

Investor Presentation May 2014 NASDAQ: TNXP

TONIX PHARMACEUTICALS 2 CONFIDENTIAL TONIX PHARMACEUTICALS Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as "anticipate," "believe," "forecast," "estimate" and "intend," among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our ability to continue as a going concern ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payer reimbursement ; limited sales and marketing efforts and dependence upon third parties ; and risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the amended Annual Report on Form 10 - K for the year ended December 31 , 2013 , as filed with the Securities and Exchange Commission (the “SEC”) on March 28 , 2014 and future periodic reports filed with the SEC on or after the date hereof . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

TONIX PHARMACEUTICALS 3 CONFIDENTIAL TONIX PHARMACEUTICALS High impact prescription drug candidates for common and complex disorders First - in - class medicines New treatment paradigms Large unmet medical needs Fibromyalgia (FM): BESTFIT trial of TNX - 102 SL fully enrolled Top line results to be reported in 4Q 2014 Strong evidence of clinical benefit in Phase 2a Measuring impact on pain relief Additional clinical - stage pipeline Post - traumatic stress disorder (PTSD) – targeting sleep pathology Tension - type headache – novel molecular target All intellectual property owned by Tonix outright – no royalties Experienced team, strong balance sheet Track record of success in drug approvals and value creation Well - capitalized to execute on key near - term milestones
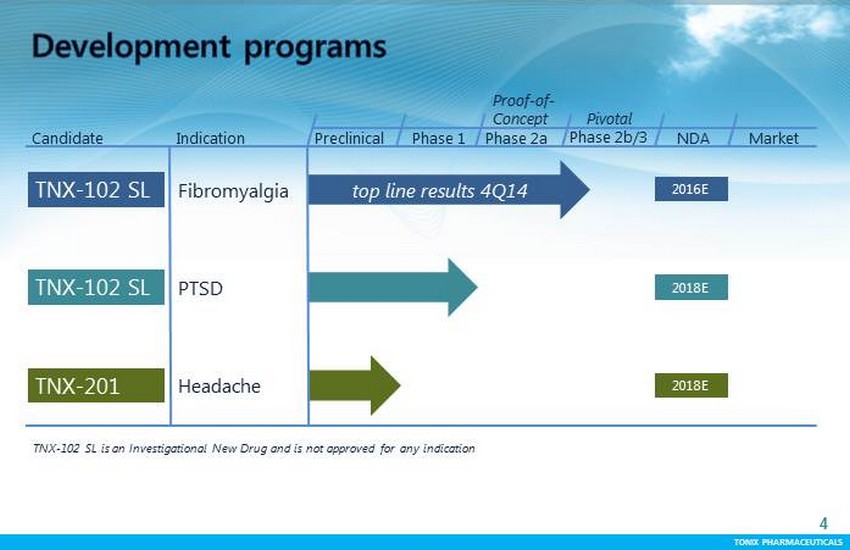
TONIX PHARMACEUTICALS 4 CONFIDENTIAL TONIX PHARMACEUTICALS Preclinical Phase 1 Proof - of - Concept Pivotal NDA Market Candidate Indication TNX - 102 SL is an Investigational New Drug and is not approved for any indication Phase 2a Phase 2b/3 PTSD TNX - 102 SL 2018E TNX - 102 SL Fibromyalgia t op line results 4Q14 2016E TNX - 201 Headache 2018E

TONIX PHARMACEUTICALS 5 CONFIDENTIAL TONIX PHARMACEUTICALS Targeting sleep quality is a new approach to FM Non - restorative sleep linked to pain and fatigue Restorative sleep improves FM symptoms Goal – to introduce an effective and well - tolerated new therapeutic with the potential to decrease use of opiates Targeting sleep quality is a new approach to PTSD Non - restorative sleep linked to hyper - vigilance and arousals Restorative sleep improves processing of emotionally charged memories Goal – to introduce an effective and well - tolerated new therapeutic with the potential to decrease use of opiates Novel molecular target in tension headache Targets of acetaminophen and barbiturates are not understood Goal – to introduce an effective and well - tolerated new therapeutic with the potential to decrease use of barbiturates
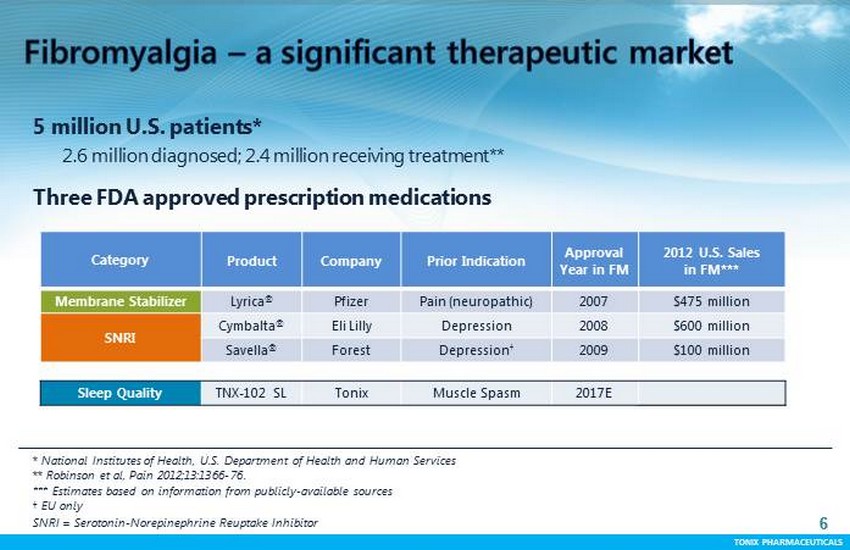
TONIX PHARMACEUTICALS 6 CONFIDENTIAL TONIX PHARMACEUTICALS * National Institutes of Health, U.S. Department of Health and Human Services ** Robinson et al, Pain 2012;13:1366 - 76 . *** Estimates based on information from publicly - available sources † EU only SNRI = Serotonin - Norepinephrine Reuptake Inhibitor 6 5 million U.S. patients* 2.6 million diagnosed; 2.4 million receiving treatment ** Three FDA approved prescription medications Category Product Company Prior Indication Approval Year in FM 2012 U.S. S ales in FM*** Membrane Stabilizer Lyrica ® Pfizer Pain (neuropathic) 2007 $475 million SNRI Cymbalta ® Eli Lilly Depression 2008 $600 million Savella ® Forest Depression † 2009 $100 million Sleep Quality TNX - 102 SL Tonix Muscle Spasm 2017E

TONIX PHARMACEUTICALS 7 CONFIDENTIAL TONIX PHARMACEUTICALS Chronic , widespread pain with sleep, fatigue, mood, and memory problems Impairs daily function and productivity: poor quality of life ”Central pain" - originates in the brain Typical patient has onset at 30 - 40 years of age with persistence for rest of life Predominantly female Patients remain unsatisfied despite approved products Patients often take multiple medications (“ polypharmacy ”) ‘ Off - label’ use of opioids and sedative - hypnotics despite no sustained benefit FM featured within FDA’s Patient - Focused Drug Development initiative Expensive, burdensome condition for the healthcare system Health utilization and medication costs are substantial Managed care / payers recognize need for new therapies
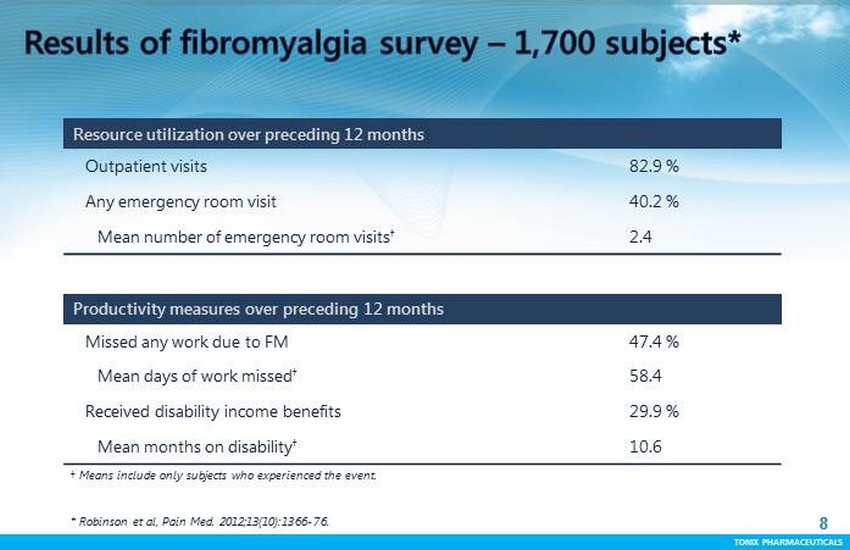
TONIX PHARMACEUTICALS 8 CONFIDENTIAL TONIX PHARMACEUTICALS * Robinson et al, Pain Med. 2012;13(10):1366 - 76. 8 Resource utilization over preceding 12 months Outpatient visits 82.9 % Any emergency room visit 40.2 % Mean number of emergency room visits † 2.4 Productivity measures over preceding 12 months Missed any work due to FM 47.4 % Mean days of work missed † 58.4 Received disability income benefits 29.9 % Mean months on disability † 10.6 † Means include only subjects who experienced the event.
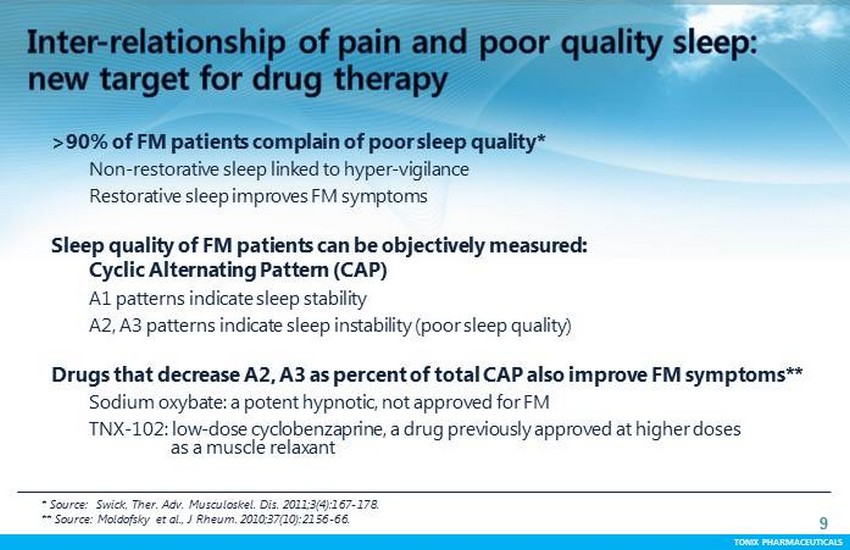
TONIX PHARMACEUTICALS 9 CONFIDENTIAL TONIX PHARMACEUTICALS >90% of FM patients complain of poor sleep quality* Non - restorative sleep linked to hyper - vigilance Restorative sleep improves FM symptoms Sleep quality of FM patients can be objectively measured: Cyclic Alternating P attern (CAP) A1 patterns indicate sleep stability A2, A3 patterns indicate sleep instability (poor sleep quality) Drugs that decrease A2, A3 as percent of total CAP also improve FM symptoms** Sodium oxybate : a potent hypnotic, not approved for FM TNX - 102: low - dose cyclobenzaprine, a drug previously approved at higher doses as a muscle relaxant * Source : Swick , Ther . Adv. Musculoskel . Dis. 2011;3(4):167 - 178. ** S ource : Moldofsky et al., J Rheum. 2010;37(10):2156 - 66.

TONIX PHARMACEUTICALS 10 CONFIDENTIAL TONIX PHARMACEUTICALS Double - blind, randomized, placebo - controlled Conducted at two academic centers in Canada Enrolled 36 subjects with fibromyalgia; 18 per arm TNX - 102 capsules or placebo taken between dinner and bedtime daily Eight - week, dose - escalating study Daily dosing ranged from 1 – 4 mg of TNX - 102 Source: Moldofsky et al., J Rheum. 2011;38(12):2653 - 63 - http ://jrheum.org/content/early/2011/08/30/jrheum.110194.full.pdf+html
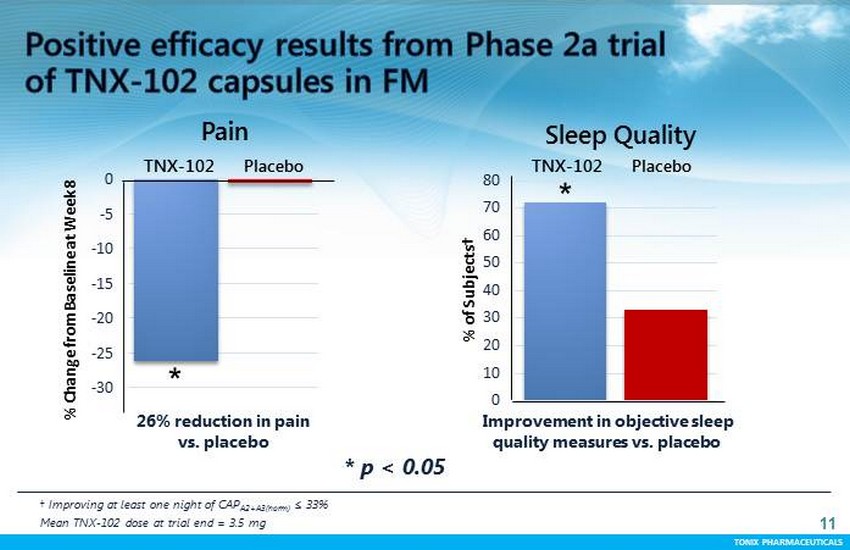
TONIX PHARMACEUTICALS 11 CONFIDENTIAL TONIX PHARMACEUTICALS -30 -25 -20 -15 -10 -5 0 % Change from Baseline at Week 8 Pain TNX - 102 Placebo † Improving at least one night of CAP A2+A3(norm) ≤ 3 3% Mean TNX - 102 dose at trial end = 3.5 mg 26% reduction in pain vs. placebo Improvement in objective sleep quality measures vs. placebo * 0 10 20 30 40 50 60 70 80 % of Subjects† Sleep Quality TNX - 102 Placebo * * p < 0.05
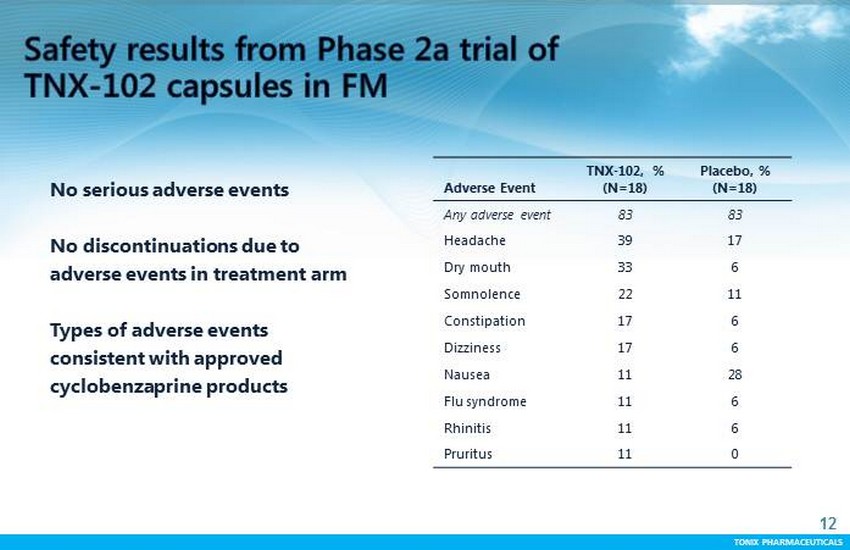
TONIX PHARMACEUTICALS 12 CONFIDENTIAL TONIX PHARMACEUTICALS No serious adverse events No discontinuations due to adverse events in treatment arm Types of adverse events consistent with approved cyclobenzaprine products Adverse Event TNX - 102, % (N=18) Placebo, % (N=18) Any adverse event 83 83 Headache 39 17 Dry mouth 33 6 Somnolence 22 11 Constipation 17 6 Dizziness 17 6 Nausea 11 28 Flu syndrome 11 6 Rhinitis 11 6 Pruritus 11 0
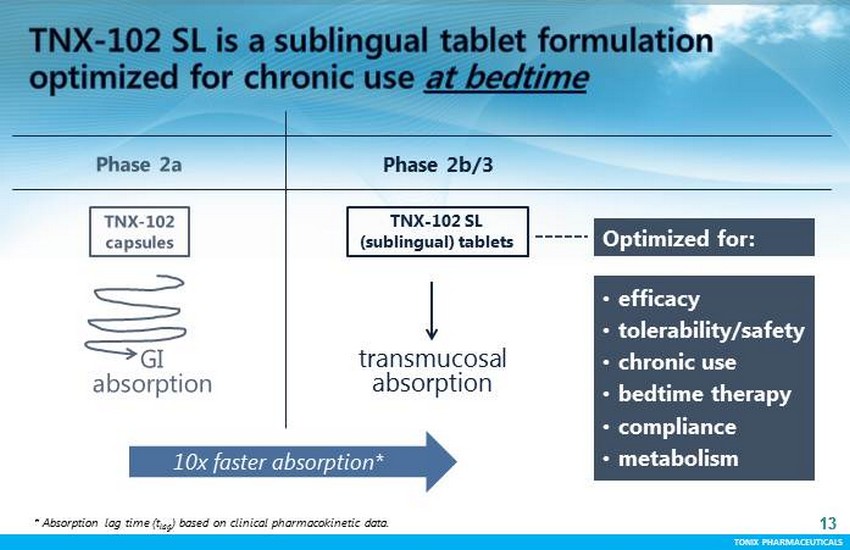
TONIX PHARMACEUTICALS 13 CONFIDENTIAL TONIX PHARMACEUTICALS 13 t ransmucosal absorption • efficacy • tolerability/safety • chronic use • bedtime therapy • compliance • metabolism Optimized for: TNX - 102 SL (sublingual) tablets Phase 2b/3 10x faster absorption* * Absorption lag time ( t lag ) based on clinical pharmacokinetic data.

TONIX PHARMACEUTICALS 14 CONFIDENTIAL TONIX PHARMACEUTICALS 14 Pre - Phase 3 meeting held with FDA in February 2013 Remaining clinical work to support New Drug Application: Two adequate and well - controlled efficacy and safety trials in FM patients Primary efficacy endpoint = pain □ First trial has completed enrollment – “BESTFIT” □ Top line BESTFIT data expected in Q4 Long - term exposure data to support chronic use label 100 subjects for six months, 50 subjects for one year □ Open - label extension study is underway Definitive repeat dose pharmacokinetic “bridging” study x x
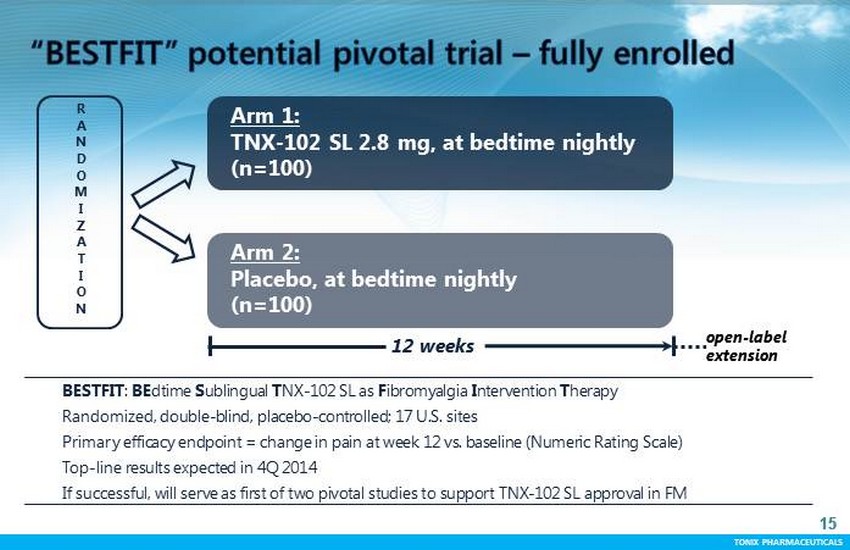
TONIX PHARMACEUTICALS 15 CONFIDENTIAL TONIX PHARMACEUTICALS BESTFIT : BE dtime S ublingual T NX - 102 SL as F ibromyalgia I ntervention T herapy Randomized, double - blind, placebo - controlled; 17 U.S. sites Primary efficacy endpoint = change in pain at week 12 vs. baseline (Numeric Rating Scale) Top - line results expected in 4Q 2014 If successful, will serve as first of two pivotal studies to support TNX - 102 SL approval in FM 15 R A N D O M I Z A T I O N Arm 1: TNX - 102 SL 2.8 mg, at bedtime nightly (n=100) Arm 2: Placebo, at bedtime nightly (n=100) 12 weeks open - label extension

TONIX PHARMACEUTICALS 16 CONFIDENTIAL TONIX PHARMACEUTICALS * National Institutes of Health, U.S. Department of Health and Human Services ** Wang et al., Arch Gen Psych. 2005;62(6):167 - 78. SSRI = Selective Serotonin Reuptake Inhibitor 16 8.4 million U.S. patients* 4.2 million receiving medical treatment** Two FDA approved prescription medications Category Product Company Prior Indication Approval Year in PTSD SSRI Paxil ® Glaxo Depression 2001 Zoloft ® Pfizer Depression 1999 Sleep Quality TNX - 102 SL Tonix Muscle Spasm NDA 2018E P hase 2 efficacy study of TNX - 102 SL to begin in 3Q 2014 Leverage fibromyalgia formulation, clinical experience, manufacturing know - how

TONIX PHARMACEUTICALS 17 TONIX PHARMACEUTICALS 3.5% of U.S. adult population has suffered from PTSD in past 12 months* Experiencing any trauma can lead to PTSD High incidence among U.S. soldiers and veterans Associated with suicide and unpredictable violent behaviors Patients desperate despite two FDA approved drugs; no new treatment in >10 years Overlap between PTSD and FM ~50% of FM or PTSD patients meet criteria for the other disorder Patients experience disturbed sleep W idespread pain is considered “co - morbid” with PTSD Opioid and sedative - hypnotic drug misuse common * National Institutes of Mental Health & National Institutes of Health 2010 17

TONIX PHARMACEUTICALS 18 CONFIDENTIAL TONIX PHARMACEUTICALS PTSD patients complain of poor sleep quality as a core symptom Distressing dreams (nightmares) are part of “re - experiencing” Restless sleep is part of “hyper - arousal” Poor sleep quality after trauma is linked to onset of PTSD Poor sleep correlates with depression, substance abuse and suicide Drugs used off - label in PTSD share mechanisms with TNX - 102 SL Trazodone is an antidepressant used at bedtime ; blocks the 5 - HT2A receptor Prazosin is a high blood pressure medicine used at bedtime ; blocks the α - 1 adrenergic receptor
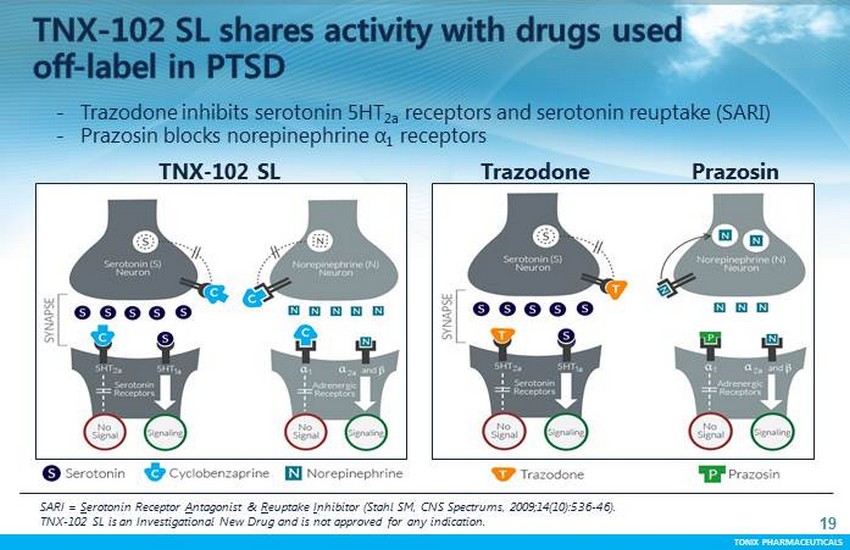
TONIX PHARMACEUTICALS 19 TNX - 102 SL Trazodone - Trazodone inhibits serotonin 5HT 2a receptors and serotonin reuptake (SARI) - Prazosin blocks norepinephrine α 1 receptors Prazosin SARI = S erotonin Receptor A ntagonist & R euptake I nhibitor (Stahl SM, CNS Spectrums, 2009;14(10):536 - 46). TNX - 102 SL is an Investigational New Drug and is not approved for any indication.
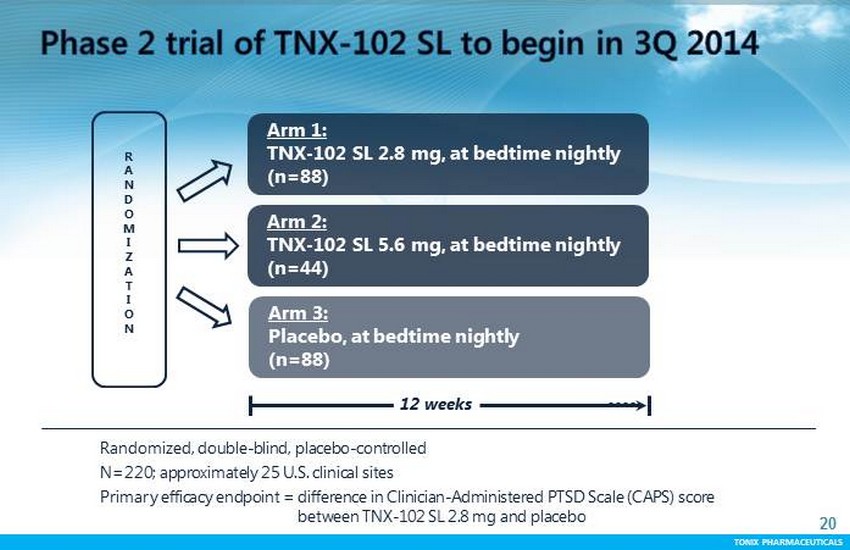
TONIX PHARMACEUTICALS 20 CONFIDENTIAL TONIX PHARMACEUTICALS Randomized , double - blind, placebo - controlled N=220; approximately 25 U.S . clinical sites Primary efficacy endpoint = difference in Clinician - Administered PTSD Scale (CAPS) score between TNX - 102 SL 2.8 mg and placebo R A N D O M I Z A T I O N Arm 1: TNX - 102 SL 2.8 mg, at bedtime nightly (n=88) Arm 3: Placebo, at bedtime nightly (n=88) 12 weeks Arm 2: TNX - 102 SL 5.6 mg, at bedtime nightly (n=44)
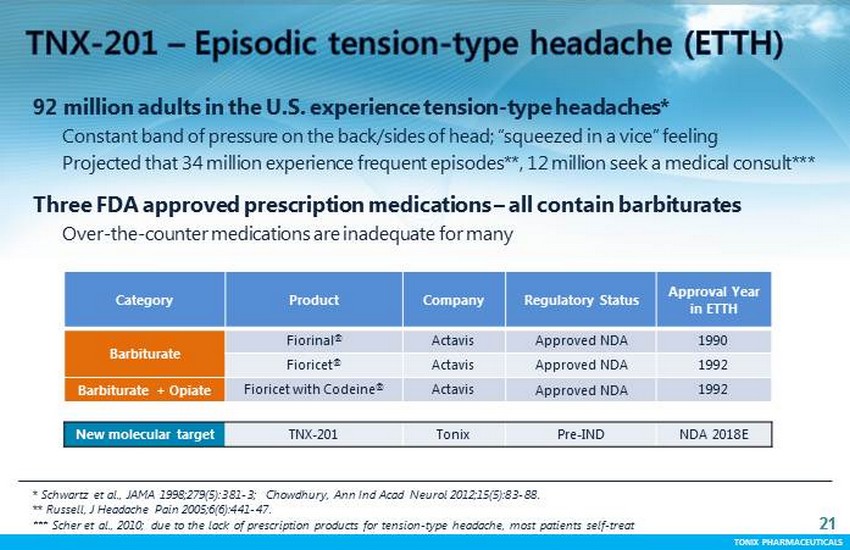
TONIX PHARMACEUTICALS 21 CONFIDENTIAL TONIX PHARMACEUTICALS * Schwartz et al., JAMA 1998;279(5):381 - 3; Chowdhury, Ann Ind Acad Neurol 2012;15(5):83 - 88. ** Russell, J Headache Pain 2005;6(6):441 - 47. *** Scher et al., 2010; due to the lack of prescription products for tension - type headache, most patients self - treat 21 92 million adults in the U.S. experience tension - type headaches* Constant band of pressure on the back/sides of head; “squeezed in a vice” feeling Projected that 34 million experience frequent episodes**, 12 million seek a medical consult *** Three FDA approved prescription medications – all contain barbiturates Over - the - counter medications are inadequate for many Category Product Company Regulatory Status Approval Year in ETTH Barbiturate Fiorinal ® Actavis Approved NDA 1990 Fioricet ® Actavis Approved NDA 1992 Barbiturate + Opiate Fioricet with Codeine ® Actavis Approved NDA 1992 New molecular target TNX - 201 Tonix Pre - IND NDA 2018E

TONIX PHARMACEUTICALS 22 CONFIDENTIAL TONIX PHARMACEUTICALS TNX - 201 (single isomer isometheptene ) Non - barbiturate, non - opioid Racemic mixture >50 years of use in combination products for headache in the U.S . Not FDA approved for any indication * Limited availability, quality concerns via compounding pharmacies Tonix non - clinical research supports the rationale for single isomer development Pre - IND meeting with FDA held in January 2014 Comparative pharmacokinetic and safety study to be conducted in 4Q 2014 * Products containing racemic isometheptene are marketed as unapproved products in the U.S.; marketing withdrawal has been sanctioned by the FDA since 2010.

TONIX PHARMACEUTICALS 23 CONFIDENTIAL TONIX PHARMACEUTICALS 23 Pharmacokinetics (PK) Patents filed around unique PK profile Protection expected to 2033 Composition - of - matter Patent filed - “Eutectic” Protection expected to 2034 Method - of - use FM: patent issued, 3Q 2020 expiry PTSD: patent filed in 2010 TNX - 102 SL Fibromyalgia, PTSD TNX - 201 H eadache Composition - of - matter Patent filed – single isomer Protection expected to 2033 All IP wholly - owned by Tonix – no royalties / future obligations

TONIX PHARMACEUTICALS 24 CONFIDENTIAL TONIX PHARMACEUTICALS Corporate □ Jan 2014 – $40.7 million net proceeds from common stock offering TNX - 102 SL □ 3Q 2013 – Began BESTFIT trial in FM □ 4Q 2013 – Began open - label extension study in FM □ 3Q 2014 – Start Phase 2 efficacy study in PTSD □ 4Q 2014 – Report top line results of BESTFIT trial in FM TNX - 201 □ Jan 2014 – Held Pre - IND meeting for tension - type headache □ 3Q 2014 – File IND for tension - type headache □ 4Q 2014 – Conduct clinical pharmacology study x x x x

TONIX PHARMACEUTICALS 25 CONFIDENTIAL TONIX PHARMACEUTICALS Bruce Daugherty, PhD CSO Seth Lederman, MD CEO Leland Gershell, MD, PhD CFO Don Kellerman, PharmD SVP, Clinical Development & Regulatory Affairs

TONIX PHARMACEUTICALS 26 CONFIDENTIAL TONIX PHARMACEUTICALS John Rhodes NYSERDA, NRDC Booz Allen Hamilton Donald Landry, MD, PhD Chair, Department of Medicine Columbia University Stuart Davidson Alkermes Combion Seth Lederman, MD (Chair) Targent Pharmaceuticals Vela Pharmaceuticals Samuel Saks, MD ALZA Jazz Pharmaceuticals Patrick Grace WR Grace Chemed Ernest Mario, PhD Glaxo, ALZA Reliant Pharmaceuticals Charles Mather Janney Montgomery Scott Cowen, Smith Barney

TONIX PHARMACEUTICALS 27 CONFIDENTIAL TONIX PHARMACEUTICALS 27 NASDAQ: TNXP Cash reported at March 31 , 2014 $ 49.5 million Net cash used in operations in 1Q14 $ 4.0 million Shares outstanding † 9.9 million † As of May 16, 2014

TONIX PHARMACEUTICALS 28 CONFIDENTIAL TONIX PHARMACEUTICALS 28 • TNX - 102 SL: late - stage clinical program in large market indication • Strong evidence of clinical benefit in Phase 2a • Current FM treatment options leave many patients unsatisfied • Fibromyalgia is a current focus of the FDA • Multiple opportunities (fibromyalgia , PTSD, headache) • Team distinguished by track record of drug development success • Well - capitalized to execute on key near - term milestones

NASDAQ: TNXP