
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
EXHIBIT 99.02

© 2019 Tonix Pharmaceuticals Holding Corp. June 2019 Version P0184 6 - 3 - 19 (Doc 0495) Investor Presentation

© 2019 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2018 , as filed with the Securities and Exchange Commission (the “SEC”) on March 18 , 2019 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
© 2019 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals Who we are: • A clinical stage biopharmaceutical company dedicated to developing innovative treatments for patients and making meaningful contributions to society • Focusing on small molecules and biologics to treat psychiatric, pain and addiction conditions as well as potential medical counter - measures to improve biodefense What we do: • Target therapeutic areas with high need for improvement − Conditions, with no or inadequate treatments − Significant patient segments not well served by existing therapies • Develop innovative treatment options with possibility to be a “game changer” − Scientifically unique and innovative − Supported by strong scientific rationale − Supported by preliminary clinical evidence and published literature − Utilize proven regulatory pathway and established clinical endpoint − Built on a foundation of proprietary intellectual property
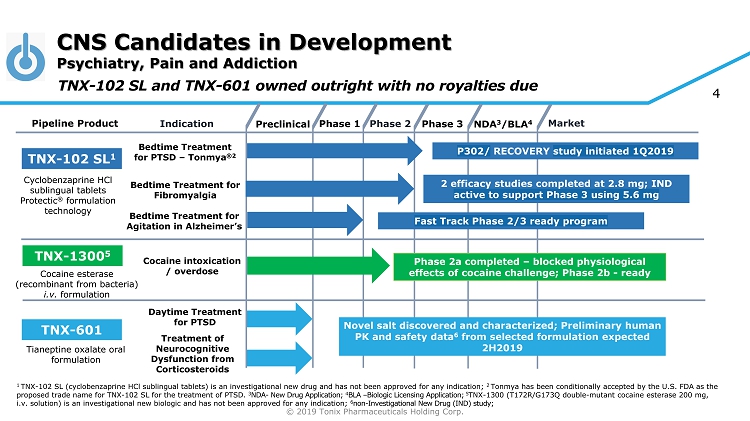
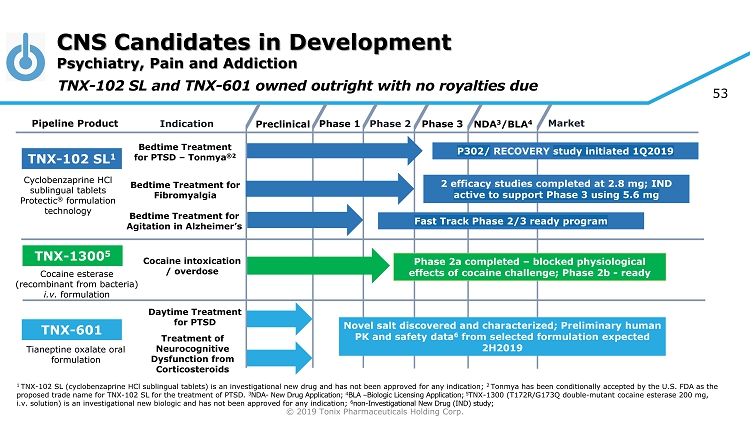
© 2019 Tonix Pharmaceuticals Holding Corp. 4 CNS Candidates in Development Psychiatry, Pain and Addiction Preclinical Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime Treatment for PTSD – Tonmya ®2 Daytime Treatment for PTSD TNX - 601 Novel salt discovered and characterized; Preliminary human PK and safety data 6 from selected formulation expected 2H2019 TNX - 1300 5 Phase 2a completed – blocked physiological effects of cocaine challenge; Phase 2b - ready Cocaine intoxication / overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 6 non - Investigational New Drug (IND) study; Bedtime Treatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due P302/ RECOVERY study initiated 1Q2019 Bedtime Treatment for Agitation in Alzheimer’s Fast Track Phase 2/3 ready program Treatment of Neurocognitive Dysfunction from Corticosteroids 2 efficacy studies completed at 2.8 mg; IND active to support Phase 3 using 5.6 mg
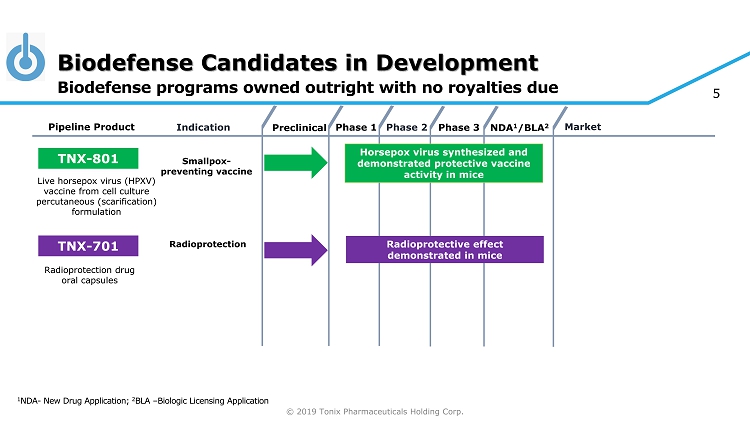
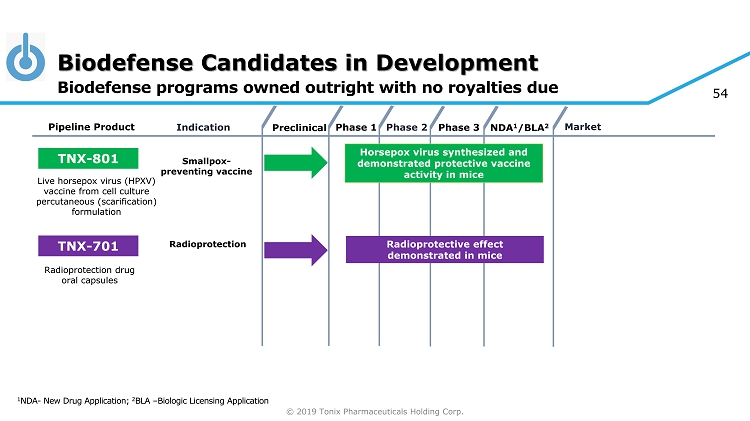
© 2019 Tonix Pharmaceuticals Holding Corp. 5 Biodefense Candidates in Development 1 NDA - New Drug Application; 2 BLA – Biologic Licensing Application Biodefense programs owned outright with no royalties due Preclinical Phase 2 NDA 1 /BLA 2 Market Pipeline Product Indication Phase 3 TNX - 801 Horsepox virus synthesized and demonstrated protective vaccine activity in mice Smallpox - preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture percutaneous (scarification) formulation Phase 1 TNX - 701 Radioprotective effect demonstrated in mice Radioprotection Radioprotection drug oral capsules
© 2019 Tonix Pharmaceuticals Holding Corp. 6 Lead Program: TNX - 102 SL – Product Concept Sleep disturbances are associated with a constellation of disorders • Considered co - morbid or a key symptom in these disorders • Believed to have a role in the onset, progression and severity of these disorders The focus of TNX - 102 SL development is both unique and innovative • Testing the therapeutic benefit of sleep (‘sleep quality’) − Restorative sleep, in contrast to time spent sleeping (‘sleep quantity’) • Targeting clinical conditions for which improved sleep quality may have a therapeutic benefit − Reduction in disease - specific symptoms, with sleep improvement as a secondary endpoint
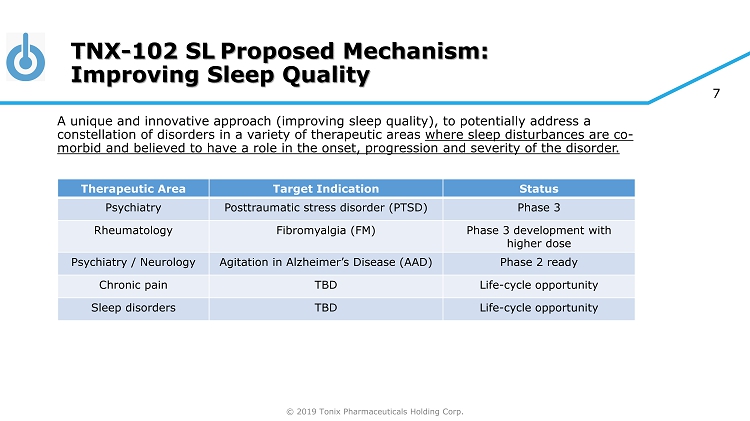
© 2019 Tonix Pharmaceuticals Holding Corp. 7 A unique and innovative approach (improving sleep quality), to potentially address a constellation of disorders in a variety of therapeutic areas where sleep disturbances are co - morbid and believed to have a role in the onset, progression and severity of the disorder. Therapeutic Area Target Indication Status Psychiatry Posttraumatic stress disorder (PTSD) Phase 3 Rheumatology Fibromyalgia (FM) Phase 3 development with higher dose Psychiatry / Neurology Agitation in Alzheimer’s Disease (AAD) Phase 2 ready Chronic pain TBD Life - cycle opportunity Sleep disorders TBD Life - cycle opportunity TNX - 102 SL Proposed Mechanism: Improving Sleep Quality
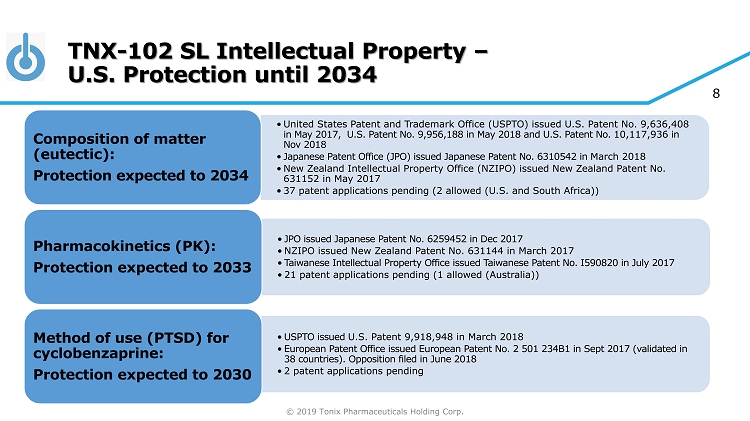
© 2019 Tonix Pharmaceuticals Holding Corp. 8 TNX - 102 SL Intellectual Property – U.S. Protection until 2034 • United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 9,636,408 in May 2017, U.S. Patent No. 9,956,188 in May 2018 and U.S. Patent No. 10,117,936 in Nov 2018 • Japanese Patent Office (JPO) issued Japanese Patent No. 6310542 in March 2018 • New Zealand Intellectual Property Office (NZIPO) issued New Zealand Patent No. 631152 in May 2017 • 37 patent applications pending (2 allowed (U.S. and South Africa)) Composition of matter (eutectic): Protection expected to 2034 • JPO issued Japanese Patent No. 6259452 in Dec 2017 • NZIPO issued New Zealand Patent No. 631144 in March 2017 • Taiwanese Intellectual Property Office issued Taiwanese Patent No. I590820 in July 2017 • 21 patent applications pending (1 allowed (Australia)) Pharmacokinetics (PK): Protection expected to 2033 • USPTO issued U.S. Patent 9,918,948 in March 2018 • European Patent Office issued European Patent No. 2 501 234B1 in Sept 2017 (validated in 38 countries). Opposition filed in June 2018 • 2 patent applications pending Method of use (PTSD) for cyclobenzaprine: Protection expected to 2030
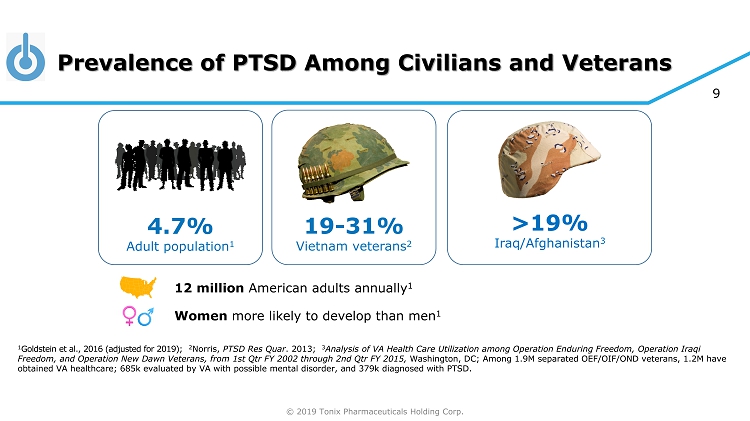
© 2019 Tonix Pharmaceuticals Holding Corp. 9 Prevalence of PTSD Among Civilians and Veterans 1 Goldstein et al., 2016 (adjusted for 2019) ; 2 Norris, PTSD Res Quar . 2013; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; Among 1.9M separated OEF/OIF/OND veterans, 1.2M have obtained VA healthcare; 685k evaluated by VA with possible mental disorder, and 379k diagnosed with PTSD. >19% Iraq/Afghanistan 3 4.7% Adult population 1 19 - 31% Vietnam veterans 2 12 million American adults annually 1 Women more likely to develop than men 1
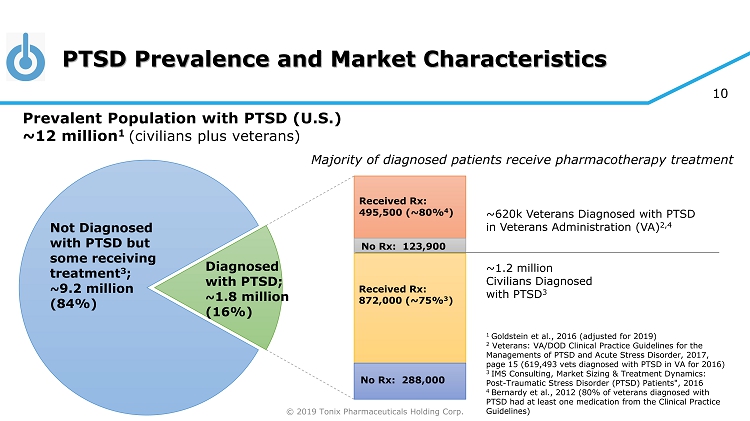
© 2019 Tonix Pharmaceuticals Holding Corp. 10 PTSD Prevalence and Market Characteristics 1 Goldstein et al., 2016 (adjusted for 2019) 2 Veterans: VA/DOD Clinical Practice Guidelines for the Managements of PTSD and Acute Stress Disorder, 2017, page 15 (619,493 vets diagnosed with PTSD in VA for 2016) 3 IMS Consulting, Market Sizing & Treatment Dynamics: Post - Traumatic Stress Disorder (PTSD) Patients", 2016 4 Bernardy et al., 2012 (80% of veterans diagnosed with PTSD had at least one medication from the Clinical Practice Guidelines) ~620k Veterans Diagnosed with PTSD in Veterans Administration (VA) 2,4 ~1.2 million Civilians Diagnosed with PTSD 3 Not Diagnosed with PTSD but some receiving treatment 3 ; ~ 9.2 million ( 84% ) Received Rx : 495,500 (~80% 4 ) No Rx : 123,900 Received Rx : 872,000 (~75% 3 ) No Rx : 288,000 Diagnosed with PTSD; ~ 1.8 million ( 16% ) Prevalent Population with PTSD (U.S.) ~12 million 1 (civilians plus veterans) Majority of diagnosed patients receive pharmacotherapy treatment

© 2019 Tonix Pharmaceuticals Holding Corp. 11 PTSD: Not Well - Served by Approved Treatments FDA - approved SSRIs, paroxetine and sertraline, are indicated as a treatment for PTSD • Neither drug has shown efficacy in military - related PTSD • Majority of male PTSD patients unresponsive or intolerant to current treatments • Side effects relating to sexual dysfunction, sleep disturbance and weight gain are commonly reported Characteristics of an ideal drug therapy that would be compatible and complementary with behavioral therapy • Lack of retrograde amnesia (e.g., unlike off - label use of benzodiazepines and non - benzodiazepines) • Lack of interference on sleep (e.g., unlike approved SSRIs) Tonmya is being investigated in both military and civilian PTSD and is expected to be indicated as a “treatment for PTSD”
© 2019 Tonix Pharmaceuticals Holding Corp. 12 Tonmya : a Potential Bedtime Treatment for PTSD First investigational new drug to show treatment effect in military - related PTSD in two potential pivotal efficacy studies • Phase 2 study (P201/ AtEase ) showed Tonmya 5.6 mg had a strong signal of treatment effect at Week 12 as measured by CAPS - 5 1 • Phase 3 study (P301/HONOR) provided evidence of effectiveness as early as 4 weeks after treatment but diminished over time due to high placebo response − Retrospective analysis showed persistent effectiveness at Week 12 in subgroup with Time Since Trauma ≤9 years from screening • Both studies can be used as supportive evidence of efficacy and safety for Tonmya NDA submission • No serious or unexpected adverse events related to Tonmya were reported FDA feedback and acceptance on new Phase 3 study (P302/RECOVERY) received in November 2018 2 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 2 FDA Meeting Minutes, November 26, 2018

© 2019 Tonix Pharmaceuticals Holding Corp. 13 No Recognized Abuse Potential in Clinical Studies Active ingredient is cyclobenzaprine, which is structurally related to tricyclic antidepressants • Cyclobenzaprine interacts with receptors that regulate sleep quality: 5 - HT 2A, a 1 - adrenergic and histamine H 1 receptors • Cyclobenzaprine does NOT interact with the same receptors as traditional hypnotic sleep drugs, benzodiazepines or non - benzodiazepines that are associated with retrograde amnesia • Cyclobenzaprine - containing product was approved 40 years ago and current labeling (May 2018) indicates no abuse or dependence concern Tonmya NDA can be filed without drug abuse and dependency assessment studies • Discussed at March 9, 2017 meeting with the FDA

© 2019 Tonix Pharmaceuticals Holding Corp. 14 TNX - 102 SL: Sublingual Formulation is Designed for Bedtime Administration TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption • Innovation by design with patent protected CBP/mannitol eutectic • Rapid systemic exposure • Increases bioavailability during sleep • Avoids first - pass metabolism • Lowers exposure to long - lived active major metabolite, norcyclobenzaprine ( norCBP ) CBP undergoes extensive first - pass hepatic metabolism when orally ingested • Active major metabolite, norCBP 1 • Long half - life (~72 hours) • Less selective for target receptors ( 5 - HT 2A, a 1 - adrenergic, histamine H 1 ) • More selective for norepinephrine transporter and muscarinic M 1 TNX - 102 SL 505(b)(2) NDA approval can rely on the safety of the reference listed drug (AMRIX ® ) 2 1 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015, Toronto Ont ario, Canada 2 FDA Minutes (November 26, 2018)

© 2019 Tonix Pharmaceuticals Holding Corp. 15 Tonmya: Hypothesized Novel Mechanism Targets Sleep Quality for Recovery from PTSD PTSD is a disorder of recovery • Most people exposed to extreme trauma recover over a few weeks • In PTSD, recovery process impeded due to insufficient sleep - dependent memory processing 1,2 Memory processing is essential to recovery • Vulnerability to memory intrusions and trauma triggers remains if no consolidation of new learning (extinction) Tonmya targets sleep quality 3 • The active ingredient in Tonmya, cyclobenzaprine, interacts with receptors that regulate sleep quality: strongly binds and potently blocks 5 - HT 2A , a 1 - adrenergic and histamine H 1 receptors, permissive to sleep - dependent recovery processes 1 Straus LD, Acheson DT, Risbrough VB, Drummond SPA. Sleep Deprivation Disrupts Recall of Conditioned Fear Extinction. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017; 2(2):123 - 129. 2 Murkar ALA, De Koninck J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med Rev. 2018; 41:173 - 184. 3 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015, Toronto Ont ario, Canada
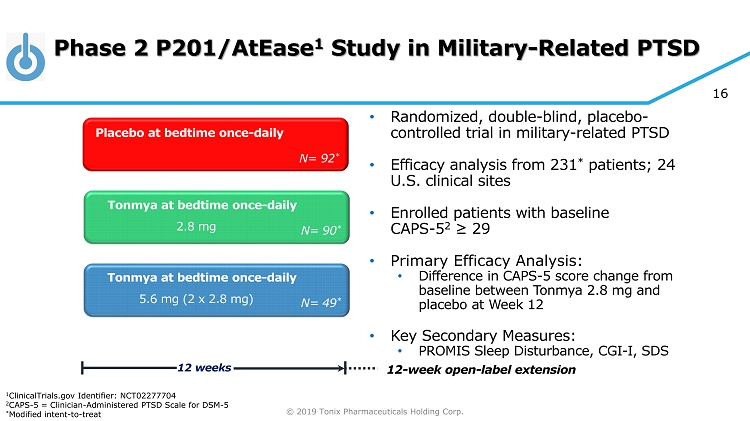
© 2019 Tonix Pharmaceuticals Holding Corp. 16 Phase 2 P201/AtEase 1 Study in Military - Related PTSD • Randomized, double - blind, placebo - controlled trial in military - related PTSD • Efficacy analysis from 231 * patients; 24 U.S. clinical sites • Enrolled patients with baseline CAPS - 5 2 ≥ 29 • Primary Efficacy Analysis: • Difference in CAPS - 5 score change from baseline between Tonmya 2.8 mg and placebo at Week 12 • Key Secondary Measures: • PROMIS Sleep Disturbance, CGI - I, SDS Tonmya at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 90 * Tonmya at bedtime once - daily N= 92 * N= 49 * 2.8 mg 5.6 mg (2 x 2.8 mg) 12 - week open - label extension 1 ClinicalTrials.gov Identifier: NCT02277704 2 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 * Modified intent - to - treat
© 2019 Tonix Pharmaceuticals Holding Corp. 17 P201/AtEase Study P201 was a large adequate well - controlled Phase 2 study in military - related PTSD • Primary endpoint (Week 12 CAPS - 5) did not separate from placebo for TNX - 102 SL 2.8 mg • No safety or tolerability issue discovered • Retrospective analyses showed TNX - 102 SL 5.6 mg had a strong signal of treatment effect at Week 12 CAPS - 5 (P=0.053) and CGI - I (P=0.041) scores • Retrospective analyses suggested CAPS - 5 ≥ 33 enrollment criteria for Phase 3
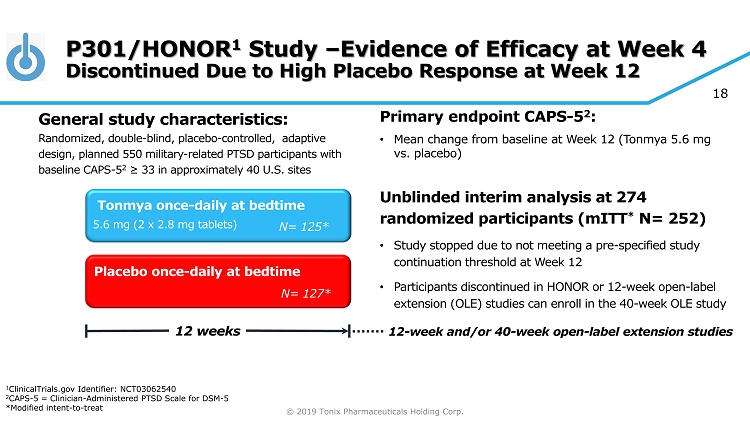
© 2019 Tonix Pharmaceuticals Holding Corp. 18 P301/HONOR 1 Study – Evidence of Efficacy at Week 4 Discontinued Due to High Placebo Response at Week 12 Primary e ndpoint CAPS - 5 2 : • Mean change from baseline at W eek 12 (Tonmya 5.6 mg vs. placebo ) Unblinded interim analysis at 274 randomized participants ( mITT * N= 252) • Study stopped due to not meeting a pre - specified study continuation threshold at Week 12 • Participants discontinued in HONOR or 12 - week open - label extension (OLE) studies can enroll in the 40 - week OLE study Placebo once - daily at bedtime 12 weeks Tonmya once - daily at bedtime N= 127* N= 125* 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: Randomized, double - blind, placebo - controlled , adaptive design, planned 550 military - related PTSD participants with baseline CAPS - 5 2 ≥ 33 in approximately 40 U.S. sites 12 - week and/or 40 - week open - label extension studies 1 ClinicalTrials.gov Identifier: NCT03062540 2 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 *Modified intent - to - treat
© 2019 Tonix Pharmaceuticals Holding Corp. 19 P301/HONOR Study Stopped After Interim Analysis (July 2018) P301 was a large adequate well - controlled Phase 3 study in military - related PTSD • Separation on primary endpoint at Week 12 did not cross pre - specified study continuation threshold at Week 12 (p=0.602) • No safety or tolerability issue discovered • Retrospective analyses showed Week 4 CAPS - 5 (P=0.019) and CGI - I (P=0.015) scores in Tonmya group had a strong signal of treatment effect P301 dataset is complex and rich • Retrospective analyses presented at Military Health System Research Symposium (MHSRS) in Kissimmee, FL on August 22, 2018 • Results discussed with the FDA 1 and helped to design the new Phase 3 P302/RECOVERY study with high probability of success 1 FDA Meeting Minutes (November 26, 2018)
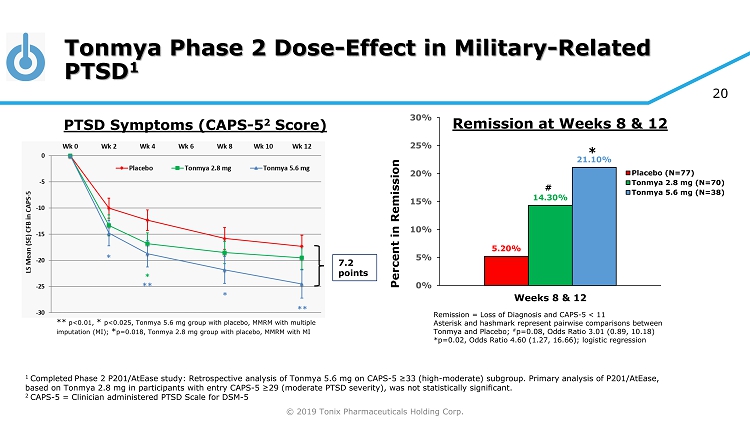
© 2019 Tonix Pharmaceuticals Holding Corp. 20 Tonmya Phase 2 Dose - Effect in Military - Related PTSD 1 1 Completed Phase 2 P201/AtEase study: Retrospective analysis of Tonmya 5.6 mg on CAPS - 5 ≥33 (high - moderate) subgroup. Primary analysis of P 201/ AtEase , based on Tonmya 2.8 mg in participants with entry CAPS - 5 ≥29 ( moderate PTSD severity), was not statistically significant. 2 CAPS - 5 = Clinician administered PTSD Scale for DSM - 5 7.2 points ** p<0.01, * p<0.025, Tonmya 5.6 mg group with placebo, MMRM with multiple imputation (MI); * p=0.018, Tonmya 2.8 mg group with placebo, MMRM with MI PTSD Symptoms (CAPS - 5 2 Score) Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between Tonmya and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # * Remission at Weeks 8 & 12
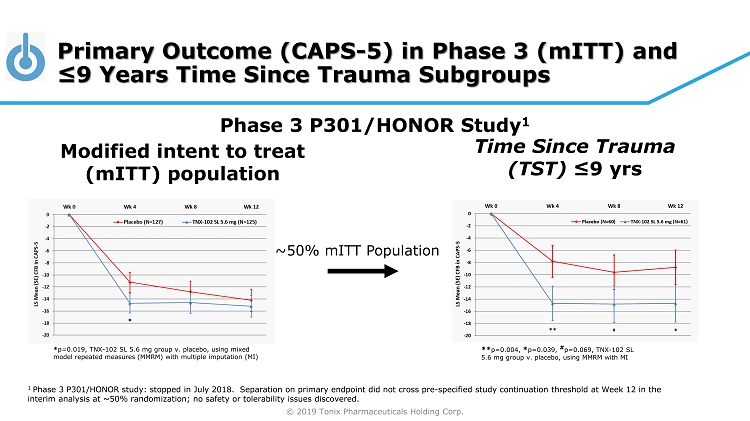
© 2019 Tonix Pharmaceuticals Holding Corp. Primary Outcome (CAPS - 5) in Phase 3 ( mITT ) and ≤9 Years Time Since Trauma Subgroups Modified intent to treat ( mITT ) population Phase 3 P301/HONOR Study 1 ** p=0.004, * p=0.039, # p=0.069, TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI ~50% mITT Population * p=0.019, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI) Time Since Trauma (TST) ≤9 yrs 1 Phase 3 P301/HONOR study: stopped in July 2018. Separation on primary endpoint did not cross pre - specified study continuation t hreshold at Week 12 in the interim analysis at ~50% randomization; no safety or tolerability issues discovered.
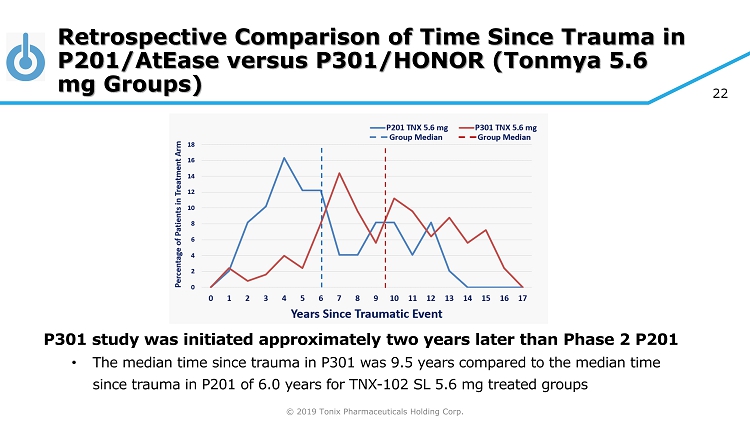
© 2019 Tonix Pharmaceuticals Holding Corp. 22 Retrospective Comparison of Time Since Trauma in P201/AtEase versus P301/HONOR (Tonmya 5.6 mg Groups) P301 study was initiated approximately two years later than Phase 2 P201 • The median time since trauma in P301 was 9.5 years compared to the median time since trauma in P201 of 6.0 years for TNX - 102 SL 5.6 mg treated groups
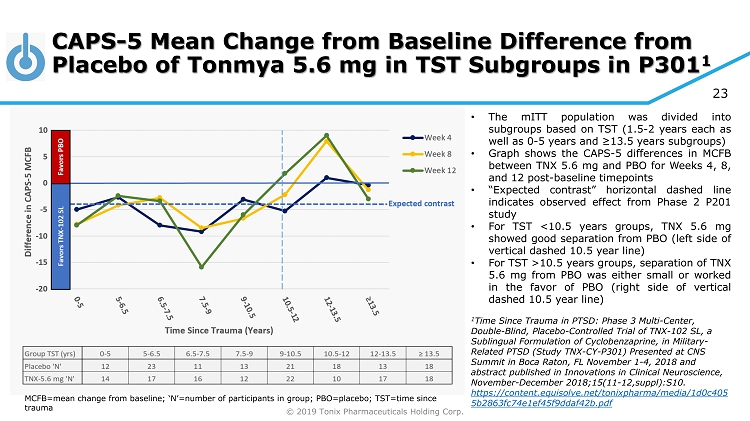
© 2019 Tonix Pharmaceuticals Holding Corp. 23 CAPS - 5 Mean Change from Baseline Difference from Placebo of Tonmya 5.6 mg in TST Subgroups in P301 1 MCFB=mean change from baseline; ‘N’=number of participants in group; PBO=placebo; TST=time since trauma • The mITT population was divided into subgroups based on TST ( 1 . 5 - 2 years each as well as 0 - 5 years and ≥ 13 . 5 years subgroups) • Graph shows the CAPS - 5 differences in MCFB between TNX 5 . 6 mg and PBO for Weeks 4 , 8 , and 12 post - baseline timepoints • “Expected contrast” horizontal dashed line indicates observed effect from Phase 2 P 201 study • For TST < 10 . 5 years groups, TNX 5 . 6 mg showed good separation from PBO (left side of vertical dashed 10 . 5 year line) • For TST > 10 . 5 years groups, separation of TNX 5 . 6 mg from PBO was either small or worked in the favor of PBO (right side of vertical dashed 10 . 5 year line) 1 Time Since Trauma in PTSD: Phase 3 Multi - Center, Double - Blind, Placebo - Controlled Trial of TNX - 102 SL, a Sublingual Formulation of Cyclobenzaprine, in Military - Related PTSD (Study TNX - CY - P301) Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018 and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12,suppl):S10. https://content.equisolve.net/tonixpharma/media/1d0c405 5b2863fc74e1ef45f9ddaf42b.pdf
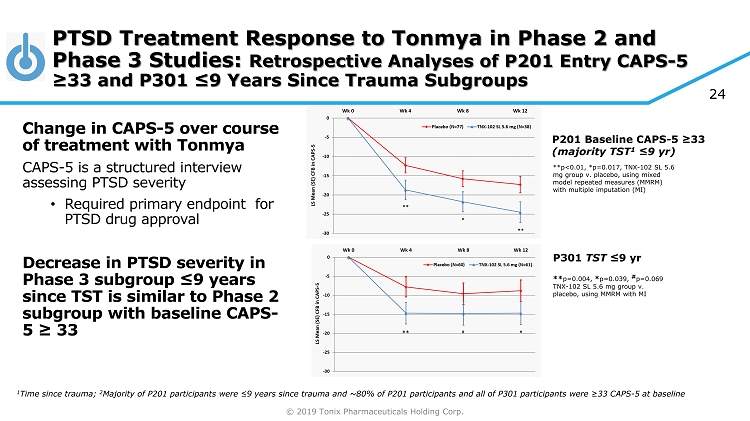
© 2019 Tonix Pharmaceuticals Holding Corp. P201 Baseline CAPS - 5 ≥33 (majority TST 1 ≤9 yr ) **p<0.01, *p=0.017, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI) P301 TST ≤9 yr ** p=0.004, * p=0.039, # p=0.069 TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI PTSD Treatment Response to Tonmya in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Change in CAPS - 5 over course of treatment with Tonmya CAPS - 5 is a structured interview assessing PTSD severity • Required primary endpoint for PTSD drug approval Decrease in PTSD severity in Phase 3 subgroup ≤9 years since TST is similar to Phase 2 subgroup with baseline CAPS - 5 ≥ 33 1 Time since trauma; 2 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥33 CAPS - 5 at baseline 24
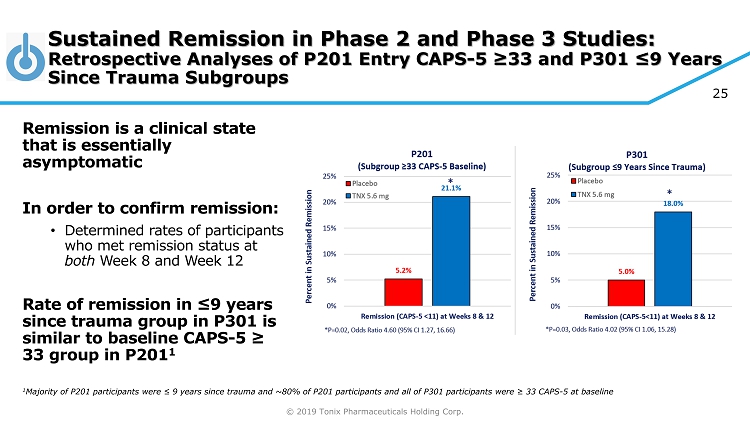
© 2019 Tonix Pharmaceuticals Holding Corp. 25 Sustained Remission in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Remission is a clinical state that is essentially asymptomatic In order to confirm remission: • Determined rates of participants who met remission status at both Week 8 and Week 12 Rate of remission in ≤9 years since trauma group in P301 is similar to baseline CAPS - 5 ≥ 33 group in P201 1 1 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥ 33 CAPS - 5 at baseline
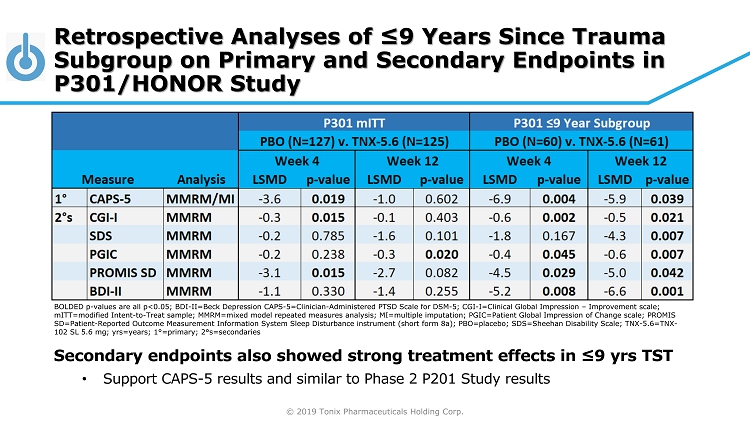
© 2019 Tonix Pharmaceuticals Holding Corp. BOLDED p - values are all p<0.05; BDI - II=Beck Depression CAPS - 5=Clinician - Administered PTSD Scale for DSM - 5; CGI - I=Clinical Global Impression – Improvement scale; mITT =modified Intent - to - Treat sample; MMRM=mixed model repeated measures analysis; MI=multiple imputation; PGIC=Patient Global Impre ssion of Change scale; PROMIS SD=Patient - Reported Outcome Measurement Information System Sleep Disturbance instrument (short form 8a); PBO=placebo; SDS=Sheeha n Disability Scale; TNX - 5.6=TNX - 102 SL 5.6 mg; yrs =years; 1 ° =primary; 2 ° s=secondaries Retrospective Analyses of ≤9 Years Since Trauma Subgroup on Primary and Secondary Endpoints in P301/HONOR Study Secondary endpoints also showed strong treatment effects in ≤9 yrs TST • Support CAPS - 5 results and similar to Phase 2 P201 Study results
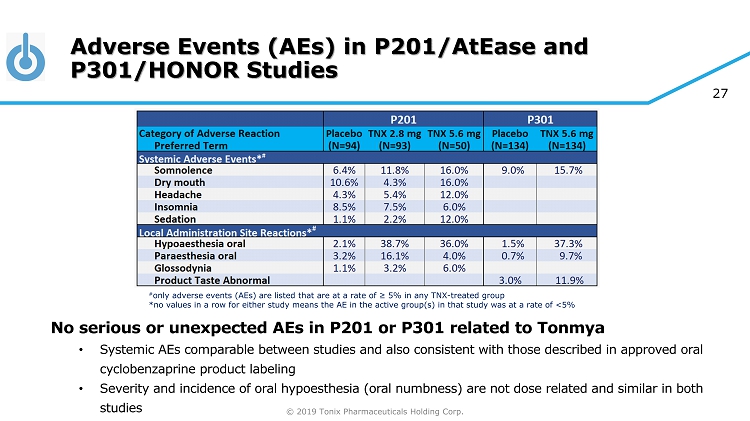
© 2019 Tonix Pharmaceuticals Holding Corp. 27 Adverse Events (AEs) in P201/AtEase and P301/HONOR Studies No serious or unexpected AEs in P201 or P301 related to Tonmya • Systemic AEs comparable between studies and also consistent with those described in approved oral cyclobenzaprine product labeling • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related and similar in both studies # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%
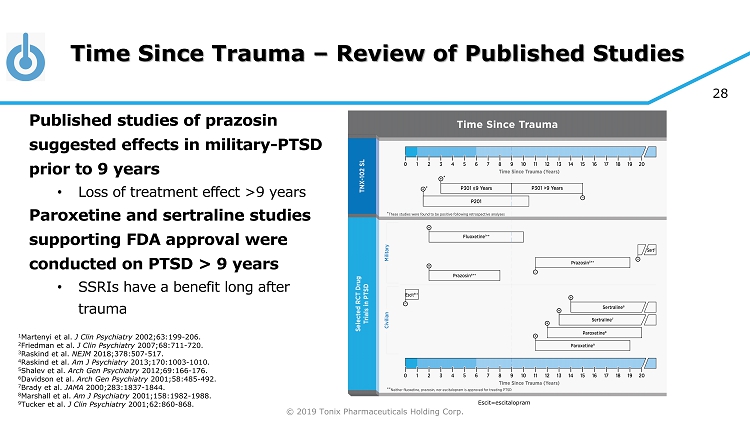
© 2019 Tonix Pharmaceuticals Holding Corp. 28 Time Since Trauma – Review of Published Studies Published studies of prazosin suggested effects in military - PTSD prior to 9 years • Loss of treatment effect >9 years Paroxetine and sertraline studies supporting FDA approval were conducted on PTSD > 9 years • SSRIs have a benefit long after trauma 1 Martenyi et al. J Clin Psychiatry 2002;63:199 - 206. 2 Friedman et al. J Clin Psychiatry 2007;68:711 - 720. 3 Raskind et al. NEJM 2018;378:507 - 517. 4 Raskind et al. Am J Psychiatry 2013;170:1003 - 1010. 5 Shalev et al. Arch Gen Psychiatry 2012;69:166 - 176. 6 Davidson et al. Arch Gen Psychiatry 2001;58:485 - 492. 7 Brady et al. JAMA 2000;283:1837 - 1844. 8 Marshall et al. Am J Psychiatry 2001;158:1982 - 1988. 9 Tucker et al. J Clin Psychiatry 2001;62:860 - 868. Escit =escitalopram
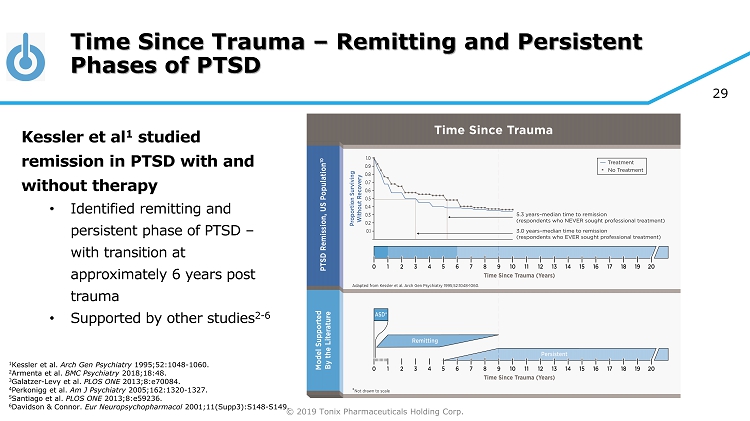
© 2019 Tonix Pharmaceuticals Holding Corp. 29 Time Since Trauma – Remitting and Persistent Phases of PTSD Kessler et al 1 studied remission in PTSD with and without therapy • Identified remitting and persistent phase of PTSD – with transition at approximately 6 years post trauma • Supported by other studies 2 - 6 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327. 5 Santiago et al. PLOS ONE 2013;8:e59236. 6 Davidson & Connor. Eur Neuropsychopharmacol 2001;11(Supp3):S148 - S149.
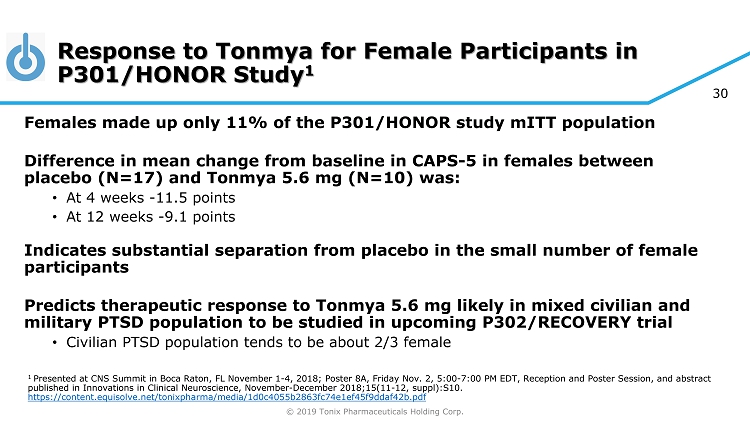
© 2019 Tonix Pharmaceuticals Holding Corp. 30 Response to Tonmya for Female Participants in P301/HONOR Study 1 Females made up only 11% of the P301/HONOR study mITT population Difference in mean change from baseline in CAPS - 5 in females between placebo (N=17) and Tonmya 5.6 mg (N=10) was: • At 4 weeks - 11.5 points • At 12 weeks - 9.1 points Indicates substantial separation from placebo in the small number of female participants Predicts therapeutic response to Tonmya 5.6 mg likely in mixed civilian and military PTSD population to be studied in upcoming P302/RECOVERY trial • Civilian PTSD population tends to be about 2/3 female 1 Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018; Poster 8A, Friday Nov. 2, 5:00 - 7:00 PM EDT, Reception and Poster S ession, and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12, suppl):S10. https://content.equisolve.net/tonixpharma/media/1d0c4055b2863fc74e1ef45f9ddaf42b.pdf
© 2019 Tonix Pharmaceuticals Holding Corp. 31 Non - combat traumas studied are similar to traumas experienced in civilian populations with PTSD To determine the therapeutic effects of Tonmya 5.6 mg in a mixed civilian and military population, difference in MCFB in CAPS - 5 was assessed in non - combat traumas in ≤9 years TST subgroup (placebo N=14, Tonmya 5.6 mg N=10): • At 4 weeks - 4.8 points • At 12 weeks - 4.4 points Non - combat traumas treated with Tonmya 5.6 mg showed clinically meaningful separation from placebo at Weeks 4 and 12, suggesting a mixed civilian and military sample within 9 years of index trauma may show a therapeutic response to Tonmya Response to Tonmya for Non - Combat Traumas in P301/HONOR Study in ≤9 Years Time Since Trauma Subgroup 1 1 Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018; Poster 8A, Friday Nov. 2, 5:00 - 7:00 PM EDT, Reception and Poster S ession, and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12, suppl):S10. https://content.equisolve.net/tonixpharma/media/1d0c4055b2863fc74e1ef45f9ddaf42b.pdf CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5; MCFB = mean change from baseline; mITT = modified Intent - to - Treat sample; TST = time since trauma
© 2019 Tonix Pharmaceuticals Holding Corp. 32 Summary of Clinical Experience with Tonmya/ TNX - 102 SL in PTSD Median time since trauma (TST) in TNX - 102 SL 5.6 mg group in the P301/HONOR study (9.5 years) was longer than P201/AtEase study (6 years) • Both studied military - related PTSD • Time has passed since the surge in Iraq In retrospective analysis, the ≤ 9 year subgroup of P301 study had similar results as the P201 study (primary and secondary) • TST is important in placebo - controlled clinical study • Potential enrichment in ≤ 9 years TST subgroup for treatment responders The ≤ 9 year subgroup of P301 may be enriched for “Remitting Phase” of PTSD 1 - 4 • Expect remitting phase of PTSD is more amenable to drug studies Results from retrospective analyses lead to improved Phase 3 study design 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327.
© 2019 Tonix Pharmaceuticals Holding Corp. 33 New Phase 3 P302/RECOVERY Study – Initiated 1Q 2019 Primary e ndpoint: • CAPS - 5 1 mean change from baseline at Week 4 ( Tonmya 5.6 mg vs. placebo) Key Secondary e ndpoint s include: • CAPS - 5 m ean change from baseline at W eek 12 (Tonmya 5.6 mg vs. placebo ) • Change from baseline Clinical Global Impression – Severity scale • Change from baseline Sheehan Disability Scale total score Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 12 weeks Tonmya once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study with baseline CAPS - 5 1 ≥ 33 in approximately 30 U.S. sites • Enrollment restricted to study participants with PTSD who experienced an index trauma ≤ 9 years from the date of screening • Both civilian and military - related PTSD to be included 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 N= 125 N= 125
© 2019 Tonix Pharmaceuticals Holding Corp. 34 Late - Stage PTSD Drug Candidates Tonmya • Phase 3 development focused on military - related and civilian PTSD; showed activity in treatment of military - related PTSD in large multi - center trials MDMA - assisted psychotherapy • Indication – “drug assisted psychotherapy” • Breakthrough therapy that is Phase 3 - ready; showed activity in a Phase 2 study of PTSD; enrolling in Phase 3 study Other drugs currently (or recently) in Phase 2 development • Rexulti ® ( brexpiprazole ) - Otsuka/Lundbeck; atypical antipsychotic; positive clinical results from Phase 2 study reported in November 2018 for brexpiprazole , when used in combination with an approved PTSD medication, sertraline, but not as monotherapy • NYX - 783 - Aptinyx ; NMDA receptor modulator (enrolling for 8 - week Phase 2 study of 144 patients using 50 mg either once daily or once weekly) • BNC - 201 – Bionomics; nicotinic receptor modulator (program planned to resume after reformulation)
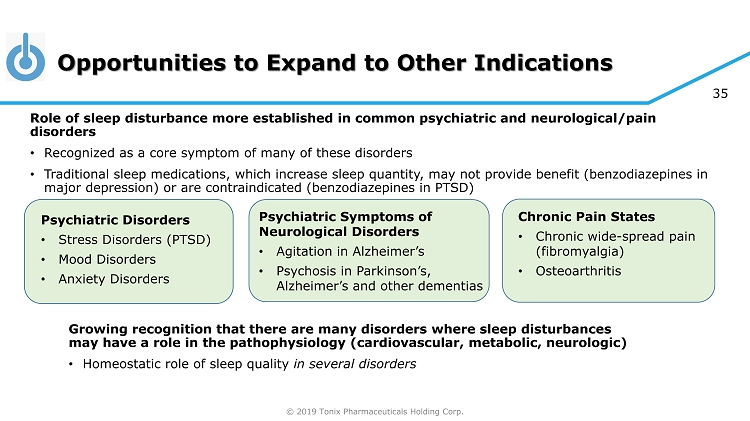
© 2019 Tonix Pharmaceuticals Holding Corp. 35 Opportunities to Expand to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Homeostatic role of sleep quality in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders • Anxiety Disorders Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated (benzodiazepines in PTSD) v Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

© 2019 Tonix Pharmaceuticals Holding Corp. 36 Volkswagen Check Engine [Photograph]. (2011, October 14). Wikipedia • Pain is a sensor system in the brain similar to a check engine light on a car’s dashboard • When the check engine light malfunctions, the light is on even though the car is not malfunctioning • Similarly, in fibromyalgia, the pain alarm is turned on even though there has been no peripheral nerve tissue injury • Fibromyalgia is considered a neurobiological disorder characterized by 1 : chronic widespread pain, non restorative sleep, fatigue, diminished cognition • Believed to result from inappropriate pain signaling in central nervous system in the absence of peripheral injury 1 • Causes significant impairment in all areas of life 2 • Lower levels of health - related quality of life – reduced daily functioning • Interference with work (loss of productivity, disability) • Inflicts substantial strain on the healthcare system • Average patient has 20 physician office visits per year 3 • Annual direct medical costs are twice those for non - fibromyalgia individuals 4 Fibromyalgia is a Chronic, Debilitating Disorder that Imposes a Significant Societal and Economic Burden 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141. 2 Schaefer et al., Pain Pract , 2015. 3 Robinson et al, Pain Medicine 2013;14:1400. 4 White et al, J Occupational Environ Med 2008;50:13.
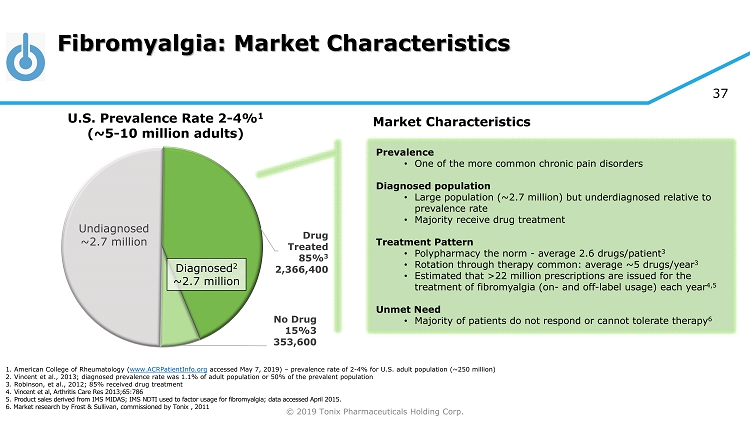
© 2019 Tonix Pharmaceuticals Holding Corp. 37 Fibromyalgia: Market Characteristics Undiagnosed ~2.7 million Drug Treated 85% 3 2,366,400 No Drug 15%3 353,600 U.S. Prevalence Rate 2 - 4% 1 (~5 - 10 million adults) Diagnosed 2 ~2.7 million Market Characteristics Prevalence • One of the more common chronic pain disorders Diagnosed population • Large population (~2.7 million) but underdiagnosed relative to prevalence rate • Majority receive drug treatment Treatment Pattern • Polypharmacy the norm - average 2.6 drugs/patient 3 • Rotation through therapy common: average ~5 drugs/year 3 • Estimated that >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 4,5 Unmet Need • Majority of patients do not respond or cannot tolerate therapy 6 1. American College of Rheumatology ( www.ACRPatientInfo.org accessed May 7, 2019) – prevalence rate of 2 - 4% for U.S. adult population (~250 million) 2. Vincent et al., 2013; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent population 3. Robinson, et al., 2012; 85% received drug treatment 4. Vincent et al, Arthritis Care Res 2013;65:786 5. Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. 6. Market research by Frost & Sullivan, commissioned by Tonix , 2011
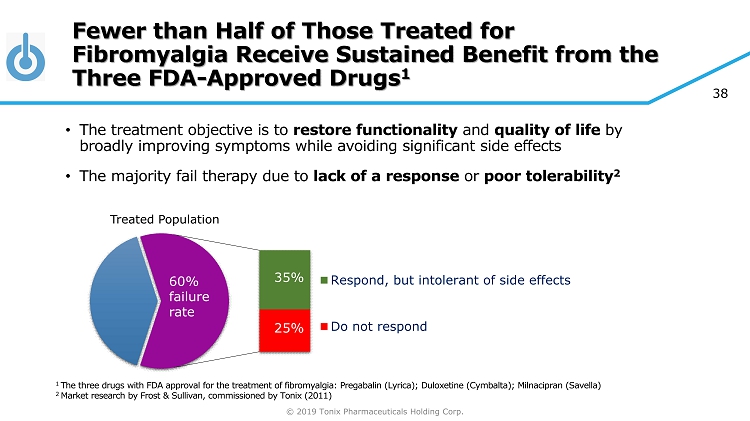
© 2019 Tonix Pharmaceuticals Holding Corp. 38 Fewer than Half of Those Treated for Fibromyalgia Receive Sustained Benefit from the Three FDA - Approved Drugs 1 • The treatment objective is to restore functionality and quality of life by broadly improving symptoms while avoiding significant side effects • The majority fail therapy due to lack of a response or poor tolerability 2 Respond, but intolerant of side effects Do not respond 25% 35% 60% failure rate 1 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 2 Market research by Frost & Sullivan, commissioned by Tonix (2011) Treated Population

© 2019 Tonix Pharmaceuticals Holding Corp. 39 Large Need for New Fibromyalgia Therapies that Provide Broad Symptom Improvement with Better Tolerability • Currently - approved medications may have side effects that limit long - term use 1 • Many patients skip doses or discontinue altogether within months of treatment initiation • Medication - related side effects may be similar to fibromyalgia symptoms • High rates of discontinuation, switching and augmentation • A ttempt to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications used simultaneously 2 • The typical patient has tried six different medications 3 • Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 • TNX - 102 SL is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources, 2011. 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J Clin Pract , 2007; 61(9):1498 – 1508.
© 2019 Tonix Pharmaceuticals Holding Corp. 40 TNX - 102 SL for Fibromyalgia: Summary of a completed Phase 3 F301 study General s tudy c haracteristics: • Randomized, 12 - week, double - blind, placebo - controlle d Phase 3 study of TNX - 102 SL 2.8 mg (half the dose being developed for PTSD) taken daily at bedtime • Patients had to satisfy the 2010 ACR Preliminary Diagnostic Classification Criteria • Primary endpoint: Weekly average pain improvement as a 30% responder analysis • Secondary endpoints: PGIC, FIQ - R Symptom Domain, FIQ - F Function Domain, Daily Sleep Quality Diary, PROMIS Sleep Disturbance Efficacy results: • mITT population: 425 (81.9%) of 519 patients • The primary analysis was not statistically significant. However, retrospective analysis showed average pain improvement (secondary endpoint) after 12 weeks of treatment showed statistical significance (P<0.05, MMRM) • Significant improvements observed in sleep quality, patient global impression of change and fibromyalgia - specific measures (secondary analyses).
© 2019 Tonix Pharmaceuticals Holding Corp. 41 TNX - 102 SL for Fibromyalgia: F301 Study Results and Program Updates Safety results: • Good tolerability and low rates of systemic AEs. • The most common AEs were generally mild and transient events related to the sublingual administration of the study drug: • hypoaesthesia (tongue or oral numbness) • glossodynia (burning sensation or other tongue discomfort) • oral paraesthesias (tingling sensations) • abnormal product taste (bitter or noticeable taste) • The severity and incidence of oral AE are similar to those reported in our PTSD studies using TNX - 102 SL 5.6 mg. Conclusion: • The promising results and highly relevant efficacy findings support further investigation of TNX - 102 SL 5.6 mg (2 x 2.8 mg tablets) as a chronic treatment for FM. Program updates: • Clear guidance and support received from FDA* to advance the FM program. The long - term safety exposure data from the PTSD program may support the fibromyalgia NDA*. • TNX - 102 SL 5.6 mg (2 x 2.8 mg tablets) will be studied in new Phase 3 study to support product registration *April 2019 FDA meeting minutes

© 2019 Tonix Pharmaceuticals Holding Corp. 42 TNX - 102 SL: Potential Treatment for Agitation in Alzheimer’s Disease (AAD) Agitation is one of the most distressing and debilitating of the behavioral complications of Alzheimer’s disease • Includes emotional lability, restlessness, irritability and aggression 1 Link between disturbed sleep and agitation in Alzheimer’s 1 - 3 • Agitation is commonly diurnal (“ sundowning ”) Prevalence • Agitation is likely to affect more than half of the 5.3 million Americans who currently suffer from moderate to severe Alzheimer’s disease; expected to nearly triple by 2050 4 FDA - designated Fast Track development program Significant unmet need with no FDA approved drugs for the treatment of AAD Proposed Phase 2 IND study can potentially serve as a pivotal efficacy study to support NDA approval 5 1 Rose, K.et al. (2015). American Journal of Alzheimer's Disease & Other Dementias , 30 :78 2 Shih, Y. H., et al. (2017). Journal of the American Medical Directors Association , 18 , 396. 3 Canevelli, M., et al. (2016). Frontiers in medicine , 3 . 4 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/ 5 FDA comments on final protocol received October 2018

© 2019 Tonix Pharmaceuticals Holding Corp. New Addition to Tonix’s Pipeline: TNX - 1300* for the Treatment of Cocaine Intoxication Recombinant protein that degrades cocaine in the bloodstream 1 • Double - mutant cocaine esterase In - licensed from Columbia University (Univ. of Kentucky and Univ. of Michigan) Phase 2 study completed by Rickett Benckiser (formerly RBP - 8000) 2 • Volunteer cocaine abusers received cocaine 50 mg i.v. infusion over 10 minutes • TNX - 1300 given one minute after completion of cocaine infusion • Rapidly reversed the physiologic effects of cocaine; cocaine plasma exposures dropped by 90% within two minutes • Well tolerated with the most frequently reported adverse events being gastrointestinal disorders (incl dry mouth, nausea); nervous systems disorders (incl headache, dizziness) and skin and subcutaneous tissue disorders (incl hyperhidrosis, dermatitis) TNX - 1300 for the treatment of cocaine intoxication has U.S. FDA Breakthrough Therapy designation (BTD) *TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication. 1 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23. 2 Nasser AF et al, J Addict Dis . 2014;33(4):289 - 302.
© 2019 Tonix Pharmaceuticals Holding Corp. 44 About TNX - 1300 Produced through rDNA technology in non - disease - producing strain of E. coli. • Cocaine Esterase ( CocE ) was identified in bacteria ( Rhodococcus ) that use cocaine as its sole source of carbon and nitrogen and that grow in soil surrounding coca plants 1 • The gene encoding CocE was identified and the protein was extensively characterized 1 - 3 • CocE catalyzes the breakdown of cocaine into metabolite ecgonine methyl ester and benzoic acid • Wild - type CocE is unstable at body temperature, so targeted mutations were introduced in the CocE gene and resulted in the T172R/G173Q Double - Mutant CocE , which is active for approximately 6 hours at body temperature 4 1 Bresler MM et al, Appl Environ Microbiol. 2000. 66(3):904 - 8. 2 Larsen NA et al, Nat Struct Biol. 2002. 9(1):17 - 21. 3 Turner JM et al, Biochemistry. 2002. 41(41):12297 - 307. 4 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23.
© 2019 Tonix Pharmaceuticals Holding Corp. 45 About Cocaine and Cocaine Intoxication Cocaine: an illegal recreational drug taken for its pleasurable effects and associated euphoria. • Cocaine blocks the reuptake of the neurotransmitter dopamine (DA) in the CNS • Results in accumulation of DA within the synapse and amplifies DA signaling • Creates positive feeling but with intense use of cocaine, results in cocaine craving • High potential for abuse/addiction (dependence), and risk of cocaine intoxication. Cocaine intoxication: deleterious effects on the body, especially cardiovascular system. • Common symptoms include tachyarrhythmias and elevated blood pressure, either of which can be life - threatening. • Known or suspected cocaine intoxication cases are sent immediately to the emergency department, preferably by ambulance in case cardiac arrest occurs during transit.
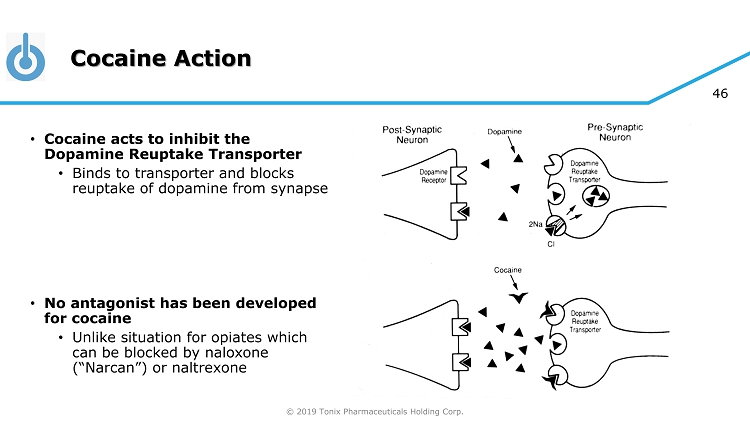
© 2019 Tonix Pharmaceuticals Holding Corp. 46 Cocaine Action • Cocaine acts to inhibit the Dopamine Reuptake Transporter • Binds to transporter and blocks reuptake of dopamine from synapse • No antagonist has been developed for cocaine • Unlike situation for opiates which can be blocked by naloxone (“Narcan”) or naltrexone
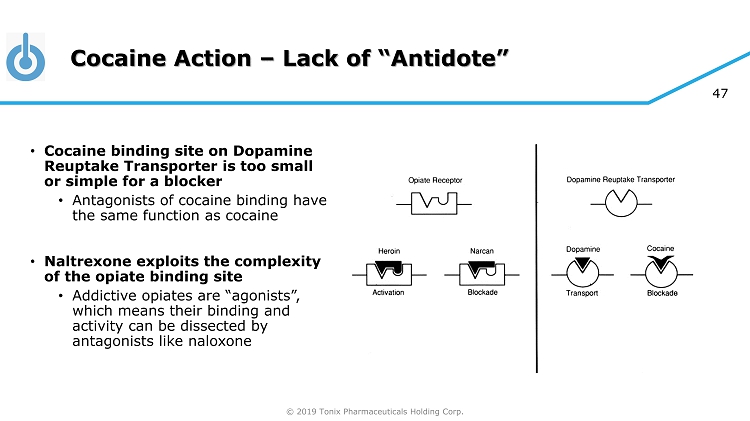
© 2019 Tonix Pharmaceuticals Holding Corp. 47 Cocaine Action – Lack of “Antidote” • Cocaine binding site on Dopamine Reuptake Transporter is too small or simple for a blocker • Antagonists of cocaine binding have the same function as cocaine • Naltrexone exploits the complexity of the opiate binding site • Addictive opiates are “agonists”, which means their binding and activity can be dissected by antagonists like naloxone
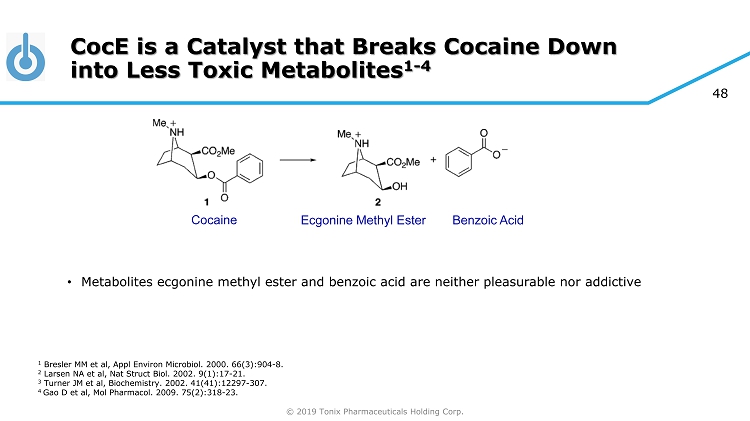
© 2019 Tonix Pharmaceuticals Holding Corp. 48 CocE is a Catalyst that Breaks Cocaine Down into Less Toxic Metabolites 1 - 4 Cocaine Ecgonine Methyl Ester Benzoic Acid • Metabolites ecgonine methyl ester and benzoic acid are neither pleasurable nor addictive 1 Bresler MM et al, Appl Environ Microbiol. 2000. 66(3):904 - 8. 2 Larsen NA et al, Nat Struct Biol. 2002. 9(1):17 - 21. 3 Turner JM et al, Biochemistry. 2002. 41(41):12297 - 307. 4 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23.
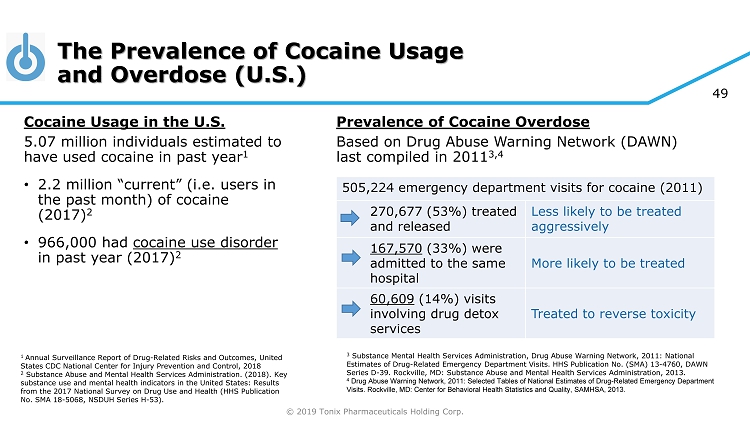
© 2019 Tonix Pharmaceuticals Holding Corp. 49 The Prevalence of Cocaine Usage and Overdose (U.S.) Cocaine Usage in the U.S. 5.07 million individuals estimated to have used cocaine in past year 1 • 2.2 million “current” (i.e. users in the past month) of cocaine (2017) 2 • 966,000 had cocaine use disorder in past year (2017) 2 1 Annual Surveillance Report of Drug - Related Risks and Outcomes, United States CDC National Center for Injury Prevention and Control, 2018 2 Substance Abuse and Mental Health Services Administration. (2018). Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18 - 5068, NSDUH Series H - 53). Prevalence of Cocaine Overdose Based on Drug Abuse Warning Network (DAWN) last compiled in 2011 3,4 505,224 emergency department visits for cocaine (2011) 270,677 (53%) treated and released Less likely to be treated aggressively 167,570 (33%) were admitted to the same hospital More likely to be treated 60,609 (14%) visits involving drug detox services Treated to reverse toxicity 3 Substance Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug - Related Emergency Department Visits. HHS Publication No. (SMA) 13 - 4760, DAWN Series D - 39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. 4 Drug Abuse Warning Network, 2011: Selected Tables of National Estimates of Drug - Related Emergency Department Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA, 2013.

© 2019 Tonix Pharmaceuticals Holding Corp. 50 Mortality Due to Cocaine Overdose Cocaine is involved in 20% of overdose deaths in the U.S. • In 2016, 10,375 deaths due to cocaine overdose 1 • In 2017, about 13,900 deaths occurred in the U.S. due to cocaine overdose. 2 Overdose deaths involving cocaine increased 34% from 2016 to 2017. 3,4 1 Overdose Death Rates - National Institute on Drug Abuse - https://www.drugabuse.gov/related - topics/trends - statistics/overdose - death - rates ; accessed May 11, 2019 2 Kariisa M et al. Drug Overdose Deaths Involving Cocaine and Psychostimulants with Abuse Potential — United States, 2003 – 2017. MMWR Weekly / May 3, 2019 / 68(17);388 – 395 - https://www.cdc.gov/mmwr/volumes/68/wr/mm6817a3.htm?s_cid=mm6817a3_w ; 3 Cocaine deaths up in U.S. and opioids are a big part of it. Associated Press. https://www.msn.com/en - us/news/us/cocaine - deaths - up - in - us - and - opioids - are - a - big - part - of - it/ar - AAAOxs8?ocid=se ; accessed May 11, 2019 4 Fottrell , Q. MarketWatch, Fatal drug overdoses involving cocaine and other stimulants have surged by over 52%, May 3, 2019 - https://www.marketwatch.com/story/fatal - drug - overdoses - involving - cocaine - and - other - stimulants - have - surged - by - over - 52 - 2019 - 05 - 03 ; accessed May 11, 2019

© 2019 Tonix Pharmaceuticals Holding Corp. 51 Treatment for Cocaine Intoxication Current Standard of Care • Patients present with acute agitation, hyperthermia, tachycardia, arrhythmias, and hypertension • Potential life - threatening sequalae of myocardial infarction, cerebrovascular accident, rhabdomyolysis, respiratory failure, and seizures • Patients are currently managed only by supportive care for the adverse effects of cocaine intoxication on the cardiovascular and central nervous systems Potential Benefit of TNX - 1300 • By reversing the cause of cocaine intoxication (rather than treating the symptoms), TNX - 1300 may offer significant advantages to the current standard of care for cocaine intoxication. • Rapid diminution in circulating cocaine • Significantly reduce time and resources required for other detox services • Reduces the risk of morbidity and mortality

© 2019 Tonix Pharmaceuticals Holding Corp. Value of TNX - 1300 to Tonix Features of the Acquired Asset: • Full rights to the IP and to develop and commercialize TNX - 1300 worldwide • FDA Breakthrough Therapy Designated product • An inventory of investigational drug product • Clinical trial results from previous Phase 2 study in which TNX - 1300 at 100 mg or 200 mg i.v. doses was well tolerated and interrupted cocaine effects after cocaine 50 mg i.v. challenge Development Plan: • Re - qualify the drug substance for Good Manufacturing Practice (GMP) purposes • Conduct non - clinical studies in reproductive toxicology • Initiate a Phase 2 study in Emergency Room cocaine intoxication Exclusivity: • Expected patent protection through 2029 • As a biologic and new molecular entity, TNX - 1300 is eligible for 12 years of U.S. market exclusivity upon approval by the FDA. Pipeline Diversification: • Brings Tonix into an additional therapeutic area: Addiction Medicine
© 2019 Tonix Pharmaceuticals Holding Corp. 53 CNS Candidates in Development Psychiatry, Pain and Addiction Preclinical Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime Treatment for PTSD – Tonmya ®2 Daytime Treatment for PTSD TNX - 601 Novel salt discovered and characterized; Preliminary human PK and safety data 6 from selected formulation expected 2H2019 TNX - 1300 5 Phase 2a completed – blocked physiological effects of cocaine challenge; Phase 2b - ready Cocaine intoxication / overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 6 non - Investigational New Drug (IND) study; Bedtime Treatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due P302/ RECOVERY study initiated 1Q2019 Bedtime Treatment for Agitation in Alzheimer’s Fast Track Phase 2/3 ready program Treatment of Neurocognitive Dysfunction from Corticosteroids 2 efficacy studies completed at 2.8 mg; IND active to support Phase 3 using 5.6 mg
© 2019 Tonix Pharmaceuticals Holding Corp. 54 Biodefense Candidates in Development 1 NDA - New Drug Application; 2 BLA – Biologic Licensing Application Biodefense programs owned outright with no royalties due Preclinical Phase 2 NDA 1 /BLA 2 Market Pipeline Product Indication Phase 3 TNX - 801 Horsepox virus synthesized and demonstrated protective vaccine activity in mice Smallpox - preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture percutaneous (scarification) formulation Phase 1 TNX - 701 Radioprotective effect demonstrated in mice Radioprotection Radioprotection drug oral capsules
© 2019 Tonix Pharmaceuticals Holding Corp. 55 TNX - 601 ( Tianeptine Oxalate): A Potential Clinical Candidate for PTSD Pre - IND Candidate Targeted as a 1 st line monotherapy for PTSD: oral formulation for daytime dosing x Leverages expertise in PTSD (clinical and regulatory experience, market analysis, etc.) x Mechanism of Action (MOA) is different from TNX - 102 SL • Tianeptine sodium (amorphous), first marketed for depression in France in 1989, is approved as an antidepressant in the EU, Russia, Asia and Latin America; established post - marketing experience • Identified new oxalate salt with improved pharmaceutical properties ideal for reformulation • Preliminary human pharmacokinetic and safety data (non - IND study) from selected formulation expected in second half 2019 Filed patent application on novel salt • Issued patent on steroid - induced cognitive impairment and memory loss issues Targeting a Condition with Significant Unmet Need Clinical evidence for PTSD • Several studies have shown tianeptine to be active in the treatment of PTSD 1 - 4 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747

© 2019 Tonix Pharmaceuticals Holding Corp. 56 TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate Pre - IND Stage Potential improvement over current biodefense tools against smallpox ✓ Leverages Tonix’s government affairs effort ✓ Collaboration with Professor David Evans and Dr. Ryan Noyce at University of Alberta ✓ Demonstrated protective vaccine activity in mice ✓ Patent application on novel vaccine submitted Regulatory strategy • We intend to meet with FDA to discuss the most efficient and appropriate investigational plan to support the licensure, either: ✓ Application of the “Animal Rule”, or ✓ Conducting an active comparator study using ACAM2000 • Good Manufacturing Practice (GMP) viral production process in development Targeting a Potential Public Health Issue Material threat medical countermeasure under 21 st Century Cures Act • Qualifies for Priority Review Voucher (PRV) upon licensure * ✓ PRVs have no expiration date, are transferrable and have sold for ~$125 M *BLA/NDA priority 6 - month review is expected.

© 2019 Tonix Pharmaceuticals Holding Corp. 57 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
© 2019 Tonix Pharmaceuticals Holding Corp. 58 Board of Directors Seth Lederman, MD Chairman Adeoye “Oye” Olukotun, MD Squibb, BMS, Mallinckrodt, Esperion John Rhodes Chair, NYS Public Service Commission, CEO, NYS Dept. of Public Service, Booz Allen James Treco First Chicago, Salomon Brothers/Citigroup Gen. David Grange (U.S. Army, ret.) Pharm - Olam, PPD, McCormick Foundation Patrick Grace ( qp ) global family offices, Grace Institute Foundation, WR Grace, Chemed Margaret Smith Bell Standard Life Investments, Putnam Investments, State Street Research Daniel Goodman, MD Psychiatrist, co - founder Psychogenics
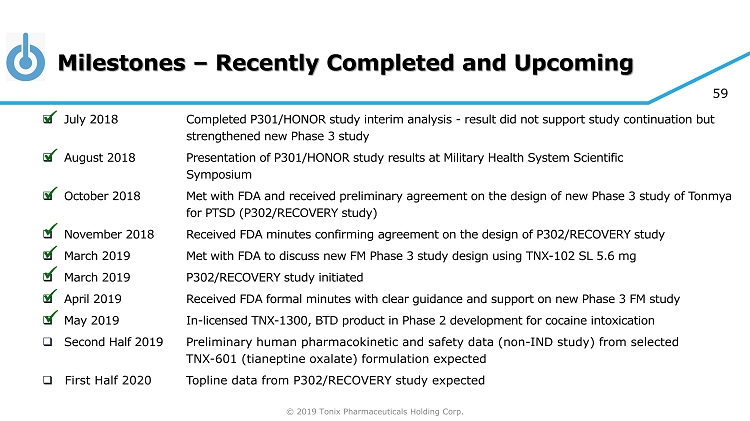
© 2019 Tonix Pharmaceuticals Holding Corp. 59 Milestones – Recently Completed and Upcoming □ July 201 8 Completed P301/HONOR study interim analysis - result did not support study continuation but strengthened new Phase 3 study □ August 201 8 Presentation of P301/HONOR study results at Military Health System Scientific Symposium □ October 201 8 Met with FDA and received preliminary agreement on the design of new Phase 3 study of Tonmya for PTSD (P302/RECOVERY study) □ November 201 8 Received FDA minutes confirming agreement on the design of P302/RECOVERY study □ March 2019 Met with FDA to discuss new FM Phase 3 study design using TNX - 102 SL 5.6 mg □ March 2019 P302/RECOVERY study initiated □ April 2019 Received FDA formal minutes with clear guidance and support on new Phase 3 FM study □ May 2019 In - licensed TNX - 1300, BTD product in Phase 2 development for cocaine intoxication □ Second Half 2019 P reliminary human pharmacokinetic and safety data (non - IND study) from selected TNX - 601 (tianeptine oxalate) formulation expected □ First Half 2020 Topline data from P302/RECOVERY study expected x x x x x x x x
© 2019 Tonix Pharmaceuticals Holding Corp. 60 Summary Two Phase 3 Programs in indications affecting millions of Americans • Tonmya for PTSD: affects 12 million adults in U.S.; currently in Phase 3 with data expected next year; bedtime treatment • TNX - 102 SL for Fibromyalgia: affects between 5 - 10 million adults in U.S.; ready for Phase 3 Two Phase 2 Programs in indications for which there is no FDA - approved drug available • TNX - 102 SL for Agitation in Alzheimer’s Disease: Fast Track designation; ready for Phase 2/3 • TNX - 1300 for Cocaine Intoxication: biologic and new molecular entity with Breakthrough Therapy designation; ready for Phase 2 Pipeline products to improve biodefense and leverage PTSD expertise • TNX - 801: smallpox - preventing vaccine in preclinical development; demonstrated protective vaccine activity in mice; GMP viral production process in development • TNX - 701: oral radioprotection drug in preclinical development; demonstrated radioprotective effect in mice • TNX - 601: tianeptine oxalate in formulation development for daytime treatment of PTSD

© 2019 Tonix Pharmaceuticals Holding Corp. Thank you ! NASDAQ: TNXP