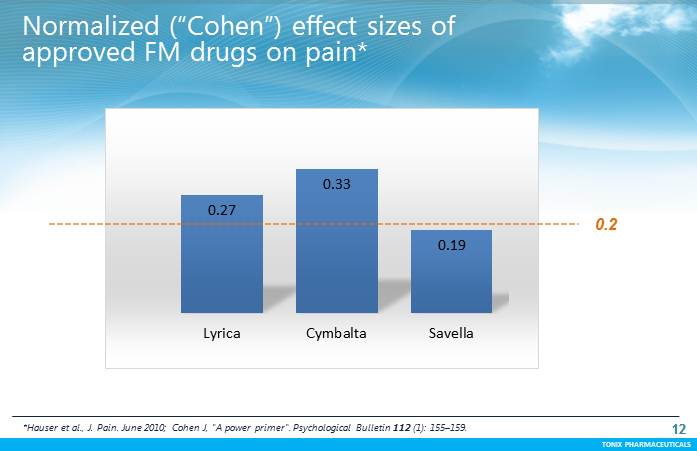
TONIX PHARMACEUTICALS 12 CONFIDENTIAL TONIX PHARMACEUTICALS 0.27 0.33 0.19 Lyrica Cymbalta Savella Normalized (“Cohen”) effect sizes of approved FM drugs on pain* 12 *Hauser et al., J. Pain. June 2010;CohenJ, "A power primer". Psychological Bulletin 112(1): 155–159. 0.2
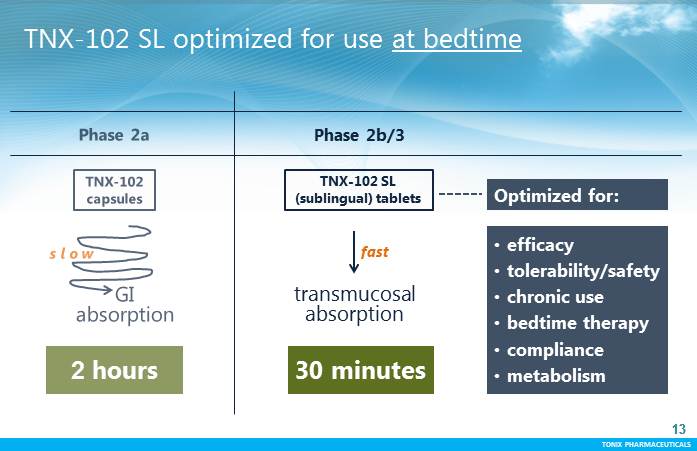
TONIX PHARMACEUTICALS 13 CONFIDENTIAL TONIX PHARMACEUTICALS 13 TNX-102 SL optimized for use at bedtime transmucosal absorption fast •efficacy •tolerability/safety •chronic use •bedtime therapy •compliance •metabolism 30 minutes 2 hours Optimized for: TNX-102 SL (sublingual) tablets Phase 2b/3

TONIX PHARMACEUTICALS 14 CONFIDENTIAL TONIX PHARMACEUTICALS Drug reformulations have generated significant value Acqui red by Allerg an for $958 M Revlimi d® annual sales of $3.7B, as of year- end 2012 Out- license d to Purdue Pharma for up to $145M Acquired by Allergan -$958 MM Acquired by Shire -$2.6 B Acquired by Johnson & Johnson -$11 B Acquired by Celgene-$2.9 B

TONIX PHARMACEUTICALS 15 CONFIDENTIAL TONIX PHARMACEUTICALS TNX-102 SL –registration program in FM 15 Held Pre-Phase 3 meeting with FDA in February 2013 Remaining clinical work to support New Drug Application: Two adequate and well-controlled safety and efficacy trials in FM patients Primary efficacy endpoint = pain Long-term exposure data to support chronic use label 100 subjects for six months, 50 subjects for one year Definitive repeat dose pharmacokinetic “bridging” study
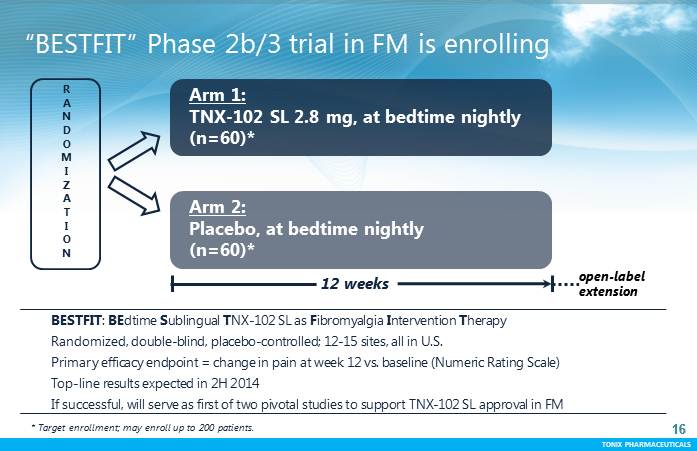
TONIX PHARMACEUTICALS 16 CONFIDENTIAL TONIX PHARMACEUTICALS “BESTFIT” Phase 2b/3 trial in FM is enrolling BESTFIT: BEdtimeSublingual TNX-102 SL as Fibromyalgia Intervention Therapy Randomized, double-blind, placebo-controlled; 12-15 sites, all in U.S. Primary efficacy endpoint = change in pain at week 12 vs. baseline (Numeric Rating Scale) Top-line results expected in 2H 2014 If successful, will serve as first of two pivotal studies to support TNX-102 SL approval in FM 16 R A N D O M I Z A T I O N Arm 1: TNX-102 SL 2.8 mg, at bedtime nightly (n=60)* Arm 2: Placebo, at bedtime nightly (n=60)* 12 weeks open-label extension * Target enrollment; may enroll up to 200 patients.

TONIX PHARMACEUTICALS 17 CONFIDENTIAL TONIX PHARMACEUTICALS TNX-102 SL: first-in-class fibromyalgia medicine Targets pain and poor sleep Unique mechanism of action among marketed FM products Sublingual tablet at bedtime Fast onset aligns exposure with sleeping period Designed to optimize ease-of-use, compliance Very low dose –2.8 mg per day Daytime tolerability Developed for long-term use Evidence of clinical benefit Positive clinical experience with TNX-102 capsules Phase 2b/3 BESTFIT study underway 17 Brain

TONIX PHARMACEUTICALS 18 TONIX PHARMACEUTICALS Post-Traumatic Stress Disorder Overlap between PTSD and FM ~50% of FM orPTSD patients meet criteria for the otherdisorder Patients experience disturbed sleep and widespread pain Core defining feature is hyper-vigilance, that can disturb sleep Painkiller abuse and addiction are common 3.5% of U.S. adult population has suffered from PTSD in past 12 months* Experiencing any trauma can lead to PTSD High incidence among U.S. soldiers and veterans Associated with suicide and unpredictable violent behaviors Patients desperate despite two FDA approved drugs; no new treatment in >10 years Phase 2 study of TNX-102 SL expected to commence in 2Q 2014 Pre-IND meeting held with FDA Leverage fibromyalgia formulation, clinical experience, manufacturing know-how * National Institutes of Mental Health & National Institutes of Health 2010 18

TONIX PHARMACEUTICALS 19 CONFIDENTIAL TONIX PHARMACEUTICALS Intellectual Property 19 Pharmacokinetics (PK) Patents filed around unique PK profile Protection expected to 2033 Composition-of-matter Patent filed -“Eutectic” Protection expected to 2034 Method-of-use FM: patent issued, 3Q 2020 expiry PTSD: patent filed in 2010 TNX-102 SL

TONIX PHARMACEUTICALS 20 CONFIDENTIAL TONIX PHARMACEUTICALS Milestones –Recent and Upcoming □ 8/9/13 –TNXP stock uplistedto NASDAQ □ 8/14/13 –Gross proceeds of $11.4 million from underwritten offering □ 3Q 2013 –Began Phase 2b/3 trial of TNX-102 SL in FM □ 4Q 2013 –Begin open-label extension study of TNX-102 SL in FM □ 1Q 2014 –File IND for TNX-102 SL in PTSD □ 1Q 2014 –Pre-IND meeting for TNX-201 for headache indications □ 2Q 2014 –Begin Phase 2a trial of TNX-102 SL in PTSD □ 2H 2014 –Top line results of Phase 2b/3 trial of TNX-102 SL in FM x x x

TONIX PHARMACEUTICALS 21 CONFIDENTIAL TONIX PHARMACEUTICALS Bruce Daugherty, PhD CSO Seth Lederman, MD CEO Management Team Leland Gershell, MD PhD CFO

TONIX PHARMACEUTICALS 22 CONFIDENTIAL TONIX PHARMACEUTICALS Board of Directors 22 Samuel Saks, MDErnest Mario, PhD Stuart Davidson Alkermes Combion Charles Mather JanneyMontgomery Scott Cowen, Smith Barney Patrick Grace WR Grace Chemed John Rhodes Booz Allen Hamilton NYSERDA Donald Landry, MD PhD Chair, Department of Medicine Columbia University Seth Lederman, MD TargentPharmaceuticals Vela Pharmaceuticals

TONIX PHARMACEUTICALS 23 CONFIDENTIAL TONIX PHARMACEUTICALS Why Invest in Tonix? 23 • Late stage clinical program in large market indication • Strong evidence of clinical benefit in Phase 2a • Active ingredient has established safety profile at higher doses • FDA 505(b)(2) regulatory pathway offers risk/reward advantage • Multiple opportunities (fibromyalgia, PTSD, headache, alcoholism) • Team distinguished by track record of drug development success • Well-capitalized to execute on key near-term milestones

NASDAQ: TNXP