
Exhibit 99.01

© 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. A Randomized Placebo - Controlled Multicenter Trial of a Low - Dose Bedtime Sublingual Formulation of Cyclobenzaprine (TNX - 102 SL) for the Treatment of Military - Related PTSD Results from the “AtEase” Study Presented by Gregory Sullivan MD at American Society of Clinical Psychopharmacology Annual Meeting, Scottsdale AZ May 31, 2016 © 2016 Tonix Pharmaceuticals Holding Corp.

1 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. The AtEase Study Why We Studied Military PTSD Characteristics of military - related PTSD population - Combat traumas but could include non - combat traumas during service (e.g. sexual assault) - Male - predominant (85:15) vs. civilian female - predominant (67:33) 1 - More commonly repeated traumas during deployments vs. discrete traumas - Both military and civilian PTSD diagnosed using DSM - 5/CAPS - 5 2 Unmet need treating military - related PTSD - No treatment response observed in US military population with the two FDA - approved therapies for PTSD - Sertraline – negative large multicenter trial in US military veterans 3 - Placebo numerically superior on CAPS - 2 - Paroxetine – not studied in military population - Inconsistent treatment response observed in males - Sertraline – FDA - conducted post - hoc analysis concluded no effect for male civilian subgroup 4 - Paroxetine – no sex - related difference in treatment outcomes in civilian population 5 - Important tolerability issues with SSRIs in this population - Sexual dysfunction - Insomnia 1 Tolin & Foa. Psychol Bull 2006;132:959 - 92. 2 Weathers FW et al . The Clinician - Administered PTSD Scale for DSM - 5 (CAP - 5), National Center for PTSD at ptsd.va.gov. 3 Friedman MJ et al. J Clin Psychiatry 2007;68:711 - 20. 4 Zoloft ® Package Insert, Pfizer , NY, NY; August 2014. 5 Paxil® Package Insert, Glaxo, June 2014
2 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. The AtEase Study Rational for TNX - 102 SL for PTSD TNX - 102 SL is a sublingual formulation of cyclobenzaprine (CBP ) - Transmucosal absorption - Tricyclic molecule – not antidepressant - Targets receptors believed to play key roles in sleep physiology - functional studies show antagonism at each of 1 - 5 - HT 2A - a 1 - adrenergic - Histamine - H 1 TNX - 102 SL is designed for bedtime administration and nighttime pharmacokinetic and pharmacodynamics effects - Rapid sublingual transmucosal absorption (reduced lag - time) - Avoidance of first - pass metabolism - reduces exposure to active metabolite, norcyclobenzaprine ( nCBP ) • Long - lived active metabolite (t 1/2 ~72 hours) • Distinct receptor binding profile less selective for target receptors • Potentially undesirable off - target functional activities • Exposure ( AUC 0 - 48 ) for CBP/ nCBP of 1.9 for TNX - 102 SL vs. 1.2 for oral IR form 2 1 Daugherty et al. Society of Biological Psychiatry 70 th Annual Scientific Convention, May 14 - 16, 2015 Toronto, Ontario, Canada . 2 Lederman et al . European Congress of Rheumatology, Rome, June 2015 T NX - 102 SL (cyclobenzaprine HCl sublingual tablets, 2.8 mg) is an Investigational New Drug and is not approved for any indication.
3 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. The AtEase Study Rational for Targeting of Sleep for Treatment of PTSD Previous work of TNX - 102 SL in a bedtime, nightly regimen improved fibromyalgia symptoms and supported a mechanism in which TNX - 102 SL improved sleep quality - PTSD has clinical overlap with fibromyalgia - PTSD has comorbidity with fibromyalgia PTSD patients complain of sleep disturbance as a core symptom - Distressing dreams (nightmares) are part of “re - experiencing” - Sleep disturbance is part of the hyperarousal cluster of PTSD diagnostic criteria - Altered autonomic and neurohormonal balance - May interfere with processing of emotionally charged memories 2 - i.e. attenuated extinction consolidation Sleep disturbance also correlates with depression, substance abuse and suicidal behaviors in PTSD 3 1 Moldofsky et al, J Rheumatol 2011, 38:2653 - 63; Lederman et al . European Congress of Rheumatology, Rome, June 2015. 2 Pace - Schott et al. Biology of Mood & Anxiety Disorders 2015;5(3):1 - 19. 3 Germain , Am J Psychiary 2013;170:372 - 382; McHugh et al, J Traumatic Stress 2014:27:82 - 89; Betts et al, Journal of Anxiety Disorders 2013;27:735 - 41. T NX - 102 SL (cyclobenzaprine HCl sublingual tablets, 2.8 mg) is an Investigational New Drug and is not approved for any indication .
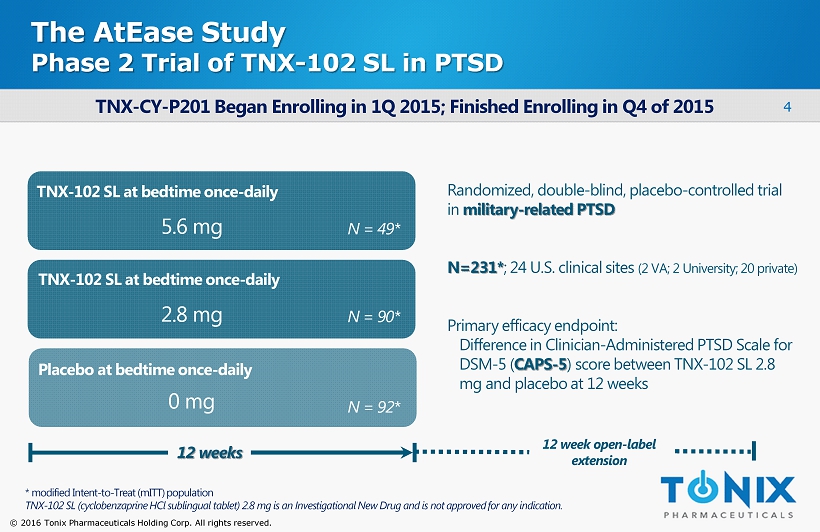
© 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. The AtEase Study Phase 2 Trial of TNX - 102 SL in PTSD Randomized, double - blind, placebo - controlled trial in military - related PTSD N=231* ; 24 U.S. clinical sites (2 VA; 2 University; 20 private) Primary efficacy endpoint: Difference in Clinician - Administered PTSD Scale for DSM - 5 ( CAPS - 5 ) score between TNX - 102 SL 2.8 mg and placebo at 12 weeks TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N = 49* TNX - CY - P201 Began Enrolling in 1Q 2015; Finished Enrolling in Q4 of 2015 TNX - 102 SL at bedtime once - daily N = 92* N = 90* 5.6 mg 2.8 mg 12 week open - label extension * modified Intent - to - Treat ( mITT ) population TNX - 102 SL (cyclobenzaprine HCl sublingual tablet) 2.8 mg is an Investigational New Drug and is not approved for any indication. 0 mg 4
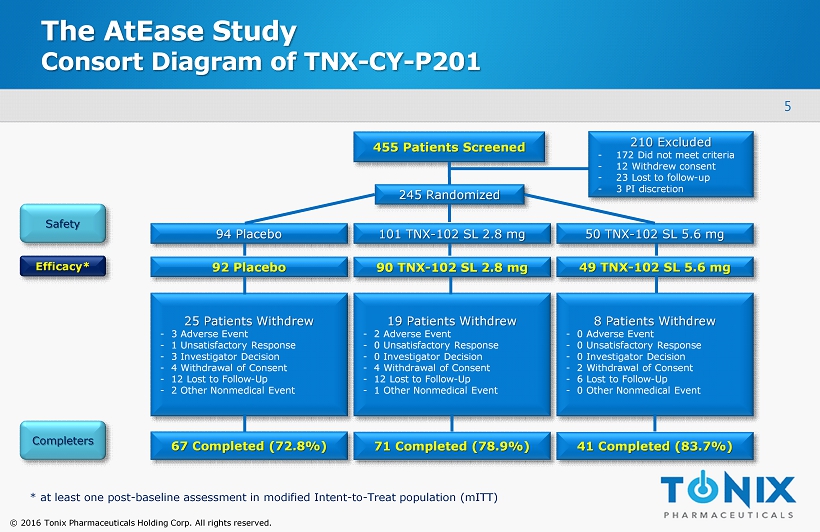
5 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. The AtEase Study Consort Diagram of TNX - CY - P201 455 Patients Screened 25 Patients Withdrew - 3 Adverse Event - 1 Unsatisfactory Response - 3 Investigator Decision - 4 Withdrawal of Consent - 12 Lost to Follow - Up - 2 Other Nonmedical Event 210 Excluded - 172 Did not meet criteria - 12 Withdrew consent - 23 Lost to follow - up - 3 PI discretion Safety Completers * at least one post - baseline assessment in modified Intent - to - Treat population ( mITT ) 67 Completed (72.8%) 71 Completed (78.9%) 41 Completed (83.7%) 245 Randomized 94 Placebo 101 TNX - 102 SL 2.8 mg 50 TNX - 102 SL 5.6 mg 92 Placebo 90 TNX - 102 SL 2.8 mg 49 TNX - 102 SL 5.6 mg 19 Patients Withdrew - 2 Adverse Event - 0 Unsatisfactory Response - 0 Investigator Decision - 4 Withdrawal of Consent - 12 Lost to Follow - Up - 1 Other Nonmedical Event 8 Patients Withdrew - 0 Adverse Event - 0 Unsatisfactory Response - 0 Investigator Decision - 2 Withdrawal of Consent - 6 Lost to Follow - Up - 0 Other Nonmedical Event Efficacy*
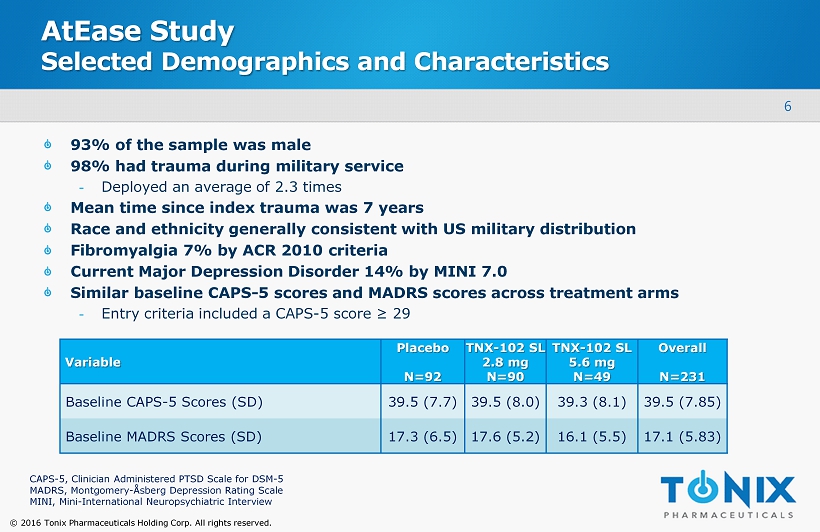
6 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Selected Demographics and Characteristics 93% of the sample was male 98% had trauma during military service - Deployed an average of 2.3 times Mean time since index trauma was 7 years Race and ethnicity generally consistent with US military distribution Fibromyalgia 7% by ACR 2010 criteria Current Major Depression Disorder 14% by MINI 7.0 Similar baseline CAPS - 5 scores and MADRS scores across treatment arms - Entry criteria included a CAPS - 5 score ≥ 29 Variable Placebo N=92 TNX - 102 SL 2.8 mg N=90 TNX - 102 SL 5.6 mg N=49 Overall N=231 Baseline CAPS - 5 Scores (SD) 39.5 (7.7) 39.5 (8.0) 39.3 (8.1) 39.5 (7.85) Baseline MADRS Scores (SD) 17.3 (6.5) 17.6 (5.2) 16.1 (5.5) 17.1 (5.83) CAPS - 5, Clinician Administered PTSD Scale for DSM - 5 MADRS, Montgomery - Å sberg Depression Rating Scale MINI, Mini - International Neuropsychiatric Interview
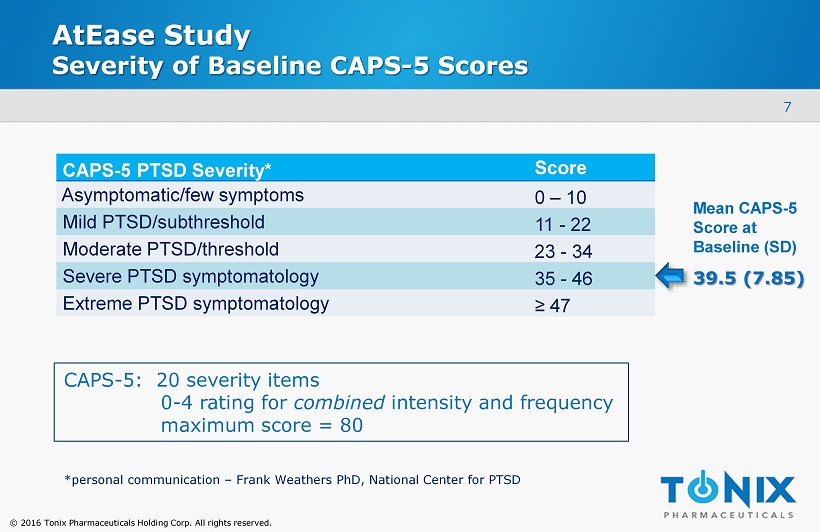
© 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Severity of Baseline CAPS - 5 Scores CAPS - 5 PTSD Severity* Score Asymptomatic/few symptoms 0 – 10 Mild PTSD/subthreshold 11 - 22 Moderate PTSD/threshold 23 - 34 Severe PTSD symptomatology 35 - 46 Extreme PTSD symptomatology ≥ 47 7 *personal communication – Frank Weathers PhD, National Center for PTSD 39.5 (7.85) Mean CAPS - 5 S core at Baseline (SD) CAPS - 5: 20 severity items 0 - 4 rating for combined intensity and frequency maximum score = 80
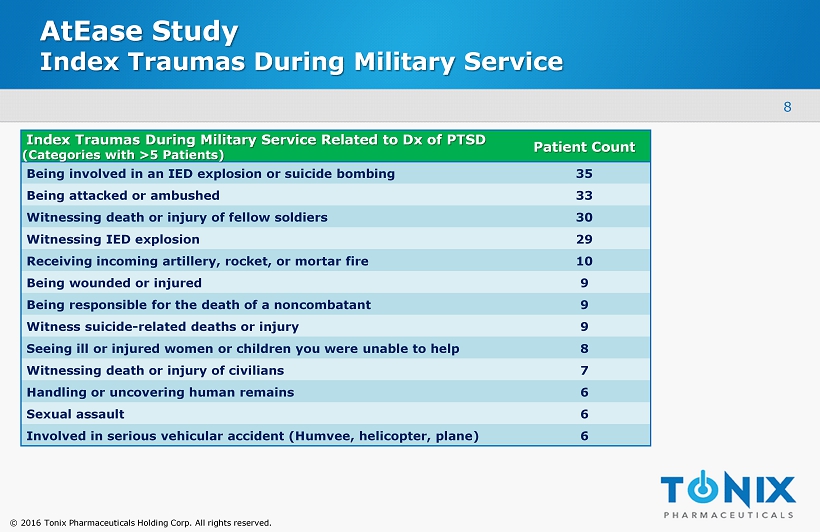
© 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Index Traumas During Military Service Index Traumas During Military Service Related to Dx of PTSD (Categories with >5 Patients) Patient Count Being involved in an IED explosion or suicide bombing 35 Being attacked or ambushed 33 Witnessing death or injury of fellow soldiers 30 Witnessing IED explosion 29 Receiving incoming artillery, rocket, or mortar fire 10 Being wounded or injured 9 Being responsible for the death of a noncombatant 9 Witness suicide - related deaths or injury 9 Seeing ill or injured women or children you were unable to help 8 Witnessing death or injury of civilians 7 Handling or uncovering human remains 6 Sexual assault 6 Involved in serious vehicular accident (Humvee, helicopter, plane) 6 8
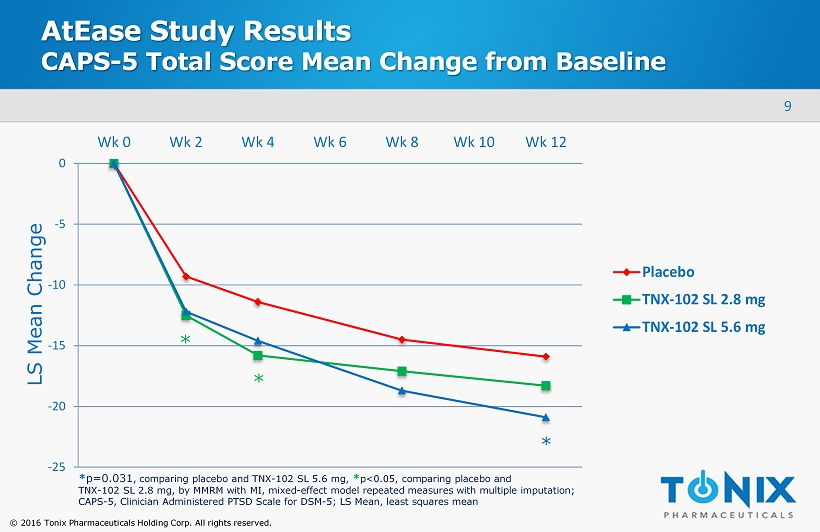
9 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results CAPS - 5 Total Score Mean Change from Baseline -25 -20 -15 -10 -5 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * p=0.031 , comparing placebo and TNX - 102 SL 5.6 mg, * p<0.05, comparing placebo and TNX - 102 SL 2.8 mg, by MMRM with MI, mixed - effect model repeated measures with multiple imputation; CAPS - 5 , Clinician Administered PTSD Scale for DSM - 5; LS Mean, least squares mean LS Mean Change * *
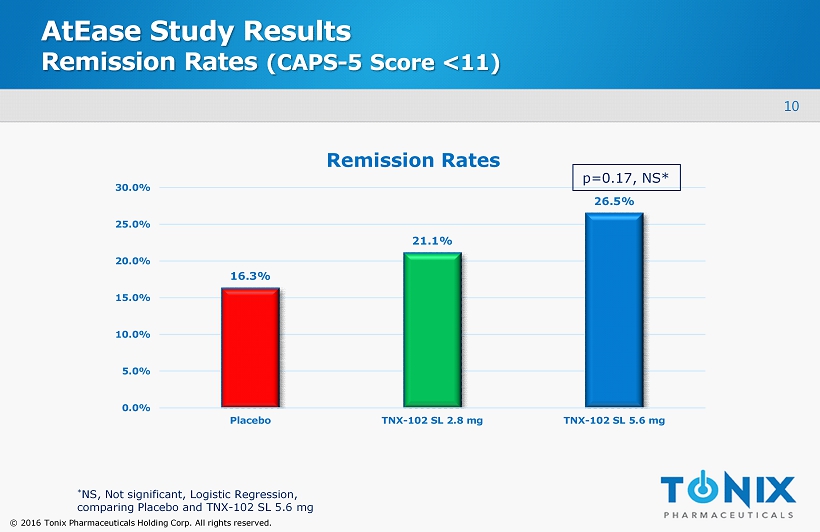
10 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results Remission Rates (CAPS - 5 Score <11) 16.3% 21.1% 26.5% 0.0% 5.0% 10.0% 15.0% 20.0% 25.0% 30.0% Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg Remission Rates * NS, Not significant, Logistic Regression, c omparing Placebo and TNX - 102 SL 5.6 mg p=0.17, NS*
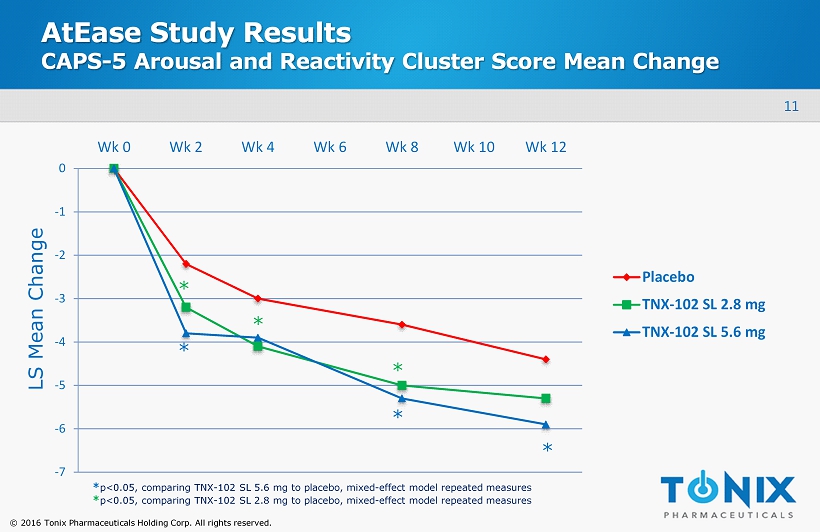
11 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results CAPS - 5 Arousal and Reactivity Cluster Score Mean Change -7 -6 -5 -4 -3 -2 -1 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * * * * * LS Mean Change * p<0.05, comparing TNX - 102 SL 5.6 mg to placebo, mixed - effect model repeated measures * p<0.05, comparing TNX - 102 SL 2.8 mg to placebo, mixed - effect model repeated measures
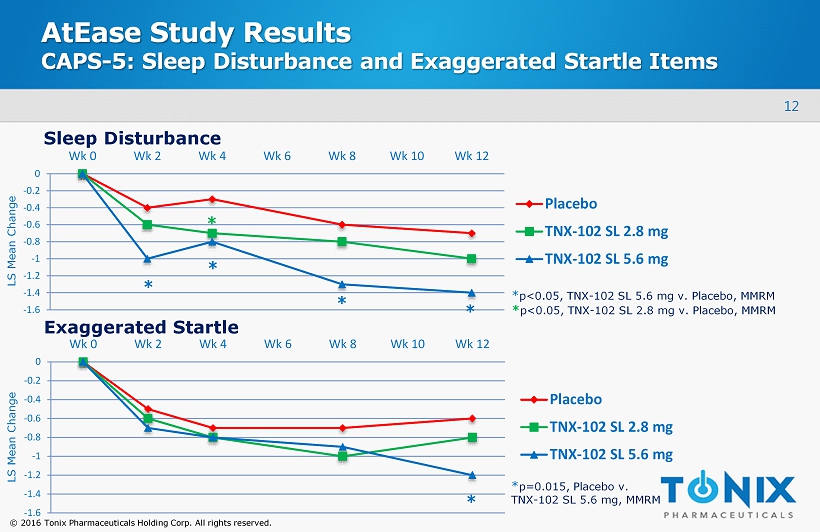
12 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results CAPS - 5: Sleep Disturbance and Exaggerated Startle Items -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg Sleep Disturbance Exaggerated Startle * * * * * * * p=0.015, Placebo v. TNX - 102 SL 5.6 mg, MMRM * p<0.05, TNX - 102 SL 5.6 mg v. Placebo , MMRM * p<0.05, TNX - 102 SL 2.8 mg v. Placebo , MMRM LS Mean Change LS Mean Change
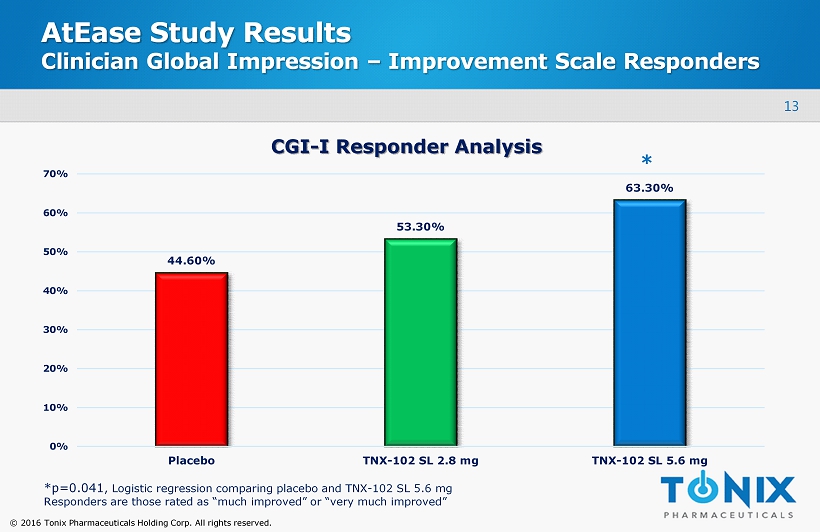
13 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results Clinician Global Impression – Improvement Scale Responders 44.60% 53.30% 63.30% 0% 10% 20% 30% 40% 50% 60% 70% Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg CGI - I Responder Analysis * *p=0.041 , Logistic regression comparing placebo and TNX - 102 SL 5.6 mg Responders are those rated as “much improved” or “very much improved”
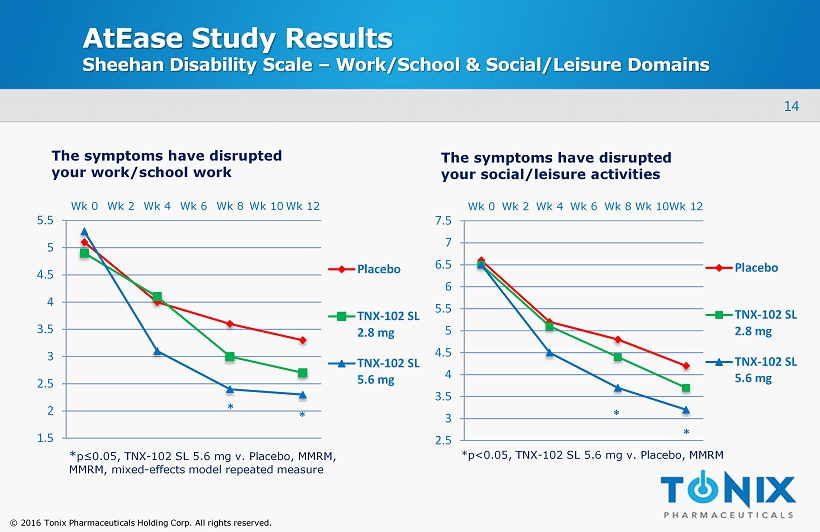
14 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results Sheehan Disability Scale – Work/School & Social/Leisure Domains 1.5 2 2.5 3 3.5 4 4.5 5 5.5 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg The symptoms have disrupted your work/school work The symptoms have disrupted your social/leisure activities * * * * * p≤0.05, TNX - 102 SL 5.6 mg v. Placebo, MMRM, MMRM, mixed - effects model repeated measure *p<0.05, TNX - 102 SL 5.6 mg v. Placebo, MMRM
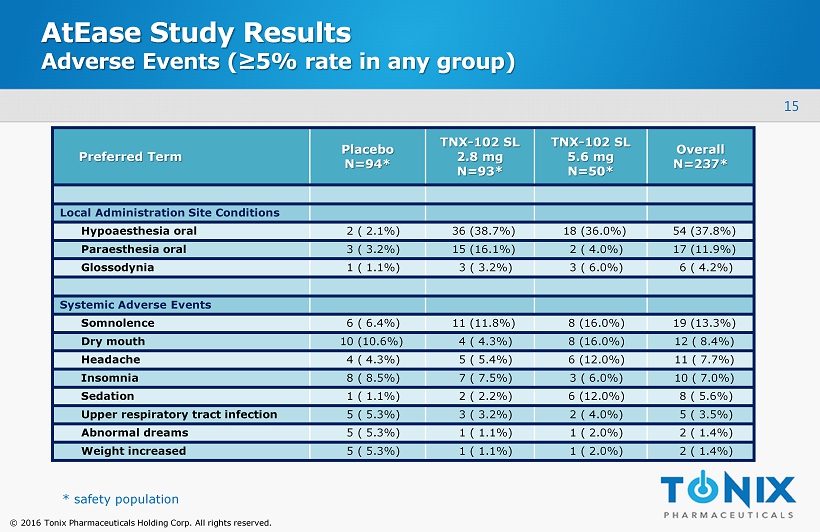
15 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Results Adverse Events (≥5% rate in any group) Preferred Term Placebo N=94* TNX - 102 SL 2.8 mg N=93* TNX - 102 SL 5.6 mg N=50* Overall N=237* Local Administration Site Conditions Hypoaesthesia oral 2 ( 2.1%) 36 (38.7%) 18 (36.0%) 54 (37.8%) Paraesthesia oral 3 ( 3.2%) 15 (16.1%) 2 ( 4.0%) 17 (11.9%) Glossodynia 1 ( 1.1%) 3 ( 3.2%) 3 ( 6.0%) 6 ( 4.2%) Systemic Adverse Events Somnolence 6 ( 6.4%) 11 (11.8%) 8 (16.0%) 19 (13.3%) Dry mouth 10 (10.6%) 4 ( 4.3%) 8 (16.0%) 12 ( 8.4%) Headache 4 ( 4.3%) 5 ( 5.4%) 6 (12.0%) 11 ( 7.7%) Insomnia 8 ( 8.5%) 7 ( 7.5%) 3 ( 6.0%) 10 ( 7.0%) Sedation 1 ( 1.1%) 2 ( 2.2%) 6 (12.0%) 8 ( 5.6%) Upper respiratory tract infection 5 ( 5.3%) 3 ( 3.2%) 2 ( 4.0%) 5 ( 3.5%) Abnormal dreams 5 ( 5.3%) 1 ( 1.1%) 1 ( 2.0%) 2 ( 1.4%) Weight increased 5 ( 5.3%) 1 ( 1.1%) 1 ( 2.0%) 2 ( 1.4%) * safety population
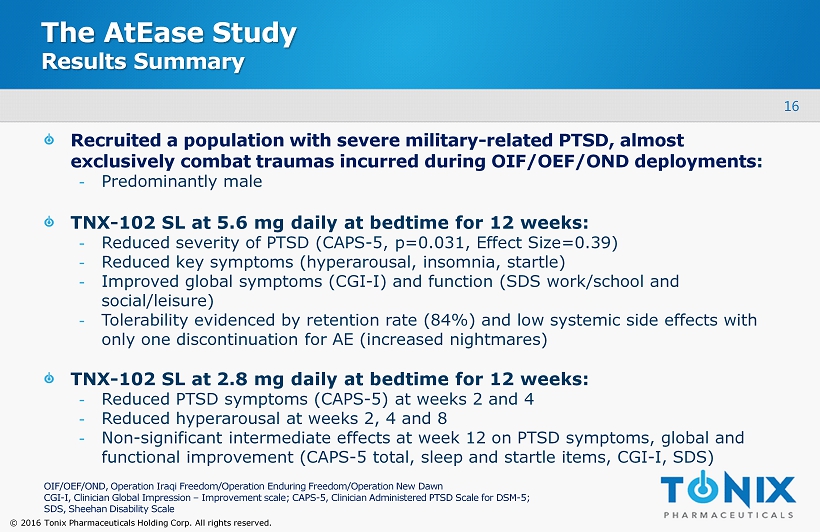
16 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. The AtEase Study Results Summary Recruited a population with severe military - related PTSD , almost exclusively combat traumas incurred during OIF/OEF/OND deployments : - Predominantly male TNX - 102 SL at 5.6 mg daily at bedtime for 12 weeks: - Reduced severity of PTSD (CAPS - 5, p=0.031, Effect Size=0.39) - Reduced key symptoms (hyperarousal, insomnia, startle) - Improved global symptoms (CGI - I) and function (SDS work/school and social/leisure) - Tolerability evidenced by retention rate (84%) and low systemic side effects with only one discontinuation for AE (increased nightmares) TNX - 102 SL at 2.8 mg daily at bedtime for 12 weeks: - Reduced PTSD symptoms (CAPS - 5) at weeks 2 and 4 - Reduced hyperarousal at weeks 2, 4 and 8 - Non - significant intermediate effects at week 12 on PTSD symptoms, global and functional improvement (CAPS - 5 total, sleep and startle items, CGI - I, SDS) OIF/OEF/OND, Operation Iraqi Freedom/Operation Enduring Freedom/Operation New Dawn CGI - I, Clinician Global Impression – Improvement scale; CAPS - 5, Clinician Administered PTSD Scale for DSM - 5; SDS, Sheehan Disability Scale

17 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Conclusions : TNX - 102 SL in Military - Related PTSD This is the first multicenter randomized clinical trial of any medication that has demonstrated efficacy in a population with military - related PTSD - Male predominant (93%) - Low incidence of comorbid fibromyalgia (7%) - Low incidence of current major depression (14%) Early effects on sleep and hyperarousal are consistent with the mechanistic hypothesis that TNX - 102 SL’s primary actions on sleep architecture and autonomic balance underlie the observed PTSD treatment effect - Late effect of TNX - 102 SL 5.6 mg on exaggerated startle consistent with longer time of recovery of sleep - related memory processing (consolidation) Next steps - Phase 3 trial in military - related PTSD - Phase 3 trial in civilian PTSD TNX - 102 SL (cyclobenzaprine HCl sublingual tablets, 2.8 mg) is an Investigational New Drug and is not approved for any indication.

18 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. AtEase Study Acknowledgements We wish to thank the military personnel, veterans, and law enforcement officers for their participation in AtEase Tonix personnel responsible for AtEase include: - Seth Lederman, Judy Gendreau, Heather Jividen, Bruce Daugherty, Ashild Peters, Perry Peters, Ron Notvest, Gregory Sullivan And key consultants to Tonix for AtEase include: - Michael Gendreau, Amy Schaberg, Pauliana Hall - Frank Weathers (Dept. National Center for PTSD) and Jonathan Davidson (Emeritus Professor, Duke University)
19 © 2016 Tonix Pharmaceuticals Holding Corp. All rights reserved. Principal Investigator Institution Arnold , Lesley UNIVERSITY OF CINCINNATI COLLEGE OF MEDICINE Bari , Mohammed SYNERGY CLINICAL RESEARCH Brenner , Ronald NEUROBEHAVIORAL RESEARCH, INC. Chueh , Daniel NRC RESEARCH INSTITUTE Croft , Harry CLINICAL TRIALS OF TEXAS Duffy , Walter PREMIER PSYCHIATRIC RESEARCH INSTITUTE, INC. Goenjian , Armen CNS , INC. Kelley , Lee Ann NOESIS PHARMA Kunovac , Jelena ALTEA RESEARCH INSTITUTE Lohr , Jim VA , San Diego Khan , Arifulla NORTHWEST CLINICAL RESEARCH CENTER McNamara , Nora UNIVERSITY HOSPITALS CASE MEDICAL CENTER Molpus , Robert CLINICAL NEUROSCIENCE SOLUTIONS, INC. Munir , Mohammad NOVEX CLINICAL RESEARC Ng , Bernardo SUN VALLEY RESEARCH CENTER Pilkinton , Patricia TUSCALOOSA VA MEDICAL CENTER Riesenberg , Robert ALTLANTA CENTER FOR MEDICAL RESEARCH (ACMR) Ross , Jeff GREAT LAKES CLINICAL TRIALS Sarkis , Elias SARKIS CLINICAL TRIALS Sedillo , Andrew MCB CLINICAL RESEARCH CENTERS Soefje , Sherry EXCELL RESEARCH, INC. Sunder , Rajagopal CITRIALS Thurman , Louise IPS RESEARCH COMPANY White , Kimberly COMPASS RESEARCH NORTH, LLC W e would also like to gratefully acknowledge the contributions of our trial sites’ principal investigators and staff AtEase Study Acknowledgements