
Exhibit 99.01

1 UPDATE Topline Results With TNX - 102 SL In Post - Traumatic Stress Disorder June 7, 2016 BIO International , San Francisco, June 7, 2016 Version 0019 6 - 7 - 16 © 2016 Tonix Pharmaceuticals Holding Corp

2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our possible need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by the Company on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2015 , as filed with the Securities and Exchange Commission (the “SEC”) on March 3 , 2016 , and future periodic reports filed with the SEC on or after the date hereof . All of the Company's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements . © 2016 Tonix Pharmaceuticals Holding Corp
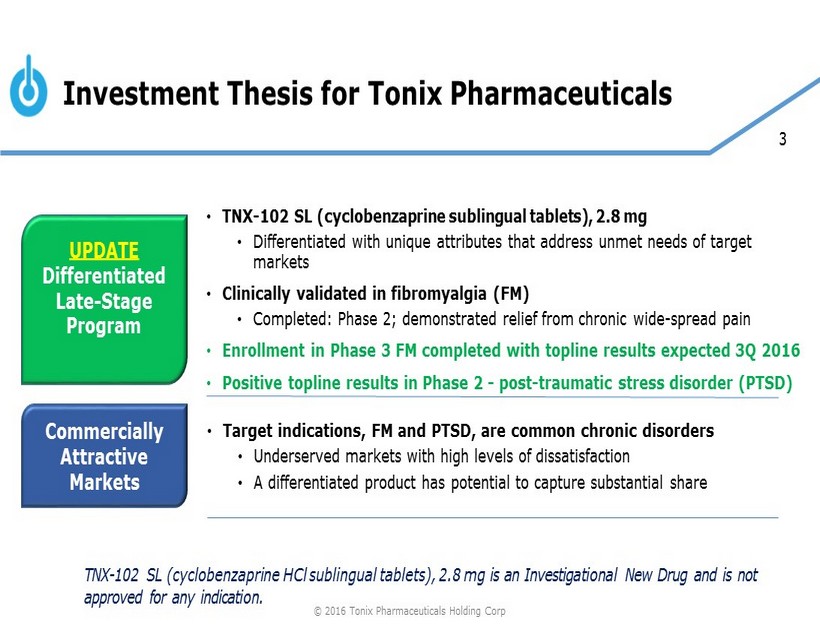
3 Investment Thesis for Tonix Pharmaceuticals UPDATE Differentiated Late - Stage Program Commercially Attractive Markets • TNX - 102 SL (cyclobenzaprine sublingual tablets), 2.8 mg • Differentiated with unique attributes that address unmet needs of target markets • Clinically validated in fibromyalgia (FM) • Completed: Phase 2; demonstrated relief from chronic wide - spread pain • Enrollment in Phase 3 FM completed with topline results expected 3Q 2016 • Positive topline results in Phase 2 - post - traumatic stress disorder (PTSD) • Target indications, FM and PTSD, are common chronic disorders • Underserved markets with high levels of dissatisfaction • A differentiated product has potential to capture substantial share TNX - 102 SL (cyclobenzaprine HCl sublingual tablets), 2.8 mg is an Investigational New Drug and is not approved for any indication. © 2016 Tonix Pharmaceuticals Holding Corp

4 CBP targets receptors with potential therapeutic effects for sleep disturbances • Multimodal: high affinity, relative selectivity and functional antagonism • 5 - HT 2A • a 1 - adrenergic • histamine H 1 • 6 - 7 fold lower affinity for SERT and NET (serotonin and norepinephrine transporters) • Profile differs from active metabolite norcyclobenzaprine, amitriptyline and other tricyclics Cyclobenzaprine: Potential To Improve Sleep Quality Cyclobenzaprine (CBP) is structurally a tricyclic molecule Tricyclics and their metabolites differ significantly in their receptor binding profiles © 2016 Tonix Pharmaceuticals Holding Corp
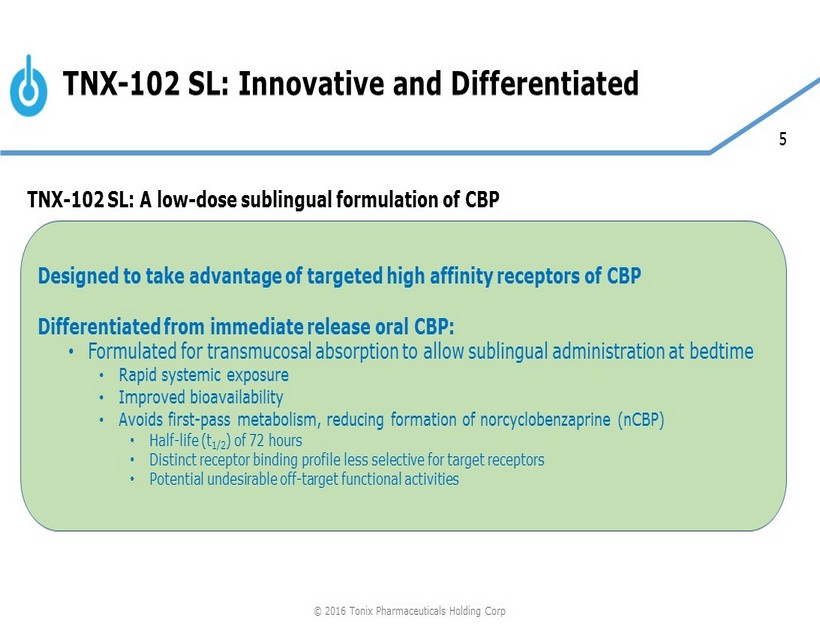
5 Designed to take advantage of targeted high affinity receptors of CBP Differentiated from immediate release oral CBP: • Formulated for transmucosal absorption to allow sublingual administration at bedtime • Rapid systemic exposure • Improved bioavailability • Avoids first - pass metabolism, reducing formation of norcyclobenzaprine (nCBP) • Half - life (t 1/2 ) of 72 hours • Distinct receptor binding profile less selective for target receptors • Potential undesirable off - target functional activities TNX - 102 SL: Innovative and Differentiated TNX - 102 SL: A low - dose sublingual formulation of CBP © 2016 Tonix Pharmaceuticals Holding Corp
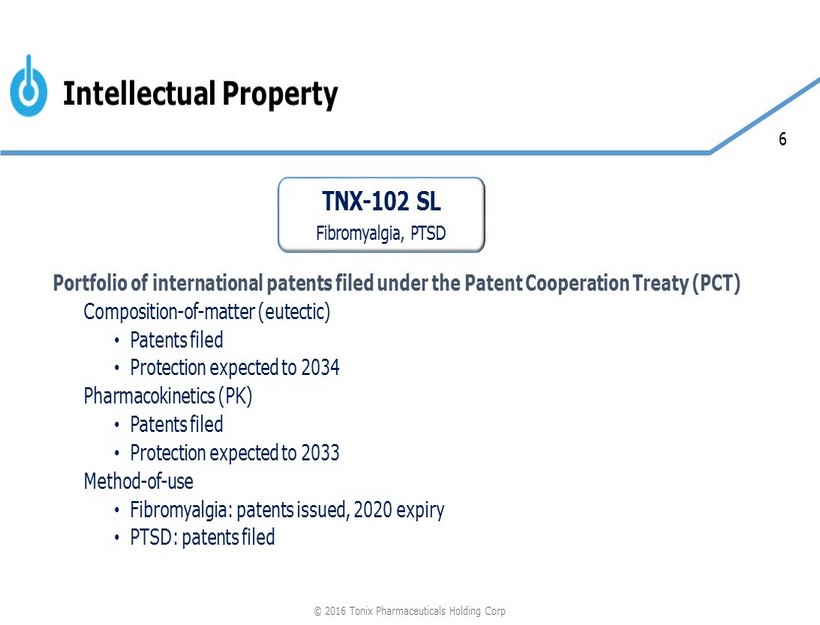
6 Intellectual Property Portfolio of international patents filed under the Patent Cooperation Treaty (PCT) C omposition - of - matter (eutectic) • Patents filed • Protection expected to 2034 Pharmacokinetics (PK) • Patents filed • Protection expected to 2033 Method - of - use • Fibromyalgia: patents issued, 2020 expiry • PTSD: patents filed TNX - 102 SL Fibromyalgia , PTSD © 2016 Tonix Pharmaceuticals Holding Corp
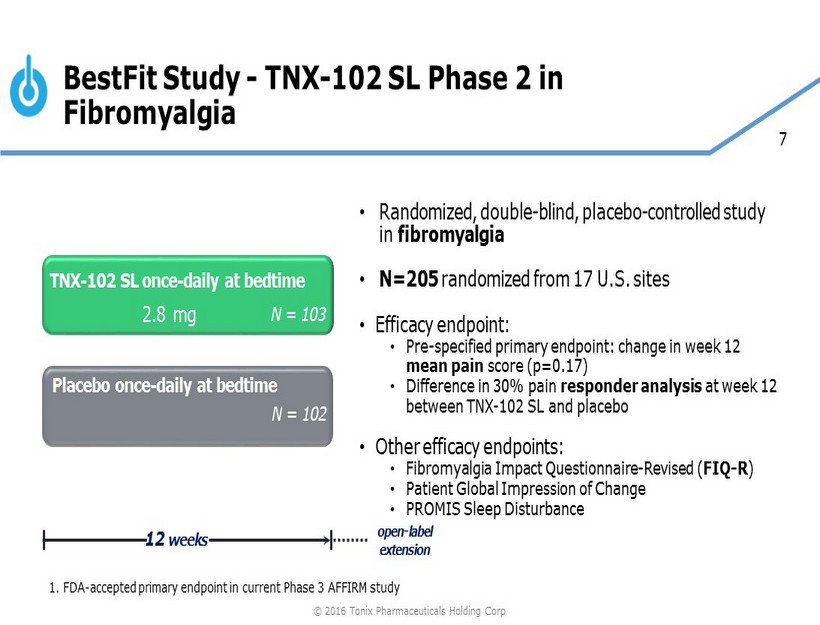
7 BestFit Study - TNX - 102 SL Phase 2 in Fibromyalgia Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime N = 102 N = 103 2.8 mg open - label extension 1. FDA - accepted primary endpoint in current Phase 3 AFFIRM study © 2016 Tonix Pharmaceuticals Holding Corp • Randomized, double - blind, placebo - controlled study in fibromyalgia • N=205 randomized from 17 U.S. sites • Efficacy endpoint: • Pre - specified primary endpoint: change in week 12 mean pain score (p=0.17) • Difference in 30% pain responder analysis at week 12 between TNX - 102 SL and placebo • Other efficacy endpoints: • Fibromyalgia Impact Questionnaire - Revised ( FIQ - R ) • Patient Global Impression of Change • PROMIS Sleep Disturbance
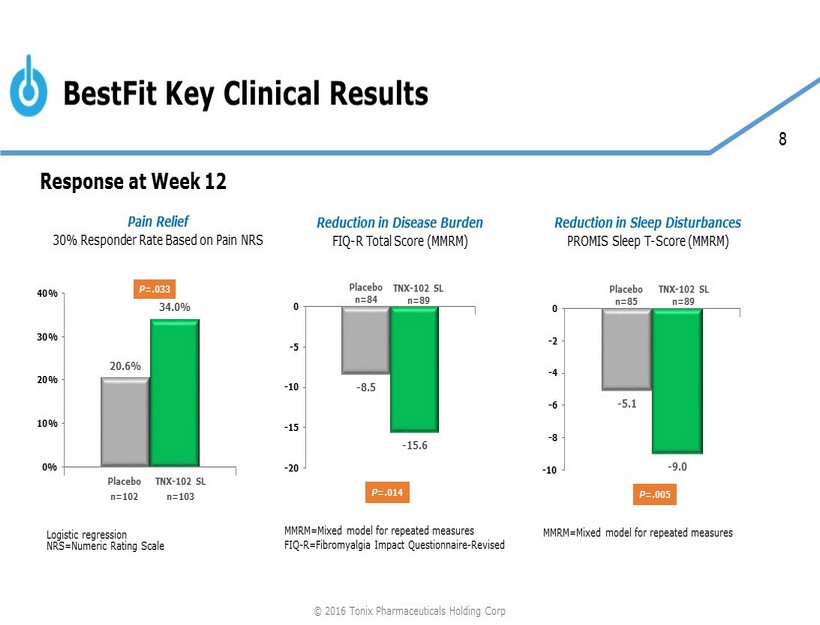
8 BestFit Key Clinical Results 20.6% 34.0% 0% 10% 20% 30% 40% Pain Relief 30% Responder Rate Based on Pain NRS Placebo n=102 TNX - 102 SL n=103 P =.033 Logistic regression NRS=Numeric Rating Scale Placebo n=85 TNX - 102 SL n=89 P =.005 - 5.1 - 9.0 -10 -8 -6 -4 -2 0 Reduction in Sleep Disturbances PROMIS Sleep T - Score (MMRM) MMRM=Mixed model for repeated measures FIQ - R=Fibromyalgia Impact Questionnaire - Revised Placebo n=84 TNX - 102 SL n=89 P =.014 - 8.5 - 15.6 -20 -15 -10 -5 0 Reduction in Disease Burden FIQ - R Total Score (MMRM) Response at Week 12 MMRM=Mixed model for repeated measures © 2016 Tonix Pharmaceuticals Holding Corp
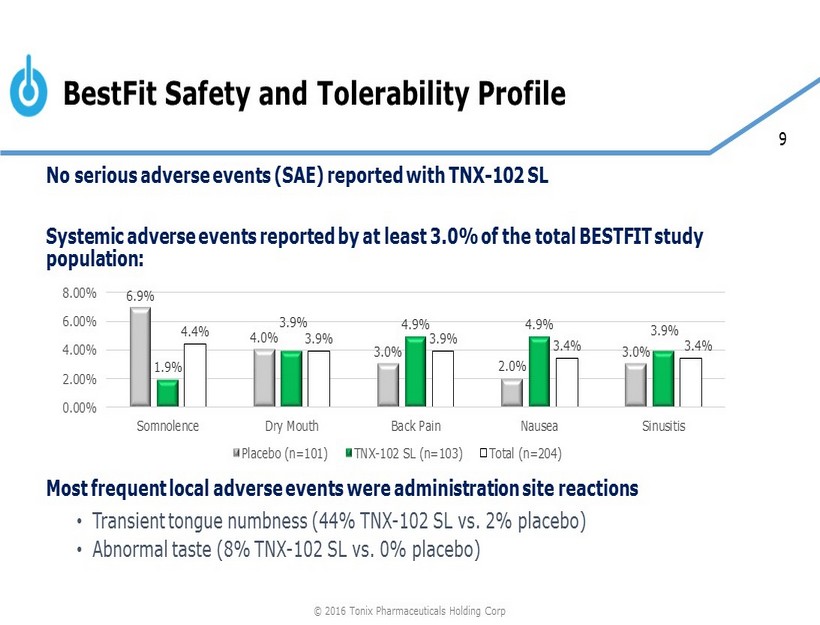
9 BestFit Safety and Tolerability Profile No serious adverse events (SAE) reported with TNX - 102 SL Systemic adverse events reported by at least 3.0% of the total BESTFIT study population : Most frequent local adverse events were administration site reactions • Transient tongue numbness (44% TNX - 102 SL vs. 2% placebo) • Abnormal taste (8% TNX - 102 SL vs. 0% placebo) 6.9% 4.0% 3.0% 2.0% 3.0% 1.9% 3.9% 4.9% 4.9% 3.9% 4.4% 3.9% 3.9% 3.4% 3.4% 0.00% 2.00% 4.00% 6.00% 8.00% Somnolence Dry Mouth Back Pain Nausea Sinusitis Placebo (n=101) TNX-102 SL (n=103) Total (n=204) © 2016 Tonix Pharmaceuticals Holding Corp
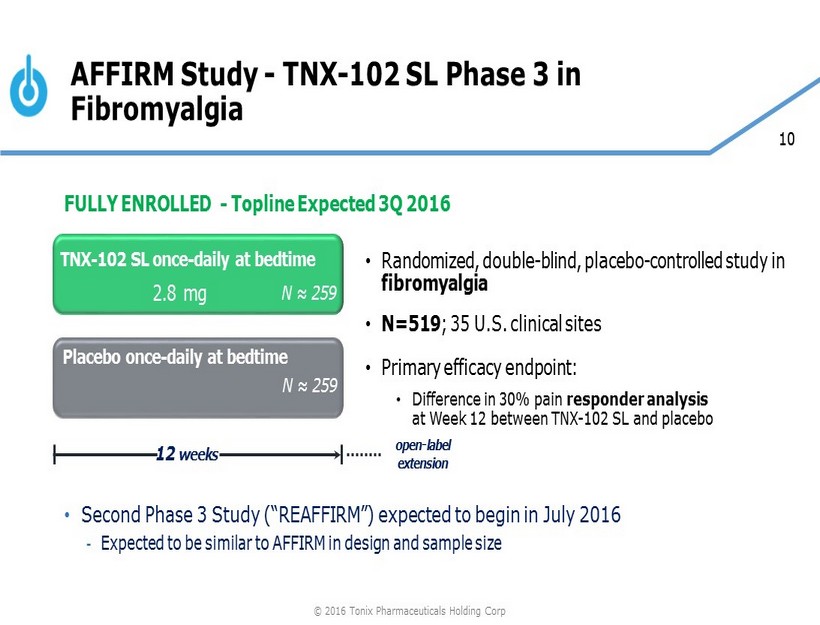
10 FULLY ENROLLED - Topline Expected 3Q 2016 • Second Phase 3 Study (“REAFFIRM”) expected to begin in July 2016 - Expected to be similar to AFFIRM in design and sample size AFFIRM Study - TNX - 102 SL Phase 3 in Fibromyalgia • Randomized, double - blind, placebo - controlled study in fibromyalgia • N=519 ; 35 U.S. clinical sites • Primary efficacy endpoint: • Difference in 30% pain responder analysis at Week 12 between TNX - 102 SL and placebo Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime N ≈ 259 N ≈ 259 2.8 mg open - label extension © 2016 Tonix Pharmaceuticals Holding Corp

11 Targeting Military - related PTSD 11 1. Friedman et al., 2007, 2. Zoloft Package Insert, August, 2014, 3. Paxil Package Insert, June, 2014 Military - related PTSD not well - served by existing FDA - approved therapies • No treatment response observed in U.S. military populatio n Sertraline: negative in large multicenter trial in U.S. military (placebo numerically better) 1 Paroxetine: not studied in military population • Inconsistent treatment response observed in males Sertraline: FDA conducted post - hoc analysis concluded no effect for male civilian subgroup 2 Paroxetine: no sex - related difference in treatment outcomes 3 • Important tolerability issues with SSRIs in this population Sexual dysfunction Insomnia © 2016 Tonix Pharmaceuticals Holding Corp

12 The AtEase Study - TNX - 102 SL Phase 2 in PTSD • Randomized, double - blind, placebo - controlled trial in military - related PTSD • N=231 ; 24 U.S. clinical sites • Primary efficacy endpoint : Difference in Clinician - Administered PTSD Scale ( CAPS - 5 ) score between TNX - 102 SL 2.8 mg and placebo at week 12 Placebo at bedtime once - daily 12 weeks N = 92 TNX - 102 SL at bedtime once - daily N = 90 2.8 mg open - label extension 0 mg TNX - 102 SL at bedtime once - daily N = 49 5.6 mg © 2016 Tonix Pharmaceuticals Holding Corp

13 AtEase Study Key Demographics / Characteristics of AtEase • 93% of the sample was male • 98% had trauma during military service and were deployed on average 2.3 times • Mean time since index trauma was 7 years • Race and ethnicity generally consistent with U.S. military distribution • Similar baseline CAPS - 5 scores and MADRS scores across treatment arms • Average CAPS - 5 scores were greater than 39 for all groups ('severe' PTSD*) *personal communication – Frank Weathers PhD, National Center for PTSD © 2016 Tonix Pharmaceuticals Holding Corp
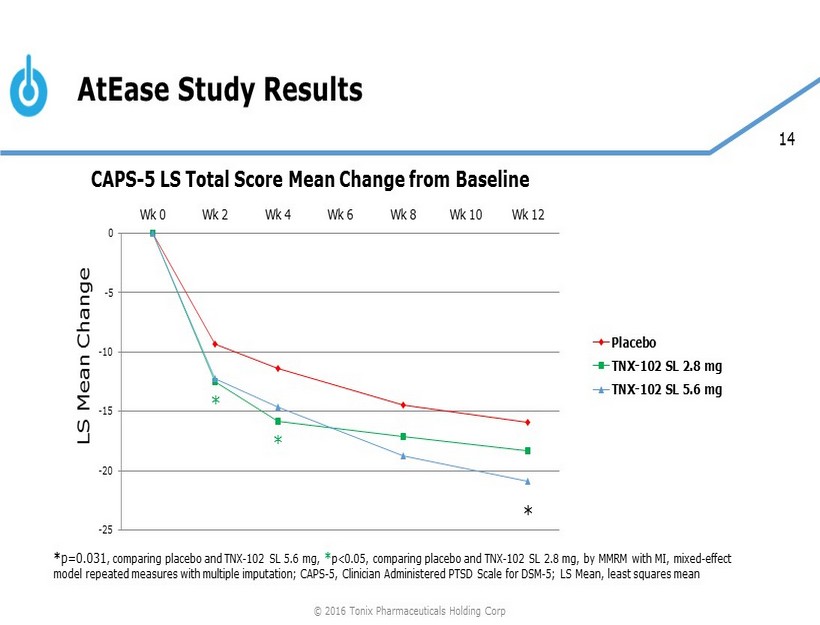
14 AtEase Study Results -25 -20 -15 -10 -5 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * p=0.031 , comparing placebo and TNX - 102 SL 5.6 mg, * p<0.05, comparing placebo and TNX - 102 SL 2.8 mg, by MMRM with MI, mixed - effect model repeated measures with multiple imputation; CAPS - 5, Clinician Administered PTSD Scale for DSM - 5; LS Mean, least squares me an LS Mean Change * * CAPS - 5 LS Total Score Mean Change from Baseline © 2016 Tonix Pharmaceuticals Holding Corp
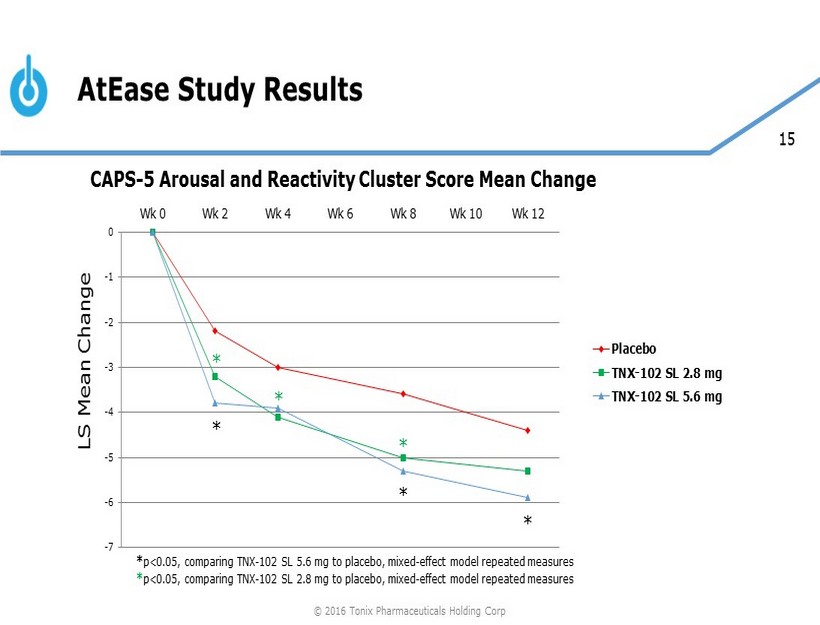
15 AtEase Study Results -7 -6 -5 -4 -3 -2 -1 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * * * * * LS Mean Change * p<0.05, comparing TNX - 102 SL 5.6 mg to placebo, mixed - effect model repeated measures * p<0.05, comparing TNX - 102 SL 2.8 mg to placebo, mixed - effect model repeated measures CAPS - 5 Arousal and Reactivity Cluster Score Mean Change © 2016 Tonix Pharmaceuticals Holding Corp
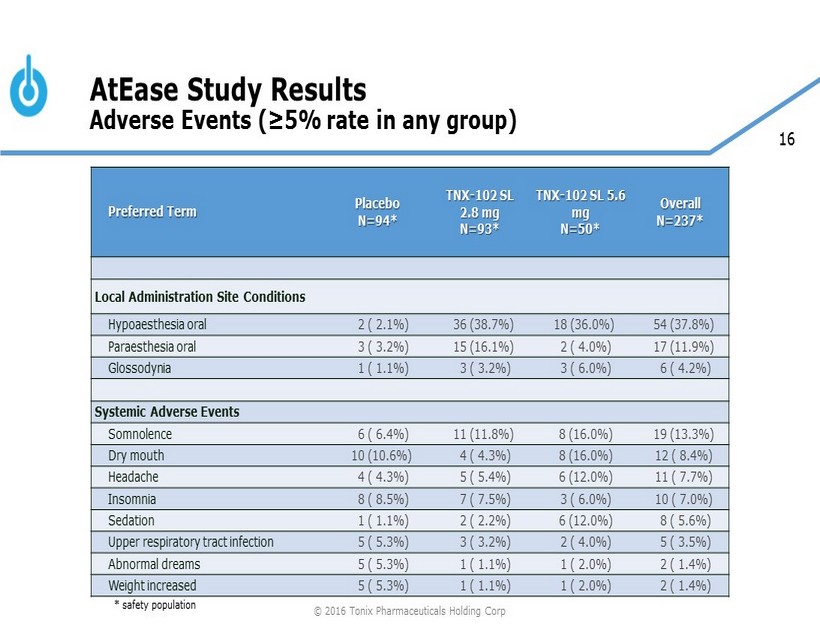
16 AtEase Study Results Adverse Events (≥5% rate in any group) * safety population © 2016 Tonix Pharmaceuticals Holding Corp Preferred Term Placebo N=94* TNX - 102 SL 2.8 mg N=93* TNX - 102 SL 5.6 mg N=50* Overall N=237* Local Administration Site Conditions Hypoaesthesia oral 2 ( 2.1%) 36 (38.7%) 18 (36.0%) 54 (37.8%) Paraesthesia oral 3 ( 3.2%) 15 (16.1%) 2 ( 4.0%) 17 (11.9%) Glossodynia 1 ( 1.1%) 3 ( 3.2%) 3 ( 6.0%) 6 ( 4.2%) Systemic Adverse Events Somnolence 6 ( 6.4%) 11 (11.8%) 8 (16.0%) 19 (13.3%) Dry mouth 10 (10.6%) 4 ( 4.3%) 8 (16.0%) 12 ( 8.4%) Headache 4 ( 4.3%) 5 ( 5.4%) 6 (12.0%) 11 ( 7.7%) Insomnia 8 ( 8.5%) 7 ( 7.5%) 3 ( 6.0%) 10 ( 7.0%) Sedation 1 ( 1.1%) 2 ( 2.2%) 6 (12.0%) 8 ( 5.6%) Upper respiratory tract infection 5 ( 5.3%) 3 ( 3.2%) 2 ( 4.0%) 5 ( 3.5%) Abnormal dreams 5 ( 5.3%) 1 ( 1.1%) 1 ( 2.0%) 2 ( 1.4%) Weight increased 5 ( 5.3%) 1 ( 1.1%) 1 ( 2.0%) 2 ( 1.4%)
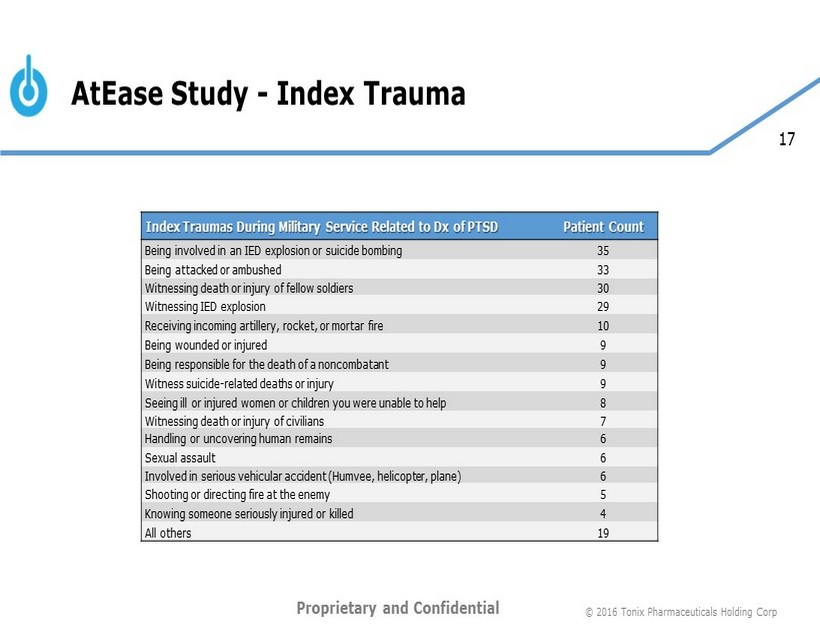
17 AtEase Study Conclusions • This is the first large, multicenter trial that demonstrated efficacy in a population with military - related PTSD • Male predominant (93%) • Low incidence of co - morbid FM (7%) • Low incidence of current major depression (14%) • Early effects on sleep and hyperarousal are consistent with the mechanistic hypothesis of TNX - 102 SL primary actions on sleep disturbance and autonomic balance. • Next steps • Phase 3 trial in military - related PTSD • Phase 3 trial in civilian PTSD © 2016 Tonix Pharmaceuticals Holding Corp
18 Summary: Tonix Pharmaceuticals UPDATE Differentiated Late - Stage Program Commercially Attractive Markets • TNX - 102 SL (cyclobenzaprine sublingual tablets), 2.8 mg • Differentiated with unique attributes that address unmet needs of target markets • Clinically validated in fibromyalgia (FM) • Completed: Phase 2; demonstrated relief from chronic wide - spread pain • Enrollment in Phase 3 FM completed with topline results expected 3Q 2016 • Positive topline results in Phase 2 - post - traumatic stress disorder (PTSD) • Target indications, FM and PTSD, are common chronic disorders • Underserved markets with high levels of dissatisfaction • A differentiated product has potential to capture substantial share © 2016 Tonix Pharmaceuticals Holding Corp

19 Thank You! © 2016 Tonix Pharmaceuticals Holding Corp