
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2019 Tonix Pharmaceuticals Holding Corp. 1 September 2019 Version P0196 9 - 16 - 19 (Doc 0535) Presentation to NAVREF

© 2019 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2018 , as filed with the Securities and Exchange Commission (the “SEC”) on March 18 , 2019 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

© 2019 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals Who we are: • A clinical stage biopharmaceutical company dedicated to developing innovative treatments for patients and making meaningful contributions to society • Focusing on small molecules and biologics to treat psychiatric, pain and addiction conditions, to improve biodefense through potential medical counter - measures and to prevent and treat organ transplant rejection What we do: • Target therapeutic areas with high need for improvement − Conditions with no, or inadequate, treatments − Significant patient populations not well served by existing therapies • Develop innovative treatment options with possibility to be a “game changer” − Scientifically unique and innovative − Strong scientific rationale supported by preliminary clinical evidence and published literature − Proven regulatory pathways and established clinical endpoints − Built on a foundation of proprietary intellectual property
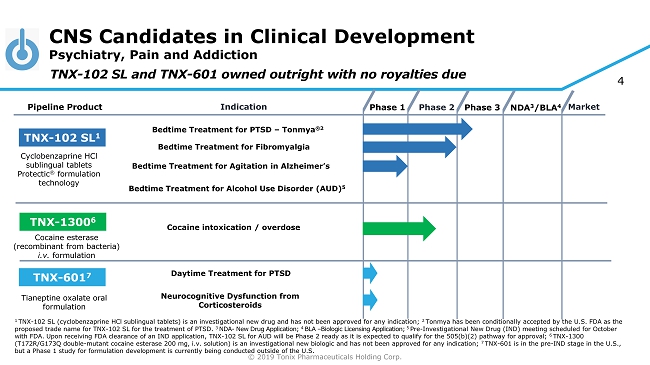
© 2019 Tonix Pharmaceuticals Holding Corp. 4 CNS Candidates in Clinical Development Psychiatry, Pain and Addiction Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime Treatment for PTSD – Tonmya ®2 Daytime Treatment for PTSD TNX - 601 7 TNX - 1300 6 Cocaine intoxication / overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 Bedtime Treatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due Bedtime Treatment for Agitation in Alzheimer’s Neurocognitive Dysfunction from Corticosteroids Bedtime Treatment for Alcohol Use Disorder (AUD) 5 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 Pre - Investigational New Drug (IND) meeting scheduled for October with FDA. Upon receiving FDA clearance of an IND application, TNX - 102 SL for AUD will be Phase 2 ready as it is expected to qual ify for the 505(b)(2) pathway for approval; 6 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 7 TNX - 601 is in the pre - IND stage in the U.S., but a Phase 1 study for formulation development is currently being conducted outside of the U.S.
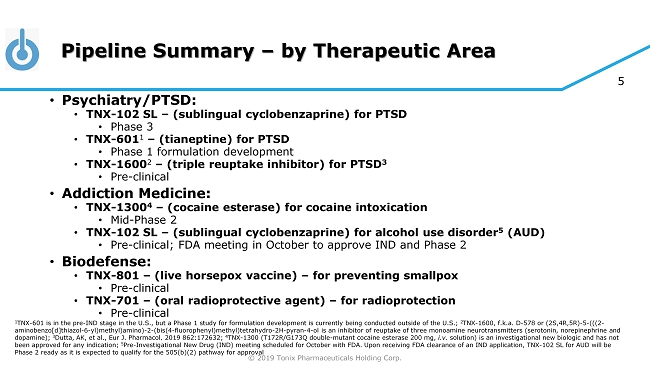
© 2019 Tonix Pharmaceuticals Holding Corp. 5 Pipeline Summary – by Therapeutic Area • Psychiatry/PTSD: • TNX - 102 SL – (sublingual cyclobenzaprine) for PTSD • Phase 3 • TNX - 601 1 – (tianeptine) for PTSD • Phase 1 formulation development • TNX - 1600 2 – (triple reuptake inhibitor) for PTSD 3 • Pre - clinical • Addiction Medicine: • TNX - 1300 4 – (cocaine esterase) for cocaine intoxication • Mid - Phase 2 • TNX - 102 SL – (sublingual cyclobenzaprine) for alcohol use disorder 5 (AUD) • Pre - clinical; FDA meeting in October to approve IND and Phase 2 • Biodefense: • TNX - 801 – (live horsepox vaccine) – for preventing smallpox • Pre - clinical • TNX - 701 – (oral radioprotective agent) – for radioprotection • Pre - clinical 1 TNX - 601 is in the pre - IND stage in the U.S., but a Phase 1 study for formulation development is currently being conducted outsid e of the U.S.; 2 TNX - 1600, f.k.a . D - 578 or (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol is an inhibitor of reuptake of thr ee monoamine neurotransmitters (serotonin, norepinephrine and dopamine); 3 Dutta, AK, et al., Eur J. Pharmacol . 2019 862:172632; 4 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 5 Pre - Investigational New Drug (IND) meeting scheduled for October with FDA. Upon receiving FDA clearance of an IND application, T NX - 102 SL for AUD will be Phase 2 ready as it is expected to qualify for the 505(b)(2) pathway for approval

© 2019 Tonix Pharmaceuticals Holding Corp. 6 Unmet Need for Effective and Safe Therapies for Treatment of PTSD No FDA - approved products for PTSD since Pfizer’s Zoloft ® (sertraline) and GSK’s Paxil ® (paroxetine) circa 2000 • Neither has shown efficacy in military - related PTSD • Male PTSD patients often unresponsive or intolerant of current treatments • Side effects relating to sexual dysfunction, sleep disruption and weight gain are commonly reported PTSD is signature wound of last 25 years of war • Affects servicemember health and performance, force readiness, retention • Believed to be the underlying cause of suicide in many cases
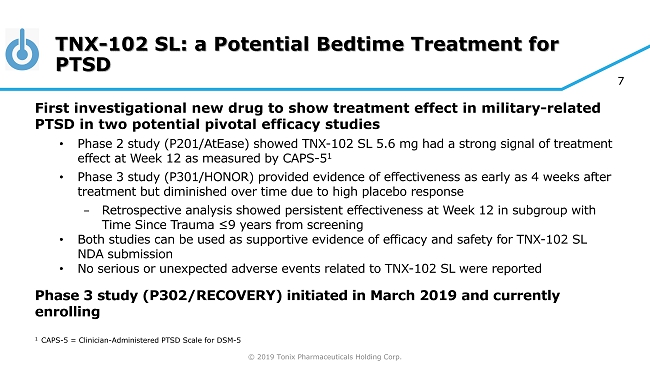
© 2019 Tonix Pharmaceuticals Holding Corp. 7 TNX - 102 SL: a Potential Bedtime Treatment for PTSD First investigational new drug to show treatment effect in military - related PTSD in two potential pivotal efficacy studies • Phase 2 study (P201/ AtEase ) showed TNX - 102 SL 5.6 mg had a strong signal of treatment effect at Week 12 as measured by CAPS - 5 1 • Phase 3 study (P301/HONOR) provided evidence of effectiveness as early as 4 weeks after treatment but diminished over time due to high placebo response − Retrospective analysis showed persistent effectiveness at Week 12 in subgroup with Time Since Trauma ≤9 years from screening • Both studies can be used as supportive evidence of efficacy and safety for TNX - 102 SL NDA submission • No serious or unexpected adverse events related to TNX - 102 SL were reported Phase 3 study (P302/RECOVERY) initiated in March 2019 and currently enrolling 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5

© 2019 Tonix Pharmaceuticals Holding Corp. 8 Potential Therapeutic Advantages of TNX - 102 SL TNX - 102 SL is believed to treat PTSD by improving sleep quality • The brain naturally processes memories during sleep • In PTSD emotionally charged memories disturb sleep and disrupt memory processing • TNX - 102 SL is believed to normalize memory processing and facilitate extinction consolidation (breaking the link between “triggers” and PTSD symptoms) TNX - 102 SL active ingredient is NEITHER a benzodiazepine nor a narcotic • Does NOT interact with the same receptors as traditional hypnotic sleep drugs associated with retrograde amnesia and is NOT an opiate TNX - 102 SL is non - addictive • Cyclobenzaprine is the active ingredient of an orally ingested immediate release tablet (Flexeril ® ), approved 40 years ago • Flexeril’s current labeling indicates no abuse and dependence concern at higher doses (15 - 30 mg/day) than TNX - 102 SL (5.6 mg/day) • TNX - 102 SL NDA can be filed without drug abuse and dependency assessment studies Once - daily sublingual dose taken at bedtime enhances patient adherence
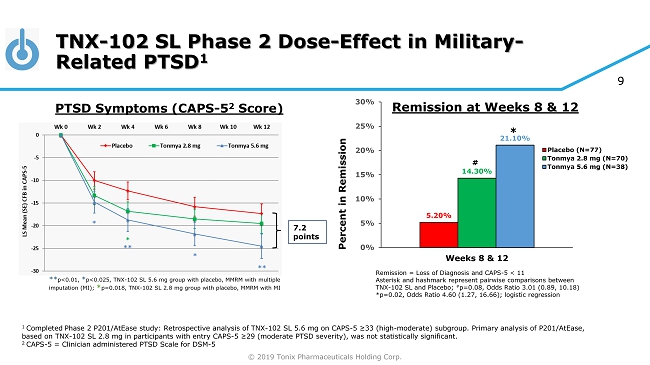
© 2019 Tonix Pharmaceuticals Holding Corp. 9 TNX - 102 SL Phase 2 Dose - Effect in Military - Related PTSD 1 1 Completed Phase 2 P201/AtEase study: Retrospective analysis of TNX - 102 SL 5.6 mg on CAPS - 5 ≥33 (high - moderate) subgroup. Primary analysis of P201/ AtEase , based on TNX - 102 SL 2.8 mg in participants with entry CAPS - 5 ≥29 ( moderate PTSD severity), was not statistically significant. 2 CAPS - 5 = Clinician administered PTSD Scale for DSM - 5 7.2 points ** p<0.01, * p<0.025, TNX - 102 SL 5.6 mg group with placebo, MMRM with multiple imputation (MI); * p=0.018, TNX - 102 SL 2.8 mg group with placebo, MMRM with MI PTSD Symptoms (CAPS - 5 2 Score) Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between TNX - 102 SL and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # * Remission at Weeks 8 & 12
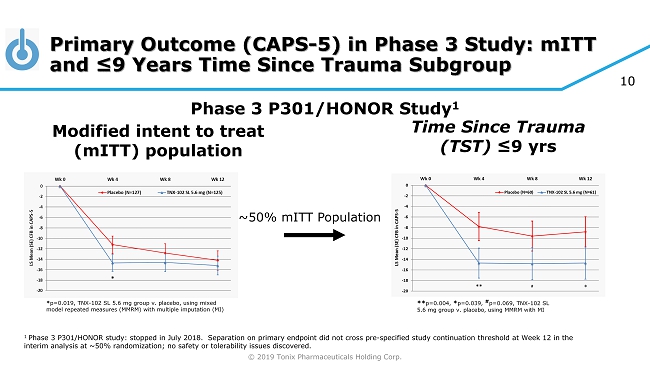
© 2019 Tonix Pharmaceuticals Holding Corp. 10 Primary Outcome (CAPS - 5) in Phase 3 Study: mITT and ≤9 Years Time Since Trauma Subgroup Modified intent to treat ( mITT ) population Phase 3 P301/HONOR Study 1 ** p=0.004, * p=0.039, # p=0.069, TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI ~50% mITT Population * p=0.019, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI) Time Since Trauma (TST) ≤9 yrs 1 Phase 3 P301/HONOR study: stopped in July 2018. Separation on primary endpoint did not cross pre - specified study continuation t hreshold at Week 12 in the interim analysis at ~50% randomization; no safety or tolerability issues discovered.
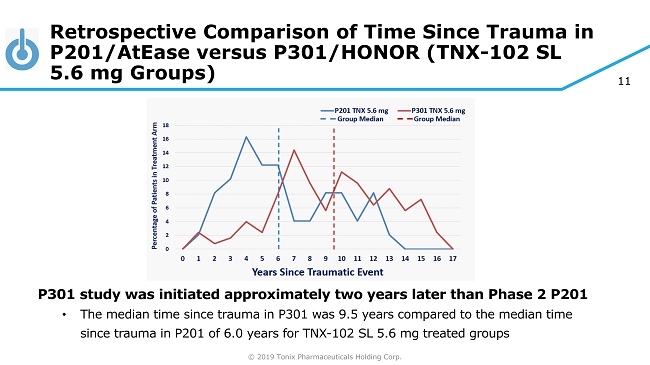
© 2019 Tonix Pharmaceuticals Holding Corp. 11 Retrospective Comparison of Time Since Trauma in P201/AtEase versus P301/HONOR (TNX - 102 SL 5.6 mg Groups) P301 study was initiated approximately two years later than Phase 2 P201 • The median time since trauma in P301 was 9.5 years compared to the median time since trauma in P201 of 6.0 years for TNX - 102 SL 5.6 mg treated groups
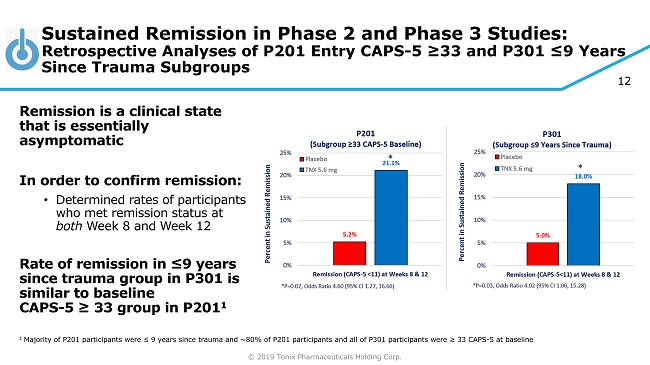
© 2019 Tonix Pharmaceuticals Holding Corp. 12 Sustained Remission in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Remission is a clinical state that is essentially asymptomatic In order to confirm remission: • Determined rates of participants who met remission status at both Week 8 and Week 12 Rate of remission in ≤9 years since trauma group in P301 is similar to baseline CAPS - 5 ≥ 33 group in P201 1 1 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥ 33 CAPS - 5 at baseline
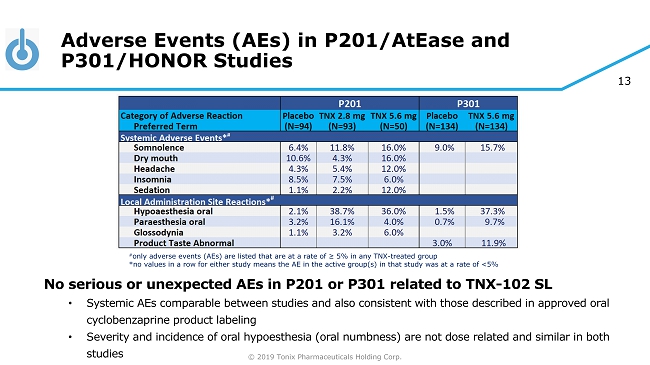
© 2019 Tonix Pharmaceuticals Holding Corp. 13 Adverse Events (AEs) in P201/AtEase and P301/HONOR Studies No serious or unexpected AEs in P201 or P301 related to TNX - 102 SL • Systemic AEs comparable between studies and also consistent with those described in approved oral cyclobenzaprine product labeling • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related and similar in both studies # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%
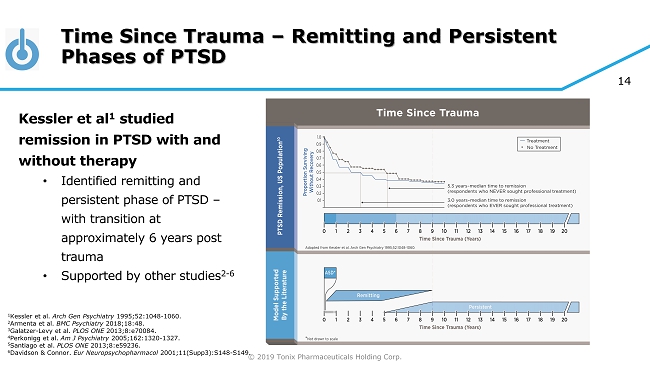
© 2019 Tonix Pharmaceuticals Holding Corp. 14 Time Since Trauma – Remitting and Persistent Phases of PTSD Kessler et al 1 studied remission in PTSD with and without therapy • Identified remitting and persistent phase of PTSD – with transition at approximately 6 years post trauma • Supported by other studies 2 - 6 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327. 5 Santiago et al. PLOS ONE 2013;8:e59236. 6 Davidson & Connor. Eur Neuropsychopharmacol 2001;11(Supp3):S148 - S149.
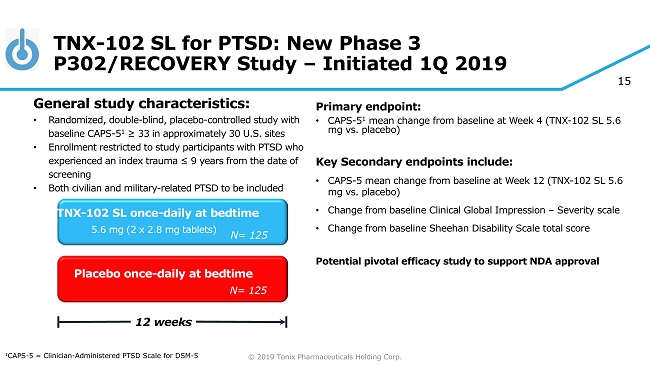
© 2019 Tonix Pharmaceuticals Holding Corp. 15 TNX - 102 SL for PTSD: New Phase 3 P302/RECOVERY Study – Initiated 1Q 2019 Primary e ndpoint: • CAPS - 5 1 mean change from baseline at Week 4 (TNX - 102 SL 5.6 mg vs. placebo) Key Secondary e ndpoint s include: • CAPS - 5 m ean change from baseline at W eek 12 (TNX - 102 SL 5.6 mg vs. placebo ) • Change from baseline Clinical Global Impression – Severity scale • Change from baseline Sheehan Disability Scale total score Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study with baseline CAPS - 5 1 ≥ 33 in approximately 30 U.S. sites • Enrollment restricted to study participants with PTSD who experienced an index trauma ≤ 9 years from the date of screening • Both civilian and military - related PTSD to be included 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 N= 125 N= 125
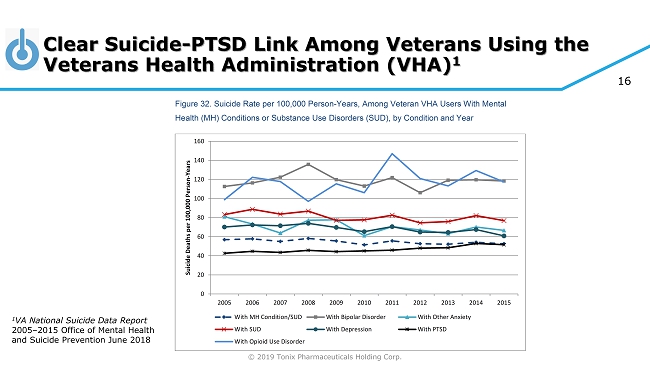
© 2019 Tonix Pharmaceuticals Holding Corp. 16 Clear Suicide - PTSD Link Among Veterans Using the Veterans Health Administration (VHA) 1 40 Between 2005 and 2015, rates of suicide remained stable across cohorts of Veteran VHA patients diagnosed with a mental health condition or substance use disorder (Figure 32). Rates were highest among those diagnosed with bipolar disorder and those diagnosed with opioid use disorder. In contrast, rates among those with any mental health or substance use disorder diagnosis decreased from 56.9 to 52.7 per 100,000 person-years between 2005 and 2015. For those diagnosed with depression, the rate decreased from 70.2 to 60.9 per 100,000 person-years between 2005 and 2015. Figure 32. Suicide Rate per 100,000 Person-Years, Among Veteran VHA Users With Mental Health (MH) Conditions or Substance Use Disorders (SUD), by Condition and Year Main finding: From 2005 through 2015, suicide rates among Veteran VHA patients diagnosed with a mental health or substance use disorder were relatively stable. Rates were highest among those with bipolar disorder and those with opioid use disorder. 0 20 40 60 80 100 120 140 160 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 S u i c i d e D e a t h s p e r 1 0 0 , 0 0 0 P e r s o n - Y e a r s With MH Condition/SUD With Bipolar Disorder With Other Anxiety With SUD With Depression With PTSD With Opioid Use Disorder 1 VA National Suicide Data Report 2005 – 2015 Office of Mental Health and Suicide Prevention June 2018
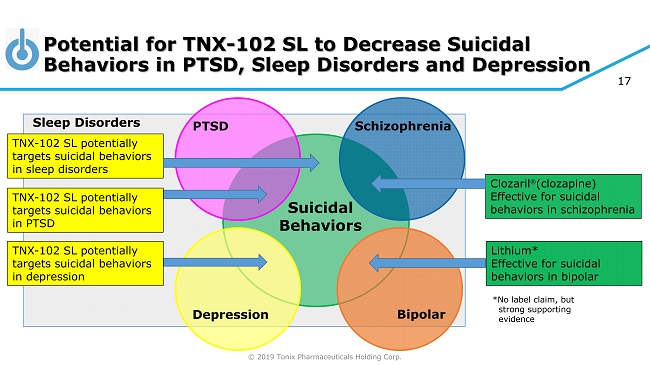
© 2019 Tonix Pharmaceuticals Holding Corp. 17 Potential for TNX - 102 SL to Decrease Suicidal Behaviors in PTSD, Sleep Disorders and Depression Suicidal Behaviors PTSD Depression Schizophrenia Bipolar Clozaril (clozapine) Effective for suicidal behaviors in schizophrenia Lithium* Effective for suicidal behaviors in bipolar *No label claim, but strong supporting evidence Sleep Disorders TNX - 102 SL potentially targets suicidal behaviors in PTSD TNX - 102 SL potentially targets suicidal behaviors in depression TNX - 102 SL potentially targets suicidal behaviors in sleep disorders

© 2019 Tonix Pharmaceuticals Holding Corp. 18 Crisis in the Pharmacotherapy of PTSD Endures “The urgent need to find effective pharmacologic treatments for PTSD should be considered a national mental health priority.” - From the Consensus Statement of the PTSD Psychopharmacology Working Group 1 • Despite the consensus about a crisis, industry’s investment in new PTSD drug treatments continues to lag • Dramatic decline in Big Pharma development of psychiatric drugs since 1990s • Small molecule drugs that enter the brain have shorter periods of market exclusivity than biologics 2 • Patent protection exclusivity has shortened because of patent law changes 3 • Psychiatry drug development is risky -- unsuccessful trials are typically seen in the clinical development programs for psychiatric drugs that ultimately receive FDA approval 4 • Psychiatry drugs do not command the prices of oncology drugs or orphan disease drugs 1 Krystal, JH., et al “It Is Time to Address the Crisis in the Pharmacotherapy of Posttraumatic Stress Disorder: A Consensus St ate ment of the PTSD Psychopharmacology Working Group. Biol. Psychiatry. 2017 Oct 1;82(7):e51 - e59. doi : 10.1016 2 The Patient Protection and Affordable Care Act. 2010 - provides 12 years of exclusivity to biologics 3 Uruguay Round Agreement Act. 1994 4 Turner, EH, NEJM 2008 358: 253

© 2019 Tonix Pharmaceuticals Holding Corp. 19 President’s Roadmap to Empower Veterans and End a National Tragedy of Suicide – “PREVENTS” 1 • Executive Order on a National Roadmap to Empower Veterans and End Suicide – March 19 2019 • Task force empaneled; VA Secretary Wilkie is Co - Chair • Dr. Barbara Van Dahlen has been appointed Executive Director • “(iii) develop strategies to better ensure the latest research discoveries are translated into practical applications and implemented quickly. • (vii) develop a public - private partnership model to foster collaborative, innovative, and effective research that accelerates these efforts.” 2 1 https://www.whitehouse.gov/presidential - actions/executive - order - national - roadmap - empower - veterans - end - suicide/ 2 Executive Order Mandate: Sec. 7(b)(iii) and (vii)

© 2019 Tonix Pharmaceuticals Holding Corp. 20 PTSD – New Learnings and Recommendations • PTSD within 9 years of trauma has a higher rate of drug responsiveness to TNX - 102 SL • Decrease in spontaneous remission after ~6 years in the National Comorbidity study supports loss of plasticity over time 1 • PTSD changes over time • Early diagnosis and treatment are likely important • Task Force is empaneled to develop public - private partnerships • PREVENTS Task Force should consider a partnerships with industry to develop PTSD therapeutics 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060

© 2019 Tonix Pharmaceuticals Holding Corp. 21 Thank you ! NASDAQ: TNXP