
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.02

© 2019 Tonix Pharmaceuticals Holding Corp. Gregory Sullivan, MD Chief Medical Officer Tonix Pharmaceuticals Inc presenting at American College of Rheumatology Annual Meeting, Atlanta, GA November 10, 2019 A Phase 3 Randomized, Double - Blind, Placebo - Controlled Trial of Bedtime Sublingual Cyclobenzaprine (TNX - 102 SL) for the Treatment of Fibromyalgia (FM): Evidence for a Broad Spectrum of Activity on the FM Syndrome

© 2019 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, substantial competition; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and risks related to failure to obtain U.S. Food and Drug Administration clearances or approvals and noncompliance with its regulations. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2018, as filed with the Securities and Exchange Commission (the “SEC”) on March 18, 2019, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

© 2019 Tonix Pharmaceuticals Holding Corp. 3 Disclosure Statements • Gregory Sullivan MD is an employee of Tonix Pharmaceuticals Inc and owns stock and stock options in the company • The co - authors on this work are employees or consultants of Tonix Pharmaceuticals Inc • TNX - 102 SL is an investigational new drug and has not been approved for any indication

© 2019 Tonix Pharmaceuticals Holding Corp. 4 TNX - 102 SL for the Treatment of Fibromyalgia Rationale for Targeting Sleep Quality • Inter - relationship between poor sleep quality and fibromyalgia (FM) described almost 50 years ago 1 • Moldofsky & Smythe identified anomalous alpha - rhythms in stage 4 NREM EEG delta wave sleep in patients with “fibrositis syndrome” (a former name for fibromyalgia) • In healthy volunteers, stage 4 sleep deprivation results in temporary appearance of myalgia, tenderness, and fatigue comparable to that in fibromyalgia (FM) • Suggests sleep disturbance in FM may not only be a consequence of pain but also pathogenic in the disorder • Sleep deprivation impairs descending pain inhibition pathways involved in controlling/coping with pain 2 • Poor sleep quality is a risk factor for the development of widespread pain through central sensitization • Reduced slow wave sleep (SWS) in FM may indicate impairment in homeostatic mechanisms of pain control • Serotonergic (5 - HT) and noradrenergic (NA) systems mediate descending inhibitory pain pathways in FM • Dysfunction in descending inhibitory activity plays a role in central sensitization in FM and the maladaptive pain expression • Enhancement of restorative sleep via modulation of 5 - HT and NA pathways may allow homeostatic plastic changes in pain processing circuitry and recovery from FM • Proof of concept study showed clinical and pharmacodynamic benefit of low dose cyclobenzaprine in FM 3 • 8 - week RCT with polysomnography (PSG) demonstrated improvement in core FM symptoms • Cyclobenzaprine treatment associated with a decreased pathological arousal pattern in the SWS EEG 1 Moldofsky & Smythe. Psychosomatic Medicine . 1975; 37 (4):341 – 351. 2 Choi EHS. Nat Rev Rheumatol 2015; 11: 513 – 520. 3 Moldofsky et al. J Rheumatol 2011; 38 :2653 - 63. CNS, central nervous system; EEG, electroencephalogram; RCT, randomized clinical trial

© 2019 Tonix Pharmaceuticals Holding Corp. 5 Non - restorative Sleep is Common in Fibromyalgia and May Contribute to Pain Symptoms 1 » Increased number of arousals leading to fragmented sleep » Non - restorative sleep or waking feeling unrefreshed » Diffuse stiffness, aching and fatigue, even after 6 - 8 hours sleep » Prolonged time to sleep onset (latency) Sleep problems and non - restorative sleep have been reported in >90% of fibromyalgia patients 2 - 5 1 Oswald I. Proc R Soc Med. 1962; 55:910 - 912; 2 Bigatti SM, et al. Arthritis Rheum 2008; 59:961 – 967; 3 Russell IJ & Bieber CS in Wall and Melzack’s Textbook of Pain 5th edn Ch. 44 (eds McMahon SB & Koltzenburg M) 669 – 682, Elsevier, 2006. 4 Moldofsky H. Joint Bone Spine 2008; 75:397 – 402. 5 Yunus MB et al. Arthritis Rheum 1991; 34:15 – 21; Illustrations from: Stahl SM. Stahl’s Illustrated Chronic Pain and Fibromyalgia . New York, NY: Cambridge University Press; 2009.
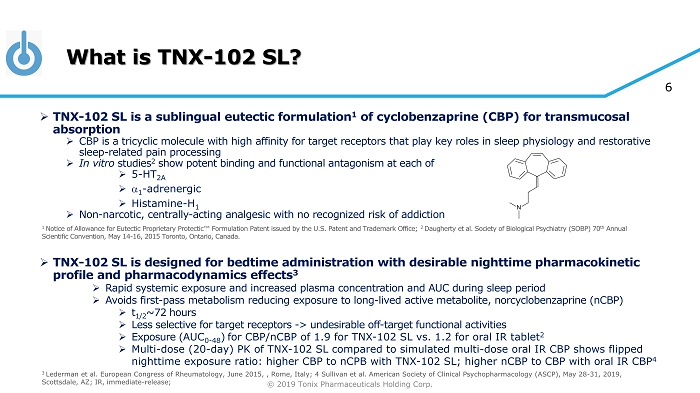
© 2019 Tonix Pharmaceuticals Holding Corp. 6 What is TNX - 102 SL? » TNX - 102 SL is a sublingual eutectic formulation 1 of cyclobenzaprine (CBP) for transmucosal absorption » CBP is a tricyclic molecule with high affinity for target receptors that play key roles in sleep physiology and restorative sleep - related pain processing » In vitro studies 2 show potent binding and functional antagonism at each of » 5 - HT 2A » a 1 - adrenergic » Histamine - H 1 » Non - narcotic, centrally - acting analgesic with no recognized risk of addiction » TNX - 102 SL is designed for bedtime administration with desirable nighttime pharmacokinetic profile and pharmacodynamics effects 3 » Rapid systemic exposure and increased plasma concentration and AUC during sleep period » Avoids first - pass metabolism reducing exposure to long - lived active metabolite, norcyclobenzaprine (nCBP) » t 1/2 ~72 hours » Less selective for target receptors - > undesirable off - target functional activities » Exposure ( AUC 0 - 48 ) for CBP/nCBP of 1.9 for TNX - 102 SL vs. 1.2 for oral IR tablet 2 » Multi - dose (20 - day) PK of TNX - 102 SL compared to simulated multi - dose oral IR CBP shows flipped nighttime exposure ratio: higher CBP to nCPB with TNX - 102 SL; higher nCBP to CBP with oral IR CBP 4 3 Lederman et al. European Congress of Rheumatology, June 2015, , Rome, Italy; 4 Sullivan et al. American Society of Clinical P syc hopharmacology (ASCP), May 28 - 31, 2019, Scottsdale, AZ; IR, immediate - release; 1 Notice of Allowance for Eutectic Proprietary Protectic ™ Formulation Patent issued by the U.S. Patent and Trademark Office; 2 Daugherty et al. Society of Biological Psychiatry (SOBP) 70 th Annual Scientific Convention, May 14 - 16, 2015 Toronto, Ontario, Canada.
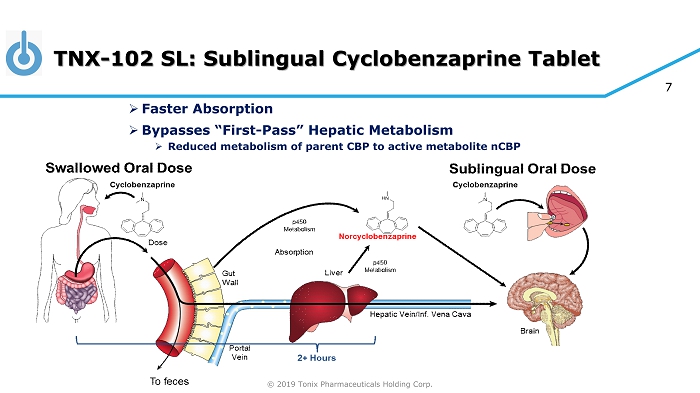
© 2019 Tonix Pharmaceuticals Holding Corp. 7 TNX - 102 SL: Sublingual Cyclobenzaprine Tablet » Faster Absorption » Bypasses “First - Pass” Hepatic Metabolism » Reduced metabolism of parent CBP to active metabolite nCBP
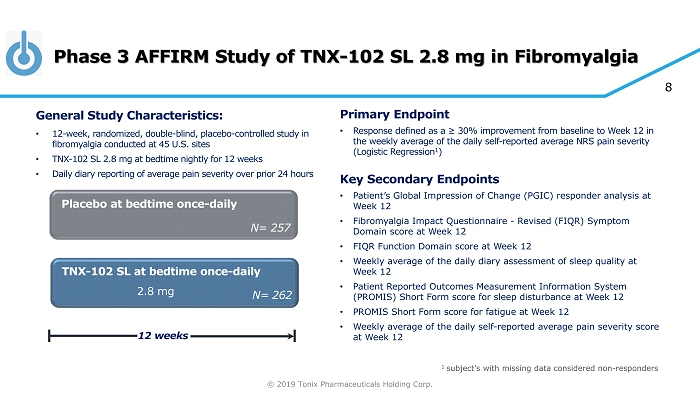
© 2019 Tonix Pharmaceuticals Holding Corp. 8 Phase 3 AFFIRM Study of TNX - 102 SL 2.8 mg in Fibromyalgia TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 262 N= 257 2.8 mg General S tudy C haracteristics: • 12 - week, r andomized, double - blind, placebo - controlle d study in fibromyalgia conducted at 45 U.S. sites • TNX - 102 SL 2.8 mg at bedtime nightly for 12 weeks • Daily diary reporting of average pain severity over prior 24 hours Primary E ndpoint • Response defined as a ≥ 30% improvement from baseline to Week 12 in the weekly average of the daily self - reported average NRS pain severity (Logistic Regression 1 ) Key Secondary E ndpoint s • Patient’s Global Impression of Change (PGIC) responder analysis at Week 12 • Fibromyalgia Impact Questionnaire - Revised (FIQR) Symptom Domain score at Week 12 • FIQR Function Domain score at Week 12 • Weekly average of the daily diary assessment of sleep quality at Week 12 • Patient Reported Outcomes Measurement Information System (PROMIS) Short Form score for sleep disturbance at Week 12 • PROMIS Short Form score for fatigue at Week 12 • Weekly average of the daily self - reported average pain severity score at Week 12 1 subject’s with missing data considered non - responders
© 2019 Tonix Pharmaceuticals Holding Corp. 9 AFFIRM Study Results: Primary Analysis » Primary Analysis of Pain Response in FM (TNX - 102 SL N=262; Placebo N=257) » Logistic regression (LR) responder analysis (≥30% pain reduction); all discontinuations considered non - responders » TNX - 102 SL 28.6% ; Placebo 22.6% ; Odds Ratio (95% CI) of 1.41 (0.94, 2.10), p=0.095, NS » AFFIRM Study did not meet its primary endpoint CI, confidence interval; LR, logistic regression; N, number; NS, not significant; OR, odds ratio
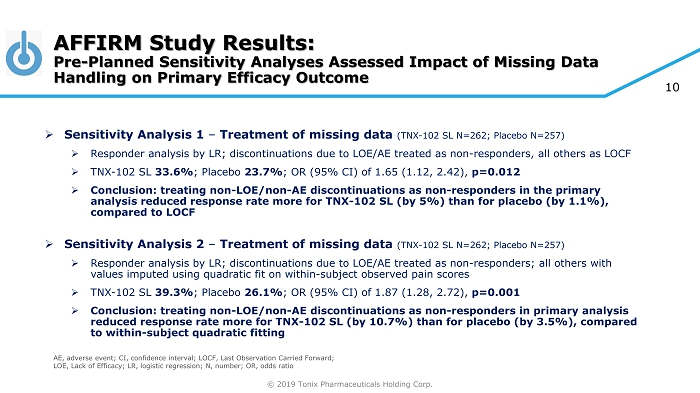
© 2019 Tonix Pharmaceuticals Holding Corp. 10 AFFIRM Study Results: Pre - Planned Sensitivity Analyses Assessed Impact of Missing Data Handling on Primary Efficacy Outcome » Sensitivity Analysis 1 – Treatment of missing data (TNX - 102 SL N=262; Placebo N=257) » Responder analysis by LR; discontinuations due to LOE/AE treated as non - responders, all others as LOCF » TNX - 102 SL 33.6% ; Placebo 23.7% ; OR (95% CI) of 1.65 (1.12, 2.42), p=0.012 » Conclusion: treating non - LOE/non - AE discontinuations as non - responders in the primary analysis reduced response rate more for TNX - 102 SL (by 5%) than for placebo (by 1.1%), compared to LOCF » Sensitivity Analysis 2 – Treatment of missing data (TNX - 102 SL N=262; Placebo N=257) » Responder analysis by LR; discontinuations due to LOE/AE treated as non - responders; all others with values imputed using quadratic fit on within - subject observed pain scores » TNX - 102 SL 39.3% ; Placebo 26.1% ; OR (95% CI) of 1.87 (1.28, 2.72), p=0.001 » Conclusion: treating non - LOE/non - AE discontinuations as non - responders in primary analysis reduced response rate more for TNX - 102 SL (by 10.7%) than for placebo (by 3.5%), compared to within - subject quadratic fitting AE, adverse event; CI, confidence interval; LOCF, Last Observation Carried Forward; LOE, Lack of Efficacy; LR, logistic regression; N, number; OR, odds ratio
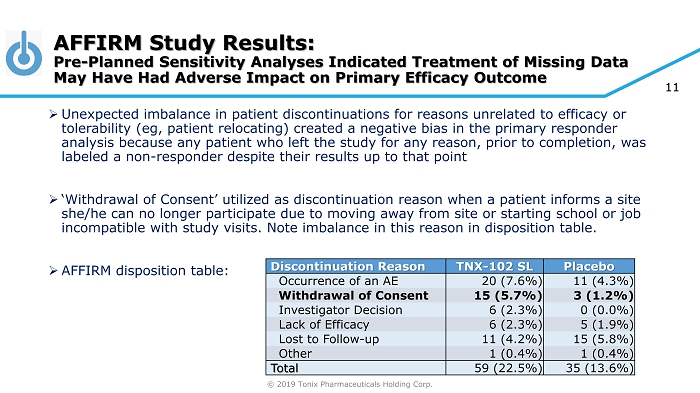
© 2019 Tonix Pharmaceuticals Holding Corp. 11 » Unexpected imbalance in patient discontinuations for reasons unrelated to efficacy or tolerability ( eg , patient relocating) created a negative bias in the primary responder analysis because any patient who left the study for any reason, prior to completion, was labeled a non - responder despite their results up to that point » ‘Withdrawal of Consent’ utilized as discontinuation reason when a patient informs a site she/he can no longer participate due to moving away from site or starting school or job incompatible with study visits. Note imbalance in this reason in disposition table. » AFFIRM disposition table: AFFIRM Study Results: Pre - Planned Sensitivity Analyses Indicated Treatment of Missing Data May Have Had Adverse Impact on Primary Efficacy Outcome Discontinuation Reason TNX - 102 SL Placebo Occurrence of an AE 20 (7.6%) 11 (4.3%) Withdrawal of Consent 15 (5.7%) 3 (1.2%) Investigator Decision 6 (2.3%) 0 (0.0%) Lack of Efficacy 6 (2.3%) 5 (1.9%) Lost to Follow - up 11 (4.2%) 15 (5.8%) Other 1 (0.4%) 1 (0.4%) Total 59 (22.5%) 35 (13.6%)
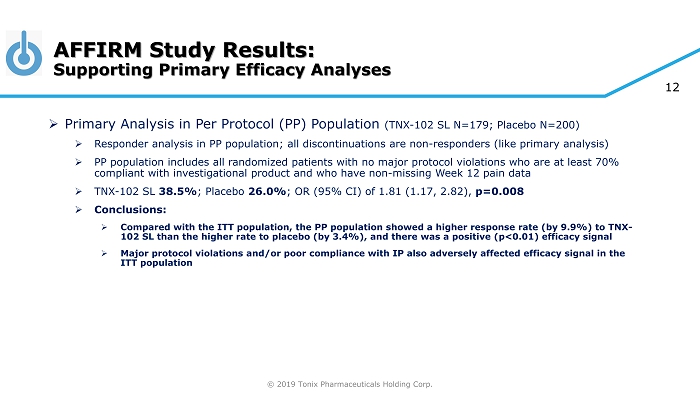
© 2019 Tonix Pharmaceuticals Holding Corp. 12 AFFIRM Study Results: Supporting Primary Efficacy Analyses » Primary Analysis in Per Protocol (PP) Population (TNX - 102 SL N=179; Placebo N=200) » Responder analysis in PP population; all discontinuations are non - responders (like primary analysis) » PP population includes all randomized patients with no major protocol violations who are at least 70% compliant with investigational product and who have non - missing Week 12 pain data » TNX - 102 SL 38.5% ; Placebo 26.0% ; OR (95% CI) of 1.81 (1.17, 2.82), p=0.008 » Conclusions: » Compared with the ITT population, the PP population showed a higher response rate (by 9.9%) to TNX - 102 SL than the higher rate to placebo (by 3.4%), and there was a positive (p<0.01) efficacy signal » Major protocol violations and/or poor compliance with IP also adversely affected efficacy signal in the ITT population
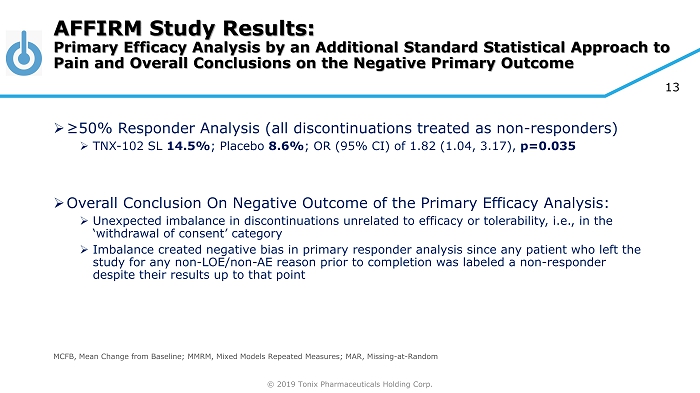
© 2019 Tonix Pharmaceuticals Holding Corp. 13 » ≥50% Responder Analysis (all discontinuations treated as non - responders) » TNX - 102 SL 14.5% ; Placebo 8.6% ; OR (95% CI) of 1.82 (1.04, 3.17), p=0.035 » Overall Conclusion On Negative Outcome of the Primary Efficacy Analysis: » Unexpected imbalance in discontinuations unrelated to efficacy or tolerability, i.e., in the ‘withdrawal of consent’ category » Imbalance created negative bias in primary responder analysis since any patient who left the study for any non - LOE/non - AE reason prior to completion was labeled a non - responder despite their results up to that point AFFIRM Study Results: Primary Efficacy Analysis by an Additional Standard Statistical Approach to Pain and Overall Conclusions on the Negative Primary Outcome MCFB, Mean Change from Baseline; MMRM, Mixed Models Repeated Measures; MAR, Missing - at - Random
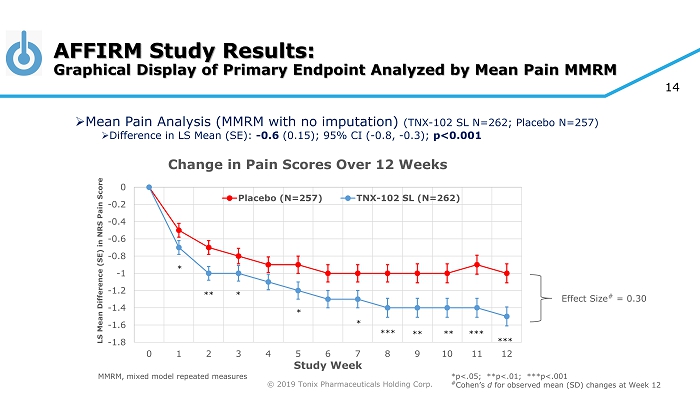
© 2019 Tonix Pharmaceuticals Holding Corp. 14 Effect Size # = 0.30 *p<.05; **p<.01; ***p<.001 # Cohen’s d for observed mean (SD) changes at Week 12 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks Placebo (N=257) TNX-102 SL (N=262) * ** *** * * * *** *** ** ** AFFIRM Study Results: Graphical Display of Primary Endpoint Analyzed by Mean Pain MMRM MMRM, mixed model repeated measures » Mean Pain Analysis (MMRM with no imputation) (TNX - 102 SL N=262; Placebo N=257) » Difference in LS Mean (SE): - 0.6 (0.15); 95% CI ( - 0.8, - 0.3); p<0.001
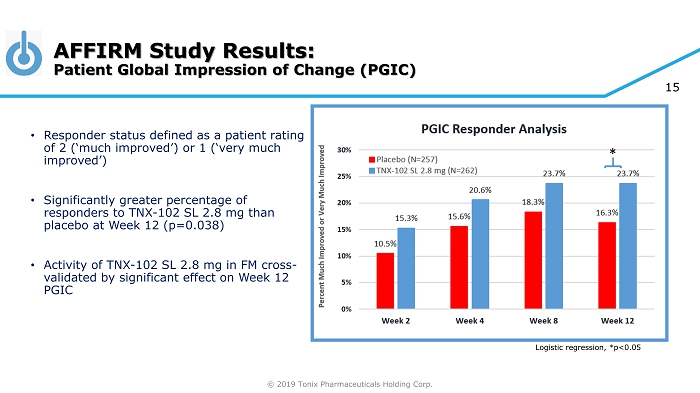
© 2019 Tonix Pharmaceuticals Holding Corp. 15 • Responder status defined as a patient rating of 2 (‘much improved’) or 1 (‘very much improved’) • Significantly greater percentage of responders to TNX - 102 SL 2.8 mg than placebo at Week 12 (p=0.038) • Activity of TNX - 102 SL 2.8 mg in FM cross - validated by significant effect on Week 12 PGIC AFFIRM Study Results: Patient Global Impression of Change (PGIC) Logistic regression, *p<0.05
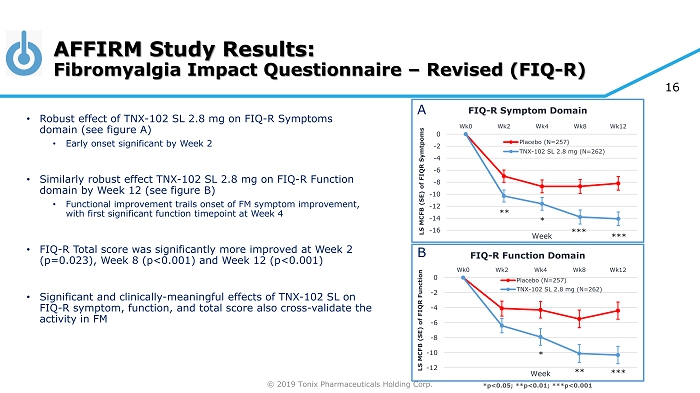
© 2019 Tonix Pharmaceuticals Holding Corp. AFFIRM Study Results: Fibromyalgia Impact Questionnaire – Revised (FIQ - R) -16 -14 -12 -10 -8 -6 -4 -2 0 Wk0 Wk2 Wk4 Wk8 Wk12 LS MCFB (SE) of FIQR Symtpoms Week FIQ - R Symptom Domain Placebo (N=257) TNX-102 SL 2.8 mg (N=262) ** *** *** * -12 -10 -8 -6 -4 -2 0 Wk0 Wk2 Wk4 Wk8 Wk12 LS MCFB (SE) of FIQR Function Week FIQ - R Function Domain Placebo (N=257) TNX-102 SL 2.8 mg (N=262) ** *** * *p<0.05; **p<0.01; ***p<0.001 • Robust effect of TNX - 102 SL 2.8 mg on FIQ - R Symptoms domain (see figure A) • Early onset significant by Week 2 • Similarly robust effect TNX - 102 SL 2.8 mg on FIQ - R Function domain by Week 12 (see figure B) • Functional improvement trails onset of FM symptom improvement, with first significant function timepoint at Week 4 • FIQ - R Total score was significantly more improved at Week 2 (p=0.023), Week 8 (p<0.001) and Week 12 (p<0.001) • Significant and clinically - meaningful effects of TNX - 102 SL on FIQ - R symptom, function, and total score also cross - validate the activity in FM A B 16
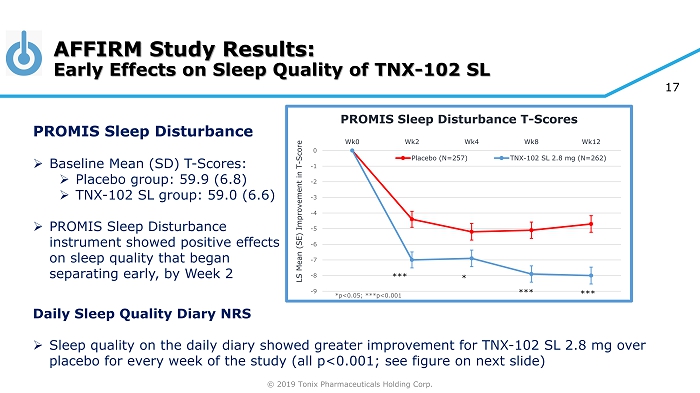
© 2019 Tonix Pharmaceuticals Holding Corp. 17 AFFIRM Study Results: Early Effects on Sleep Quality of TNX - 102 SL -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Wk0 Wk2 Wk4 Wk8 Wk12 LS Mean (SE) Improvement in T - Score PROMIS Sleep Disturbance T - Scores Placebo (N=257) TNX-102 SL 2.8 mg (N=262) *** *** *** * Daily Sleep Quality Diary NRS » Sleep quality on the daily diary showed greater improvement for TNX - 102 SL 2.8 mg over placebo for every week of the study (all p<0.001; see figure on next slide) PROMIS Sleep Disturbance » Baseline Mean (SD) T - Scores: » Placebo group: 59.9 (6.8) » TNX - 102 SL group: 59.0 (6.6) » PROMIS Sleep Disturbance instrument showed positive effects on sleep quality that began separating early, by Week 2 *p<0.05; ***p<0.001
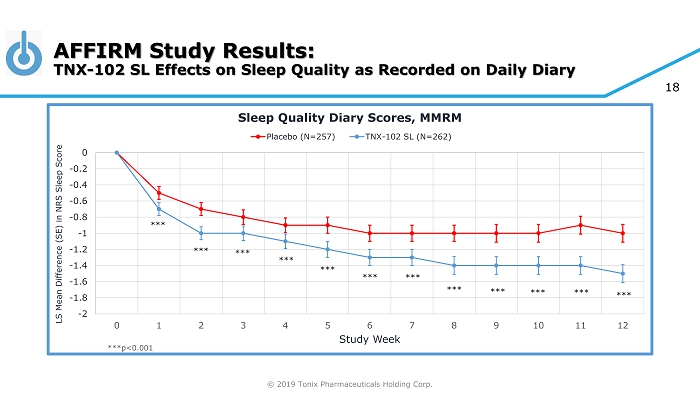
© 2019 Tonix Pharmaceuticals Holding Corp. 18 AFFIRM Study Results: TNX - 102 SL Effects on Sleep Quality as Recorded on Daily Diary -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Sleep Score Study Week Sleep Quality Diary Scores, MMRM Placebo (N=257) TNX-102 SL (N=262) *** *** *** *** *** *** *** *** *** *** *** *** ***p<0.001
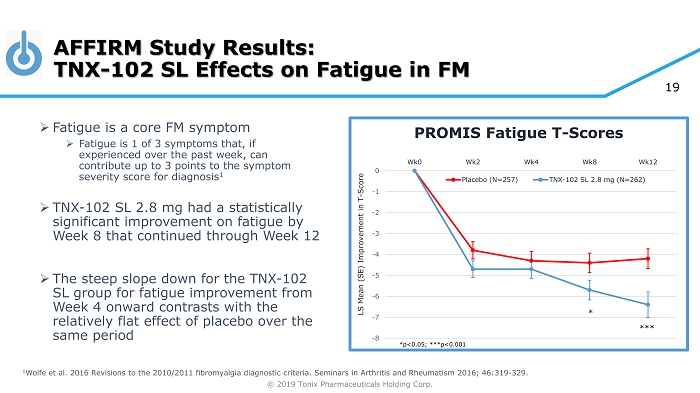
© 2019 Tonix Pharmaceuticals Holding Corp. 19 AFFIRM Study Results: TNX - 102 SL Effects on Fatigue in FM » Fatigue is a core FM symptom » Fatigue is 1 of 3 symptoms that, if experienced over the past week, can contribute up to 3 points to the symptom severity score for diagnosis 1 » TNX - 102 SL 2.8 mg had a statistically significant improvement on fatigue by Week 8 that continued through Week 12 » The steep slope down for the TNX - 102 SL group for fatigue improvement from Week 4 onward contrasts with the relatively flat effect of placebo over the same period -8 -7 -6 -5 -4 -3 -2 -1 0 Wk0 Wk2 Wk4 Wk8 Wk12 LS Mean (SE) Improvement in T - Score PROMIS Fatigue T - Scores Placebo (N=257) TNX-102 SL 2.8 mg (N=262) *** * 1 Wolfe et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Seminars in Arthritis and Rheumatism 2016; 46: 319 - 329. *p<0.05; ***p<0.001
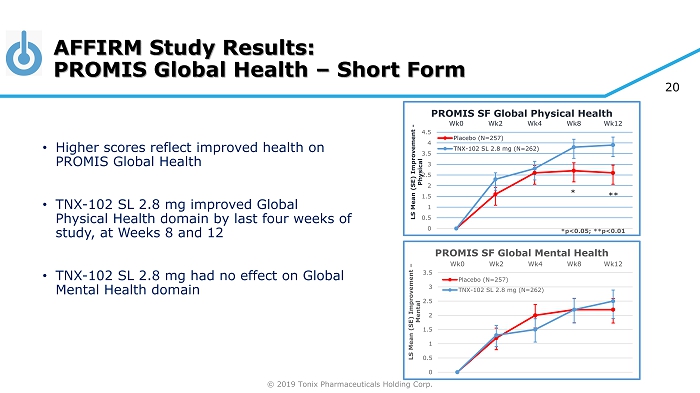
© 2019 Tonix Pharmaceuticals Holding Corp. AFFIRM Study Results: PROMIS Global Health – Short Form *p<0.05; **p<0.01 • Higher scores reflect improved health on PROMIS Global Health • TNX - 102 SL 2.8 mg improved Global Physical Health domain by last four weeks of study, at Weeks 8 and 12 • TNX - 102 SL 2.8 mg had no effect on Global Mental Health domain 0 0.5 1 1.5 2 2.5 3 3.5 4 4.5 Wk0 Wk2 Wk4 Wk8 Wk12 LS Mean (SE) Improvement - Physical PROMIS SF Global Physical Health Placebo (N=257) TNX-102 SL 2.8 mg (N=262) ** * 0 0.5 1 1.5 2 2.5 3 3.5 Wk0 Wk2 Wk4 Wk8 Wk12 LS Mean (SE) Improvement – Mental PROMIS SF Global Mental Health Placebo (N=257) TNX-102 SL 2.8 mg (N=262) 20
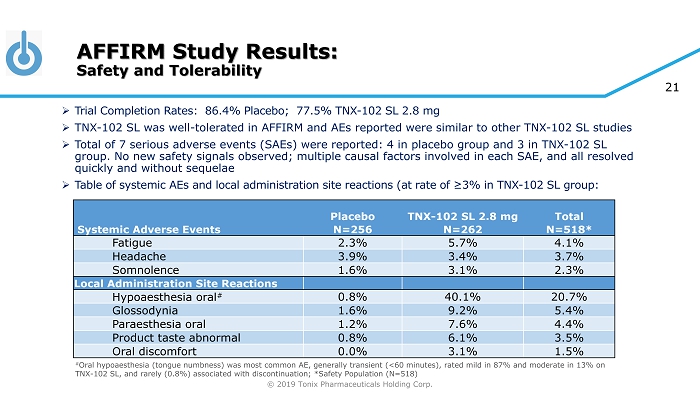
© 2019 Tonix Pharmaceuticals Holding Corp. 21 » Trial Completion Rates: 86.4% Placebo; 77.5% TNX - 102 SL 2.8 mg » TNX - 102 SL was well - tolerated in AFFIRM and AEs reported were similar to other TNX - 102 SL studies » Total of 7 serious adverse events (SAEs) were reported: 4 in placebo group and 3 in TNX - 102 SL group. No new safety signals observed; multiple causal factors involved in each SAE, and all resolved quickly and without sequelae » Table of systemic AEs and local administration site reactions (at rate of ≥3% in TNX - 102 SL group: # Oral hypoaesthesia (tongue numbness) was most common AE, generally transient (<60 minutes), rated mild in 87% and moderate in 13 % on TNX - 102 SL, and rarely (0.8%) associated with discontinuation; *Safety Population (N=518) AFFIRM Study Results: Safety and Tolerability Placebo TNX - 102 SL 2.8 mg Total Systemic Adverse Events N=256 N=262 N=518* Fatigue 2.3% 5.7% 4.1% Headache 3.9% 3.4% 3.7% Somnolence 1.6% 3.1% 2.3% Local Administration Site Reactions Hypoaesthesia oral # 0.8% 40.1% 20.7% Glossodynia 1.6% 9.2% 5.4% Paraesthesia oral 1.2% 7.6% 4.4% Product taste abnormal 0.8% 6.1% 3.5% Oral discomfort 0.0% 3.1% 1.5%
© 2019 Tonix Pharmaceuticals Holding Corp. 22 What was learned about dose and tolerability from studies of TNX - 102 SL in PTSD? • For overall PTSD symptoms, TNX - 102 SL 5.6 mg showed an efficacy signal, whereas the 2.8 mg dose did not • The most common AE with TNX - 102 SL, oral hypoaesthesia, occurred at similar rates with 2.8 mg (39%) and 5.6 mg (36%) in AtEase , and 5.6 mg (37%) in HONOR. • Rate of oral hypoaesthesia does not appear to be dose dependent • TNX - 102 SL 5.6 mg was well tolerated with only systemic AE of somnolence at a consistently higher rate (at ~16%) than in 2.8 mg • In - clinic Past 24 - hr average NRS pain ratings were collected at each visit in the military PTSD study AtEase (P201; data on next slide)
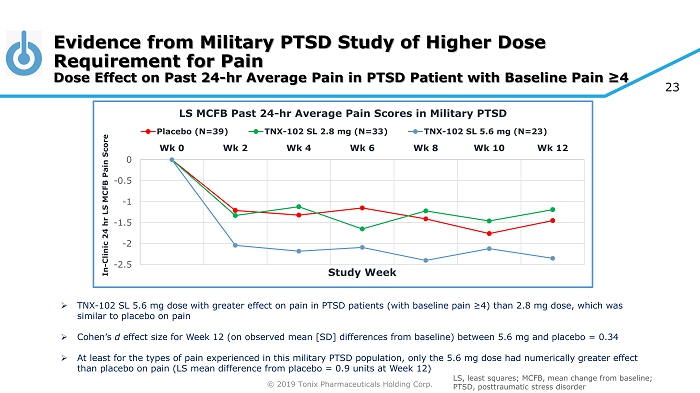
© 2019 Tonix Pharmaceuticals Holding Corp. 23 Evidence from Military PTSD Study of Higher Dose Requirement for Pain Dose Effect on Past 24 - hr Average Pain in PTSD Patient with Baseline Pain ≥4 -2.5 -2 -1.5 -1 -0.5 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 In - Clinic 24 hr LS MCFB Pain Score Study Week LS MCFB Past 24 - hr Average Pain Scores in Military PTSD Placebo (N=39) TNX-102 SL 2.8 mg (N=33) TNX-102 SL 5.6 mg (N=23) » TNX - 102 SL 5.6 mg dose with greater effect on pain in PTSD patients (with baseline pain ≥4) than 2.8 mg dose, which was similar to placebo on pain » Cohen’s d effect size for Week 12 (on observed mean [SD] differences from baseline) between 5.6 mg and placebo = 0.34 » At least for the types of pain experienced in this military PTSD population, only the 5.6 mg dose had numerically greater eff ect than placebo on pain (LS mean difference from placebo = 0.9 units at Week 12) LS, least squares; MCFB, mean change from baseline; PTSD, posttraumatic stress disorder
© 2019 Tonix Pharmaceuticals Holding Corp. 24 Conclusions » The AFFIRM Study in fibromyalgia (FM) did not achieve significance on the primary endpoint » While the study was not significant base on the pre - specified pain responder analysis, it was significant by other standard pain analysis techniques » Results were highly sensitive to methods used to handle missing data » Significant effects on PGIC and FIQ - R cross - validated the activity of TNX - 102 SL in FM » Improved effects on sleep quality, present within the 1 st week and throughout treatment, support the mechanistic hypothesis that TNX - 102 SL has positive effects on FM mediated by improvements in sleep quality » Broad based effects on FM symptoms also evident from statistically significant effects on PROMIS Fatigue Scale and Global Physical Health domain » Studies with TNX - 102 SL at 2.8 mg and 5.6 mg in PTSD suggested higher dose more effective on pain, and the 5.6 mg dose is generally well - tolerated » Therefore, the planned next phase 3 study of TNX - 102 SL in FM will utilize the 5.6 mg dose with the expectation of a larger effect on FM pain and symptoms than the 2.8 mg dose used in AFFIRM

© 2019 Tonix Pharmaceuticals Holding Corp. Thank you ! Co - Authors: R. Michael Gendreau, Gendreau Consulting Judith Gendreau, Gendreau Consulting Ashild Peters, Tonix Pharmaceuticals Inc Perry Peters, Tonix Pharmaceuticals Inc Seth Lederman, Tonix Pharmaceuticals Inc