
Exhibit 99.1

NASDAQ: TNXP March 2015 © 2015 Tonix Pharmaceuticals Holding Corp.

Safe harbor statement 2 Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our possible need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2014 , as filed with the Securities and Exchange Commission (the “SEC”) on February 27 , 2015 and future periodic reports filed with the SEC on or after the date hereof . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
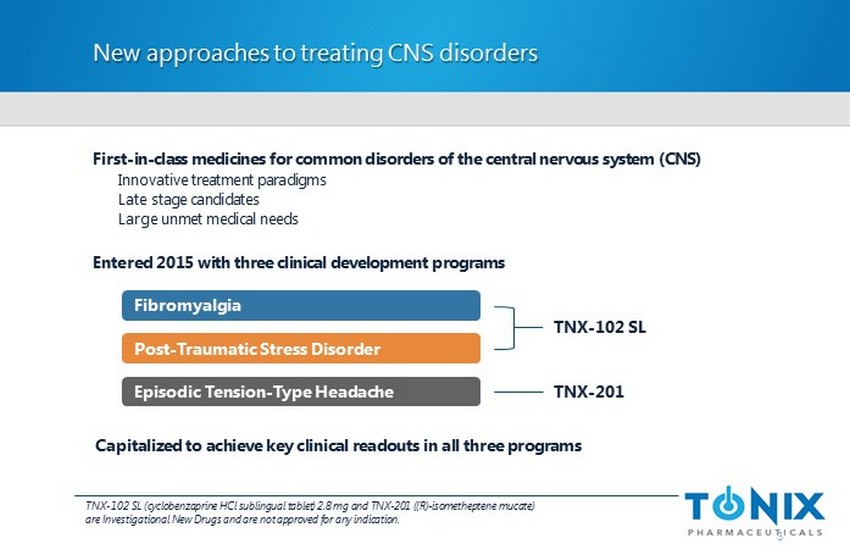
New approaches to treating CNS disorders First - in - class medicines for common disorders of the central nervous system (CNS) Innovative treatment paradigms Late stage candidates Large unmet medical needs Entered 2015 with three clinical development programs 3 Fibromyalgia Post - Traumatic Stress Disorder Episodic Tension - Type Headache TNX - 102 SL TNX - 201 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg and TNX - 201 ((R) - isometheptene mucate ) are Investigational New Drugs and are not approved for any indication. C apitalized to achieve key clinical readouts in all three programs
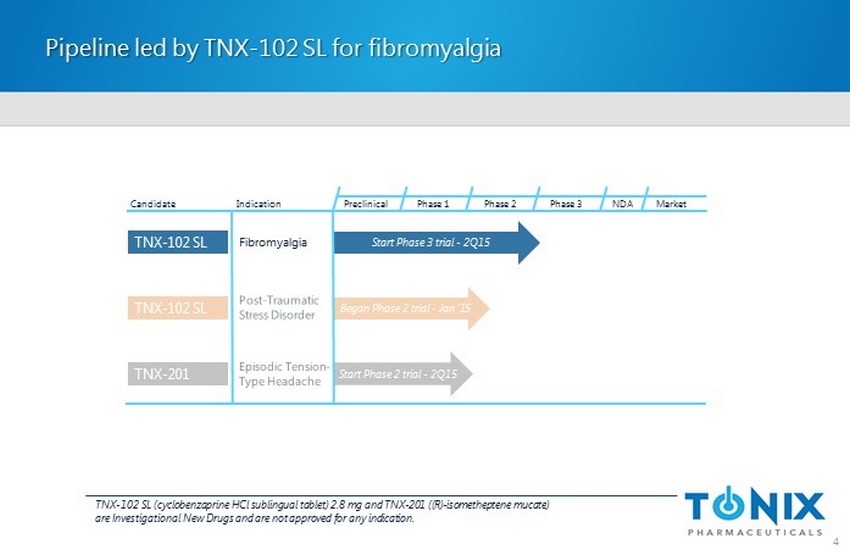
Pipeline led by TNX - 102 SL for fibromyalgia 4 Preclinical Phase 1 NDA Market Candidate Indication Phase 2 Phase 3 TNX - 102 SL Began Phase 2 trial - Jan ‘15 TNX - 102 SL Fibromyalgia Start Phase 3 trial - 2Q15 Start Phase 2 trial - 2Q15 TNX - 201 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg and TNX - 201 ( (R) - isometheptene mucate ) are Investigational New Drugs and are not approved for any indication.
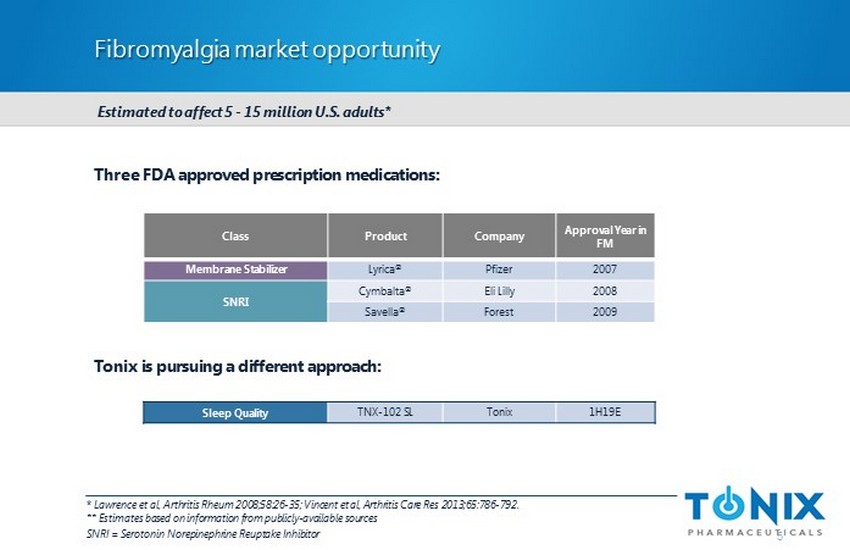
Fibromyalgia market opportunity * Lawrence et al, Arthritis Rheum 2008;58:26 - 35; Vincent et al, Arthritis Care Res 2013;65:786 - 792. ** Estimates based on information from publicly - available sources SNRI = Serotonin Norepinephrine Reuptake Inhibitor Three FDA approved prescription medications: Tonix is pursuing a different approach: Class Product Company Approval Year in FM Membrane Stabilizer Lyrica ® Pfizer 2007 SNRI Cymbalta ® Eli Lilly 2008 Savella ® Forest 2009 5 Estimated to affect 5 - 15 million U.S. adults* Sleep Quality TNX - 102 SL Tonix 1H19E
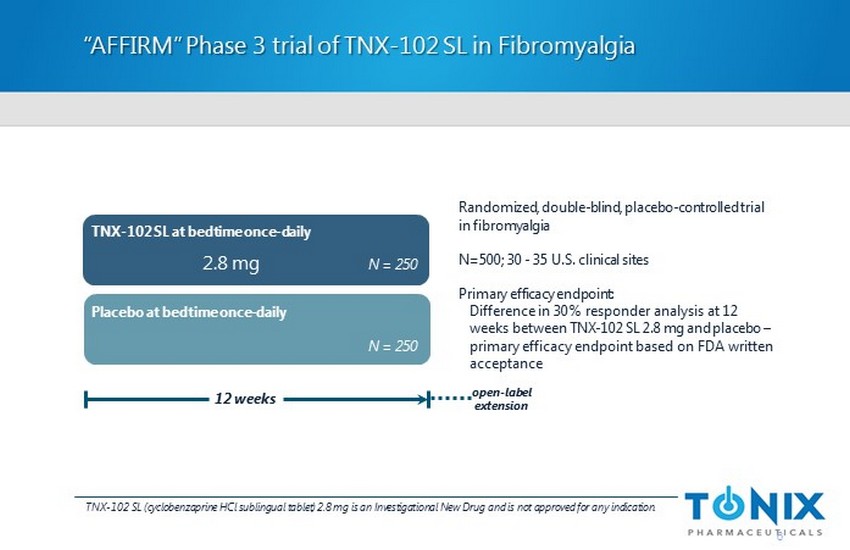
“AFFIRM” Phase 3 trial of TNX - 102 SL in Fibromyalgia Randomized, double - blind, placebo - controlled trial in f ibromyalgia N=500; 30 - 35 U.S. clinical sites Primary efficacy endpoint: Difference in 30 % responder analysis at 12 weeks between TNX - 102 SL 2.8 mg and placebo – primary efficacy endpoint based on FDA written acceptance Placebo at bedtime once - daily 12 weeks 6 TNX - 102 SL at bedtime once - daily N = 250 N = 250 2.8 mg open - label extension TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication .
Restorative sleep improves pain and other fibromyalgia symptoms >90% of fibromyalgia patients complain of poor sleep quality* Sleep quality improvement is not a feature of approved medications Phase 2a study with low - dose cyclobenzaprine (CBP) capsule showed proof - of - concept** TNX - 102 SL is a sublingual tablet formulation of CBP Pharmacokinetic profile well - suited to bedtime administration Tolerability profile well - suited to chronic use Phase 2b BESTFIT results support Phase 3 program in fibromyalgia Contribute to evidence of efficacy to support the planned NDA Phase 3 trial to begin in 2Q 2015 * Swick , Ther Adv Musculoskel Dis 2011;3:167 - 178. ** Moldofsky et al., J Rheum 2011;38:2653 - 63. TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication. Sleep quality is a new target for fibromyalgia therapy 7
BESTFIT Phase 2b trial in fibromyalgia 8 BESTFIT = BEdtime Sublingual TNX - 102 SL as Fibromyalgia Intervention Therapy Randomized, double - blind, placebo - controlled trial 2010 American College of Rheumatology diagnostic criteria for fibromyalgia 205 participants were randomized 1:1 at 17 U.S. sites One sublingual tablet of TNX - 102 SL 2.8 mg or placebo daily at bedtime for twelve weeks Primary efficacy endpoint Mean change from baseline in the daily diary pain score during week 12 11 - point (0 - 10) Numerical Rating Scale (NRS) to assess prior 24 - hour average pain intensity First Patient – First Dose September 2013 Last Patient – Last Dose August 2014 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.

TNX - 102 SL improved pain in fibromyalgia in the BESTFIT study 9 Outcome Measure at Week 12 Intent - to - Treat Population † p value Method Daily Pain Diary, NRS Mean Change** 0.086 0.172 MMRM JTC - MI Daily Pain Diary, NRS Proportion Achieving 30% Improvement* 0.033 LR Clinic NRS 7 - day pain recall Mean Change 0.029 MMRM FIQ - R Pain Item Mean Change 0.004 MMRM NRS = Numeric Rating Scale for pain; FIQ - R = Fibromyalgia Impact Questionnaire - Revised MMRM = Mixed - Effect Model Repeated Measure; JTC - MI = Jump to Control - Multiple Imputation (FDA - preferred analysis); LR = Logistic Regression ** Declared primary endpoint; was primary endpoint for FDA approvals of Lyrica and Cymbalta * Declared secondary endpoint; will be the primary endpoint in the upcoming Phase 3 study † N=205 (TNX - 102 SL N=103, placebo N=102) p < 0.05 ; a chieved statistical significance Source: Phase 2b BESTFIT study preliminary data TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.
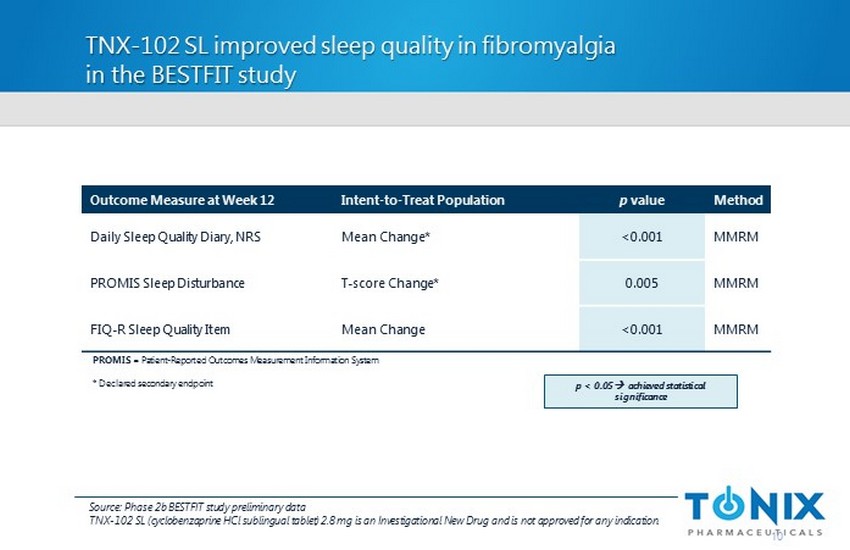
TNX - 102 SL improved sleep quality in fibromyalgia in the BESTFIT study 10 Outcome Measure at Week 12 Intent - to - Treat Population p value Method Daily Sleep Quality Diary, NRS Mean Change* <0.001 MMRM PROMIS Sleep Disturbance T - score Change* 0.005 MMRM FIQ - R Sleep Quality Item Mean Change <0.001 MMRM PROMIS = Patient - Reported Outcomes Measurement Information System * Declared secondary endpoint p < 0.05 ; a chieved statistical significance Source: Phase 2b BESTFIT study preliminary data TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.
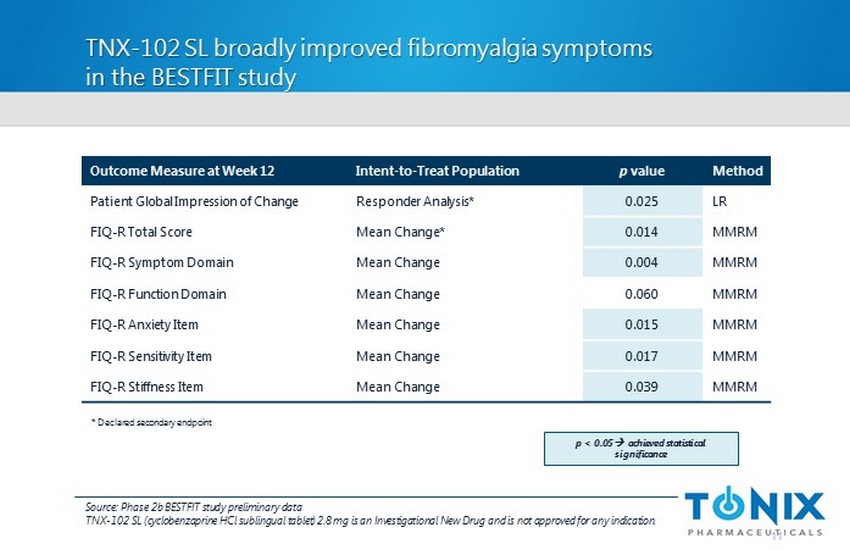
TNX - 102 SL broadly improved fibromyalgia symptoms in the BESTFIT study 11 Outcome Measure at Week 12 Intent - to - Treat Population p value Method Patient Global Impression of Change Responder Analysis* 0.025 LR FIQ - R Total Score Mean Change* 0.014 MMRM FIQ - R Symptom Domain Mean Change 0.004 MMRM FIQ - R Function Domain Mean Change 0.060 MMRM FIQ - R Anxiety Item Mean Change 0.015 MMRM FIQ - R Sensitivity Item Mean Change 0.017 MMRM FIQ - R Stiffness Item Mean Change 0.039 MMRM * Declared secondary endpoint p < 0.05 ; a chieved statistical significance Source: Phase 2b BESTFIT study preliminary data TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.
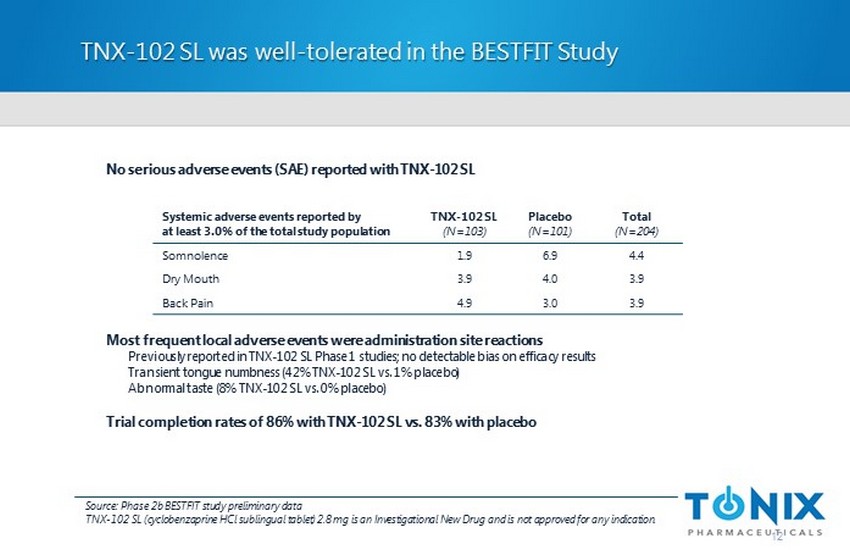
TNX - 102 SL was well - tolerated in the BESTFIT Study 12 No serious adverse events (SAE) reported with TNX - 102 SL Most frequent local adverse events were administration site reactions Previously reported in TNX - 102 SL Phase 1 studies; no detectable bias on efficacy results Transient tongue numbness (42% TNX - 102 SL vs. 1% placebo) Abnormal taste (8% TNX - 102 SL vs. 0% placebo) Trial completion rates of 86% with TNX - 102 SL vs. 83% with placebo Systemic adverse events reported by at least 3.0% of the total study population TNX - 102 SL (N=103) Placebo (N=101) Total (N=204) Somnolence 1.9 6.9 4.4 Dry Mouth 3.9 4.0 3.9 Back Pain 4.9 3.0 3.9 Source: Phase 2b BESTFIT study preliminary data TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.
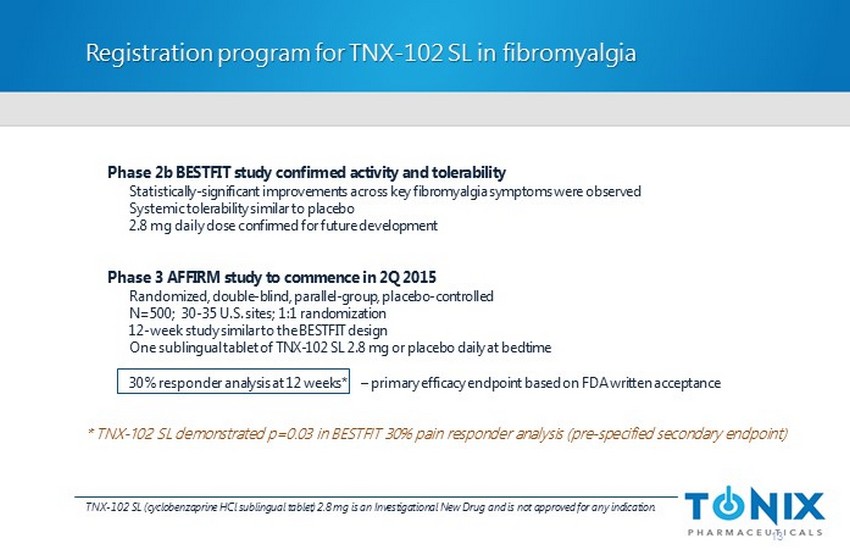
Phase 2b BESTFIT study confirmed activity and tolerability Statistically - significant improvements across key fibromyalgia symptoms were observed Systemic tolerability similar to placebo 2.8 mg daily dose confirmed for future development Phase 3 AFFIRM study to commence in 2Q 2015 Randomized, double - blind, parallel - group, placebo - controlled N=500; 30 - 35 U.S. sites; 1:1 randomization 12 - week study similar to the BESTFIT design One sublingual tablet of TNX - 102 SL 2.8 mg or placebo daily at bedtime 30% responder analysis at 12 weeks* – primary efficacy endpoint based on FDA written acceptance Registration program for TNX - 102 SL in fibromyalgia 13 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication. * TNX - 102 SL demonstrated p=0.03 in BESTFIT 30% pain responder analysis (pre - specified secondary endpoint)
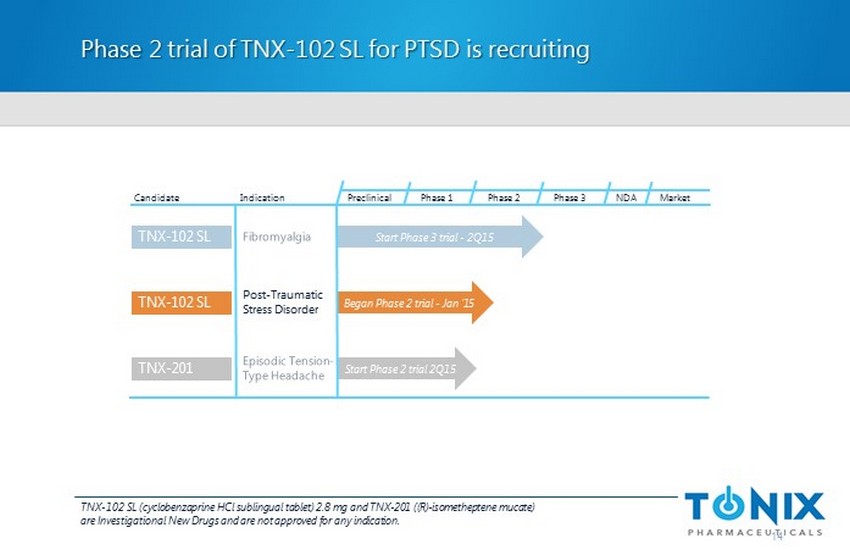
Phase 2 trial of TNX - 102 SL for PTSD is recruiting 14 Preclinical Phase 1 NDA Market Candidate Indication Phase 2 Phase 3 Post - Traumatic Stress Disorder TNX - 102 SL Began Phase 2 trial - Jan ‘15 TNX - 102 SL Start Phase 3 trial - 2Q15 Start Phase 2 trial 2Q15 TNX - 201 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg and TNX - 201 ( (R) - isometheptene mucate ) are Investigational New Drugs and are not approved for any indication.
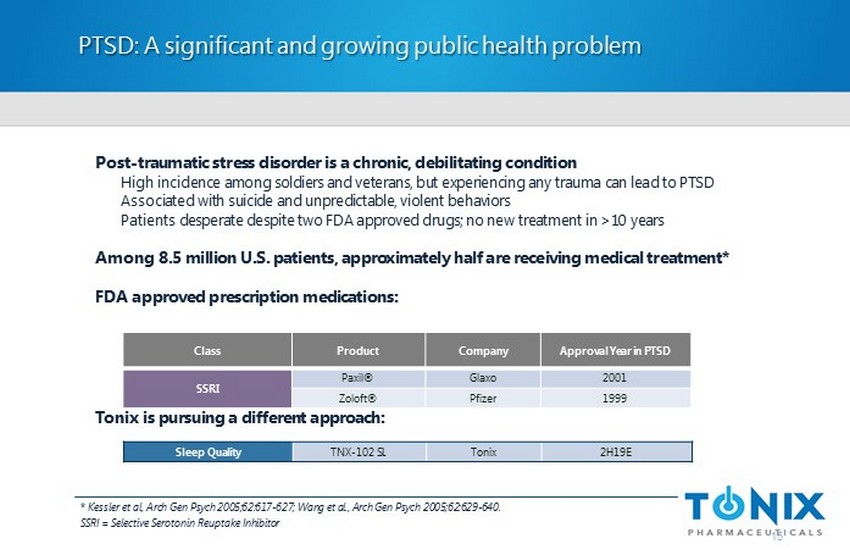
Post - traumatic stress disorder is a chronic, debilitating condition High incidence among soldiers and veterans, but experiencing any trauma can lead to PTSD Associated with suicide and unpredictable, violent behaviors Patients desperate despite two FDA approved drugs; no new treatment in >10 years Among 8.5 million U.S. patients, approximately half are receiving medical treatment* FDA approved prescription medications: Tonix is pursuing a different approach: PTSD: A significant and growing public health problem 15 Class Product Company Approval Year in PTSD SSRI Paxil ® Glaxo 2001 Zoloft ® Pfizer 1999 Sleep Quality TNX - 102 SL Tonix 2H19E * Kessler et al, Arch Gen Psych 2005;62:617 - 627; Wang et al., Arch Gen Psych 2005;62:629 - 640. SSRI = Selective Serotonin Reuptake Inhibitor

Poor sleep quality after trauma is linked to onset of PTSD PTSD patients complain of poor sleep quality as a core symptom Distressing dreams (nightmares) are part of “re - experiencing” Restless sleep is part of “hyper - arousal” Correlated with depression, substance abuse and suicide Military - related PTSD is an unmet need Evidence suggests that SSRIs may be ineffective in military - related PTSD Response of PTSD in men to SSRIs has not been adequately studied TNX - 102 SL targets mechanisms associated with treating disturbed sleep in PTSD Sleep quality is a new target for PTSD treatment 16 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.
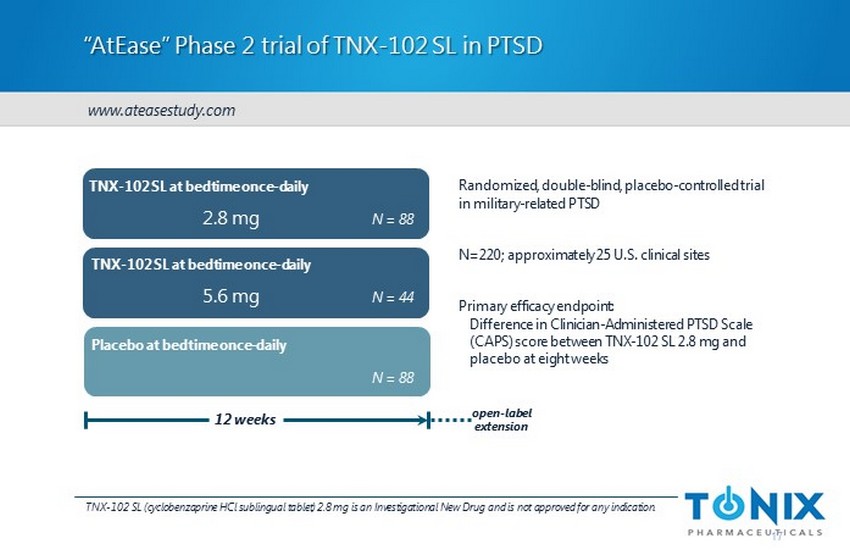
“AtEase” Phase 2 trial of TNX - 102 SL in PTSD Randomized, double - blind, placebo - controlled trial in military - related PTSD N=220; approximately 25 U.S. clinical sites Primary efficacy endpoint: Difference in Clinician - Administered PTSD Scale (CAPS) score between TNX - 102 SL 2.8 mg and placebo at eight weeks TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N = 88 17 TNX - 102 SL at bedtime once - daily N = 88 N = 44 2.8 mg 5.6 mg open - label extension TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication . www.ateasestudy.com
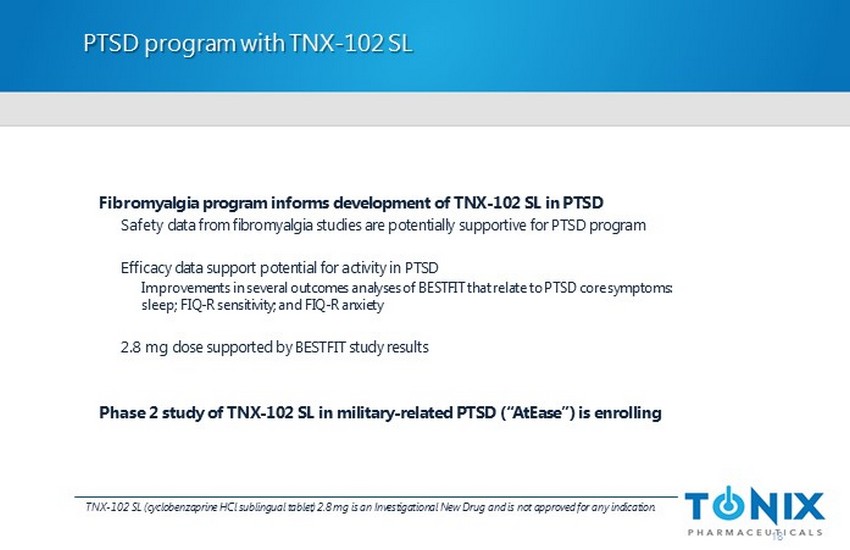
PTSD program with TNX - 102 SL 18 Fibromyalgia program informs development of TNX - 102 SL in PTSD Safety data from fibromyalgia studies are potentially supportive for PTSD program Efficacy data support potential for activity in PTSD Improvements in several outcomes analyses of BESTFIT that relate to PTSD core symptoms: sleep; FIQ - R sensitivity; and FIQ - R anxiety 2.8 mg dose supported by BESTFIT study results Phase 2 study of TNX - 102 SL in military - related PTSD (“ AtEase ”) is enrolling TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication.
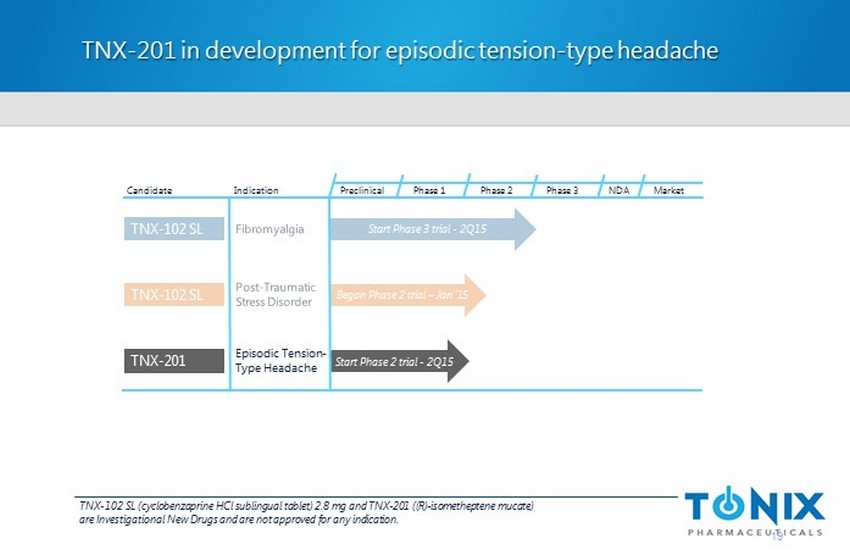
TNX - 201 in development for episodic tension - type headache 19 Preclinical Phase 1 NDA Market Candidate Indication Phase 2 Phase 3 TNX - 102 SL Began Phase 2 trial – Jan ‘15 TNX - 102 SL Start Phase 3 trial - 2Q15 Start Phase 2 trial - 2Q15 TNX - 201 Episodic Tension - Type Headache TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg and TNX - 201 ( (R) - isometheptene mucate ) are Investigational New Drugs and are not approved for any indication.
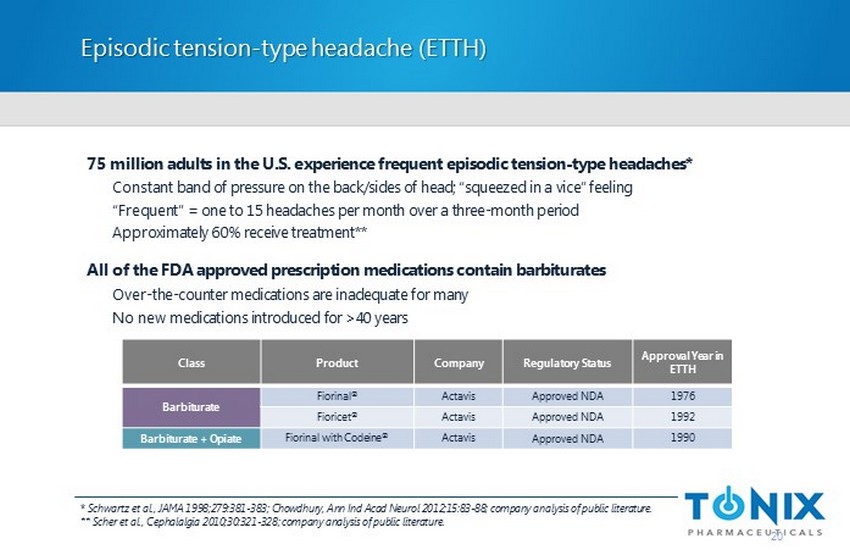
Episodic tension - type headache (ETTH) * Schwartz et al., JAMA 1998;279:381 - 383; Chowdhury, Ann Ind Acad Neurol 2012;15:83 - 88; company analysis of public literature. ** Scher et al., Cephalalgia 2010;30:321 - 328; company analysis of public literature. 75 million adults in the U.S. experience frequent episodic tension - type headaches* Constant band of pressure on the back/sides of head; “squeezed in a vice” feeling “Frequent” = one to 15 headaches per month over a three - month period Approximately 60% receive treatment** All of the FDA approved prescription medications contain barbiturates Over - the - counter medications are inadequate for many No new medications introduced for >40 years Class Product Company Regulatory Status Approval Year in ETTH Barbiturate Fiorinal ® Actavis Approved NDA 1976 Fioricet ® Actavis Approved NDA 1992 Barbiturate + Opiate Fiorinal with Codeine ® Actavis Approved NDA 1990 20
TNX - 201 in clinical development for ETTH TNX - 201 is (R) - isometheptene mucate Tonix is developing TNX - 201 for ETTH Phase 2 study to begin in 2Q 2015 Racemic isometheptene mucate is a mixture of (R) and (S) isomers Had been widely prescribed for many decades in the U.S. as: a single - agent medicine (pre - 1962) a component of combination drug products Midrin ® – NDA withdrawn Prodrin ® – marketed under “unapproved drug category” No product containing isometheptene mucate is currently FDA approved for any indication 21 TNX - 201 ((R) - isometheptene mucate ) is an Investigational New Drug and is not approved for any indication.
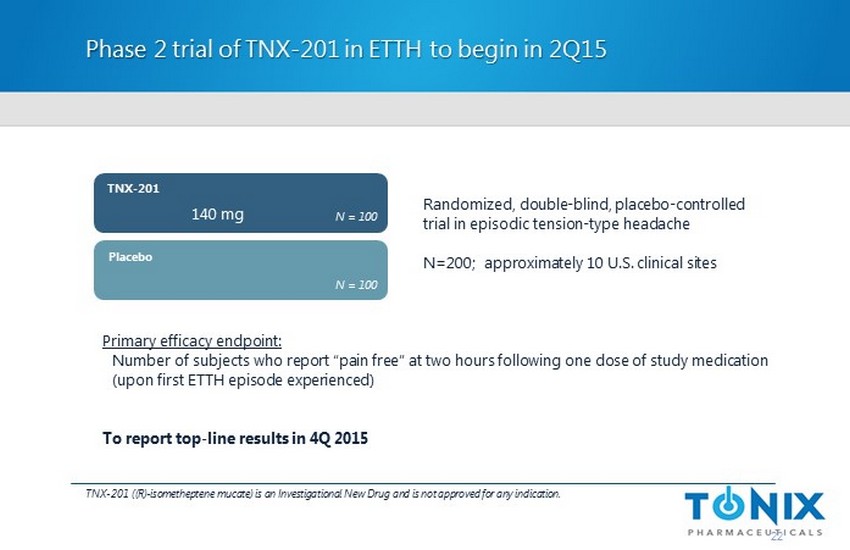
Phase 2 trial of TNX - 201 in ETTH to begin in 2Q15 22 TNX - 201 ((R) - isometheptene mucate ) is an Investigational New Drug and is not approved for any indication. Randomized, double - blind, placebo - controlled trial in episodic tension - type headache N=200 ; approximately 10 U.S. clinical sites Placebo TNX - 201 N = 100 N = 100 140 mg Primary efficacy endpoint: Number of subjects who report “pain free” at two hours following one dose of study medication (upon first ETTH episode experienced) To report top - line results in 4Q 2015
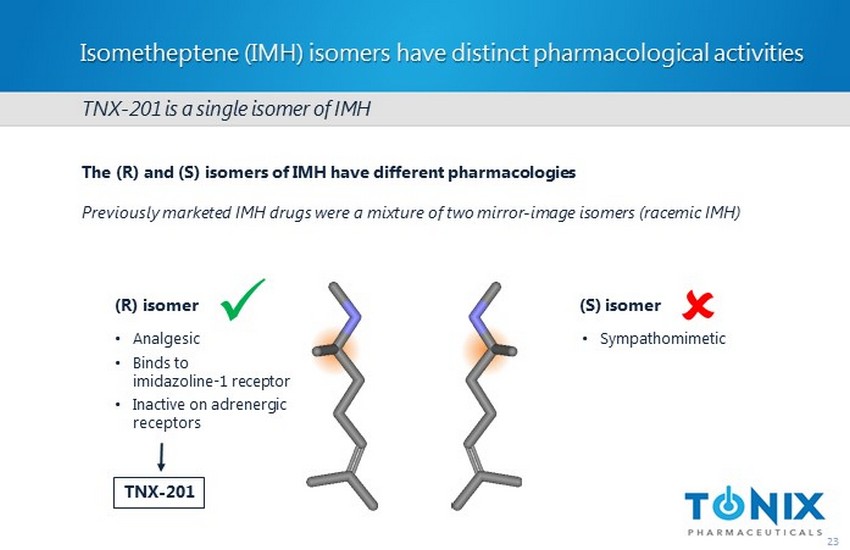
Isometheptene (IMH) isomers have distinct pharmacological activities 23 (R) isomer (S) isomer • Analgesic • Binds to imidazoline - 1 receptor • Inactive on adrenergic receptors • Sympathomimetic The (R) and (S) isomers of IMH have different pharmacologies Previously marketed IMH drugs were a mixture of two mirror - image isomers (racemic IMH) TNX - 201 is a single isomer of IMH x ; TNX - 201
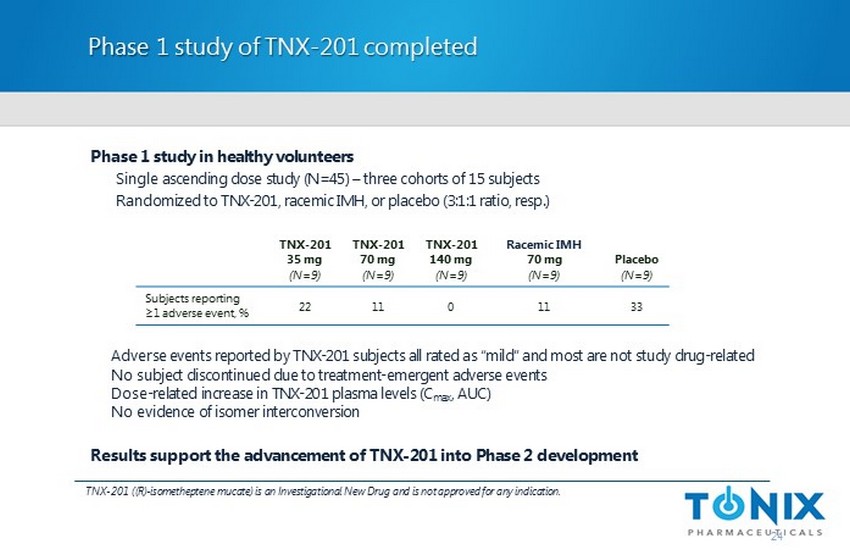
Phase 1 study of TNX - 201 completed Phase 1 study in healthy volunteers Single ascending dose study (N=45) – three cohorts of 15 subjects Randomized to TNX - 201, racemic IMH, or placebo (3:1:1 ratio, resp.) 24 TNX - 201 ((R) - isometheptene mucate ) is an Investigational New Drug and is not approved for any indication. TNX - 201 35 mg (N=9) TNX - 201 70 mg (N=9) TNX - 201 140 mg (N=9) Racemic IMH 70 mg (N=9) Placebo (N=9) Subjects reporting ≥1 adverse event, % 22 11 0 11 33 Adverse events reported by TNX - 201 subjects all rated as “mild” and most are not study drug - related No subject discontinued due to treatment - emergent adverse events Dose - related increase in TNX - 201 plasma levels ( C max , AUC) No evidence of isomer interconversion Results support the advancement of TNX - 201 into Phase 2 development
The imidazoline - 1 receptor (I 1 - R) is a novel target for the treatment of pain 25 Hot Plate Test Characteristics Transmembrane receptor Distinct from α 2 AR and MAO receptor subtypes No sequence similarity to GPCRs or ATP - sensitive K+ channels Shares similarities to ryanodine and cytokine receptors Mouse studies I 1 - R null mice show no difference in systolic blood pressure or heart rate compared to wild type I 1 - R null mice show a reduction in pain threshold compared to wild type in both the hot plate and tail flick tests Piletz JE et al, DNA Cell Biol 2000; 19:319 - 329. Zhang L et al, CNS Neurosci Ther 2013;19:978 - 981.
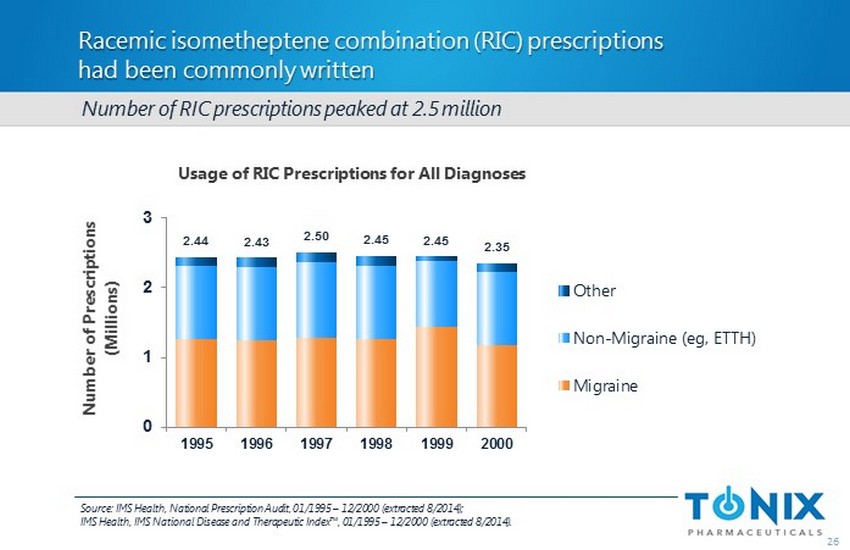
Racemic isometheptene combination (RIC) prescriptions had been commonly written 26 Usage of RIC Prescriptions for All Diagnoses Number of RIC prescriptions peaked at 2.5 million 2.44 2.43 2.50 2.45 2.45 2.35 0 1 2 3 1995 1996 1997 1998 1999 2000 Number of Prescriptions (Millions) Other Non-Migraine (eg, ETTH) Migraine Source : IMS Health, National Prescription Audit, 01/1995 – 12/2000 (extracted 8/2014); IMS Health, IMS National Disease and Therapeutic Index™, 01/1995 – 12/2000 (extracted 8/2014).
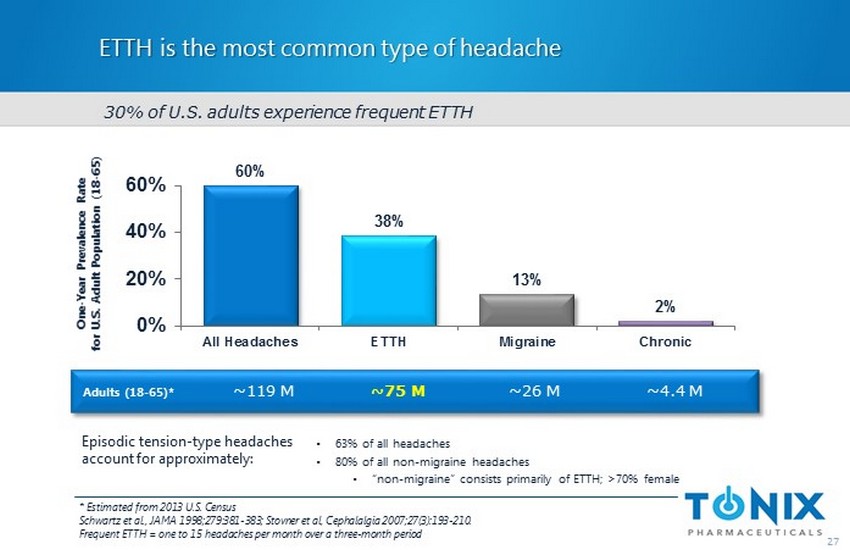
ETTH is the most common type of headache 27 60% 38% 13% 2% 0% 20% 40% 60% All Headaches ETTH Migraine Chronic One - Year Prevalence Rate for U.S. Adult Population (18 - 65) Adults (18 - 65)* * Estimated from 2013 U.S. Census Schwartz et al., JAMA 1998;279:381 - 383; Stovner et al, Cephalalgia 2007;27(3 ): 193 - 210. Frequent ETTH = one to 15 headaches per month over a three - month period • 63 % of all headaches • 80% of all non - migraine headaches • “non - migraine ” consists primarily of ETTH; >70% female ~119 M ~75 M ~26 M ~4.4 M 30% of U.S. adults experience frequent ETTH Episodic tension - type headaches account for approximately:
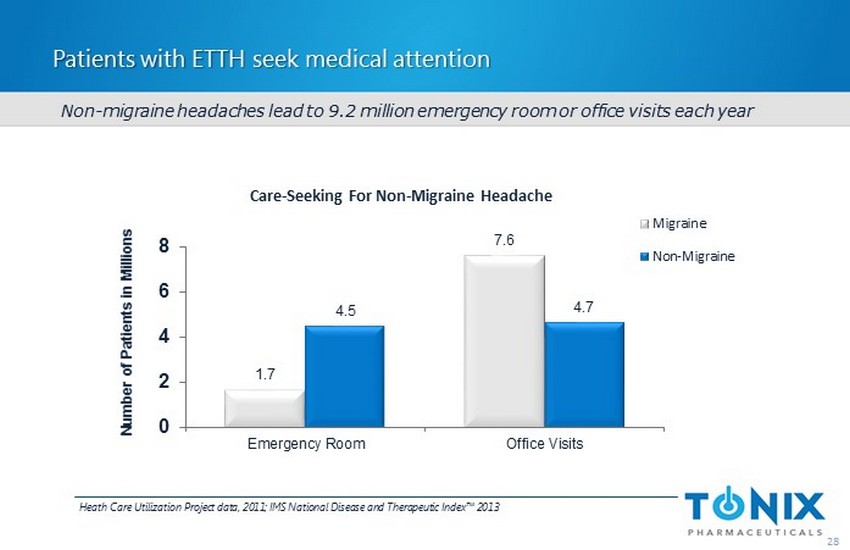
Patients with ETTH seek medical attention 1.7 7.6 4.5 4.7 0 2 4 6 8 Emergency Room Office Visits Number of Patients in Millions Care - Seeking For Non - Migraine Headache Migraine Non-Migraine 28 Heath Care Utilization Project data, 2011; IMS National Disease and Therapeutic Index™ 2013 Non - migraine headaches lead to 9.2 million emergency room or office visits each year
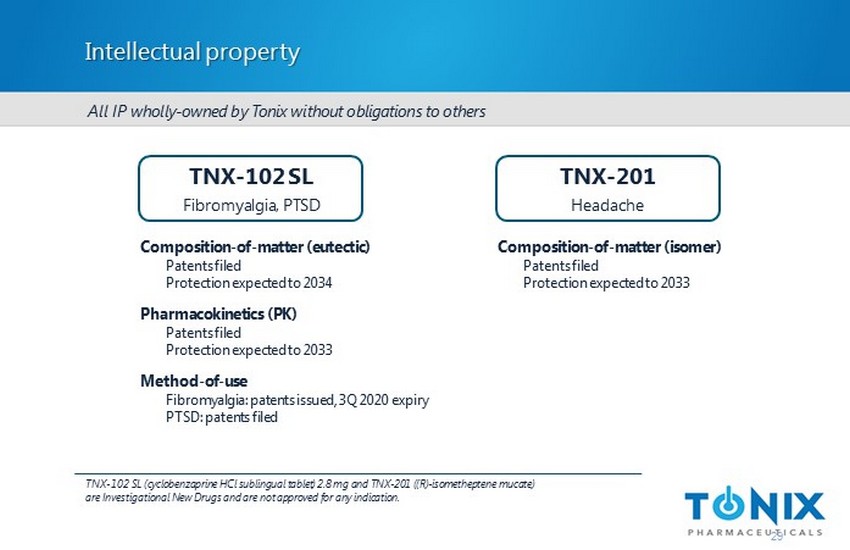
Intellectual property Composition - of - matter (eutectic) Patents filed Protection expected to 2034 Pharmacokinetics (PK) Patents filed Protection expected to 2033 Method - of - use Fibromyalgia: patents issued, 3Q 2020 expiry PTSD: patents filed TNX - 102 SL Fibromyalgia, PTSD TNX - 201 Headache Composition - of - matter (isomer) Patents filed Protection expected to 2033 All IP wholly - owned by Tonix without obligations to others 29 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg and TNX - 201 ((R) - isometheptene mucate ) are Investigational New Drugs and are not approved for any indication.
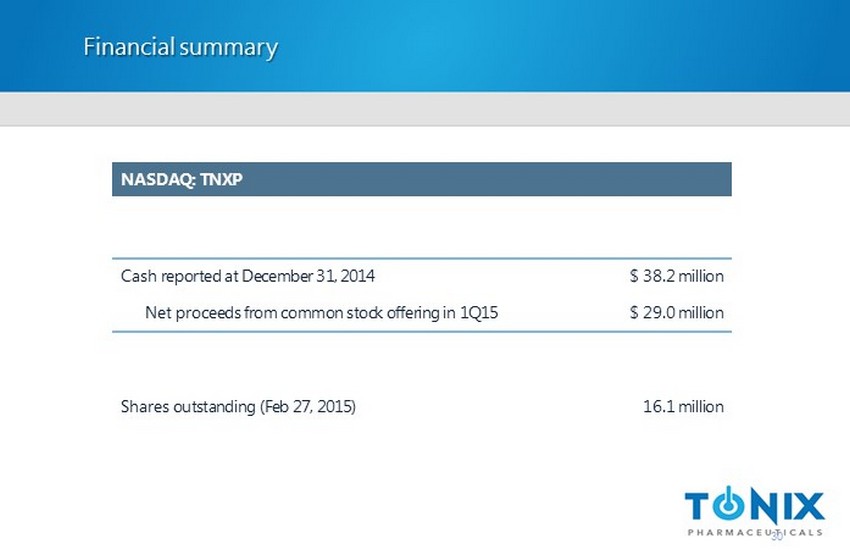
Financial summary NASDAQ: TNXP Cash reported at December 31 , 2014 $ 38.2 million Net proceeds from common stock offering in 1Q15 $ 29.0 million Shares outstanding (Feb 27, 2015) 16.1 million 30

Bruce Daugherty, PhD Chief Scientific Officer Seth Lederman, MD Chief Executive Officer Management team Leland Gershell, MD, PhD Chief Financial Officer 31

TNX - 102 SL – Fibromyalgia □ September 2014 – Reported top line results from Phase 2b BESTFIT study □ January 2015 – Reported FDA acceptance of 30% responder analysis as Phase 3 primary endpoint □ 2Q 2015 – Begin Phase 3 AFFIRM study □ 2H 2016 – R eport top - line results from AFFIRM study TNX - 102 SL – Post - Traumatic Stress Disorder □ December 2014 – Began recruiting Phase 2 AtEase study in military - related PTSD □ 1H 2015 – Provide update on enrollment and timing of results from AtEase □ 1H 2016 – Report top - line results from AtEase study TNX - 201 – Episodic Tension - Type Headache □ December 2014 – Completed Phase 1 clinical pharmacology study □ 2Q 2015 – Begin Phase 2 study in ETTH □ 4Q 2015 – Report top - line results from Phase 2 study x x x x Milestones – recent and upcoming 32 TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg and TNX - 201 ((R) - isometheptene mucate ) are Investigational New Drugs and are not approved for any indication.

NASDAQ: TNXP 509 Madison Avenue New York, NY 10022 (212) 980 - 9155 www.tonixpharma.com