
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.01

© 2020 Tonix Pharmaceuticals Holding Corp. January 2020 Version P0215 1 - 6 - 20 (Doc 0580) Investor Presentation

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2018 , as filed with the Securities and Exchange Commission (the “SEC”) on March 18 , 2019 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals Who we are: • A clinical stage biopharmaceutical company dedicated to developing innovative treatments for psychiatric, pain and addiction conditions What we do: • Target therapeutic areas with high need for improvement − Conditions with no, or inadequate, treatments − Significant patient populations not well served by existing therapies • Develop innovative treatment options with possibility to be a “game changer” − Scientifically unique and innovative − Strong scientific rationale supported by clinical evidence − Proven regulatory pathways and established clinical endpoints − Built on a foundation of proprietary intellectual property
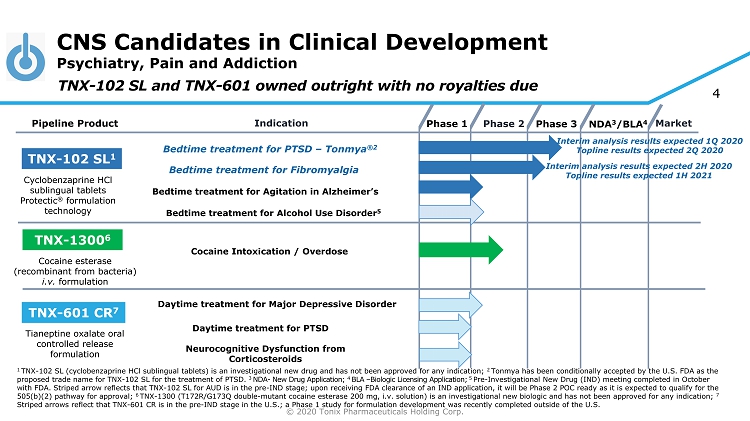
© 2020 Tonix Pharmaceuticals Holding Corp. 4 CNS Candidates in Clinical Development Psychiatry, Pain and Addiction Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime treatment for PTSD – Tonmya ®2 Daytime treatment for PTSD TNX - 601 CR 7 TNX - 1300 6 Cocaine Intoxication / Overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral controlled release formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 Bedtime t reatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due Bedtime t reatment for Agitation in Alzheimer’s Neurocognitive Dysfunction from Corticosteroids Bedtime treatment for Alcohol Use Disorder 5 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 Pre - Investigational New Drug (IND) meeting completed in October with FDA. Striped arrow reflects that TNX - 102 SL for AUD is in the pre - IND stage; upon receiving FDA clearance of an IND applica tion, it will be Phase 2 POC ready as it is expected to qualify for the 505(b)(2) pathway for approval; 6 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 7 Striped arrows reflect that TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 study for formulation development was recently completed outside of th e U.S. Interim analysis results expected 1Q 2020 Topline results expected 2Q 2020 Interim analysis results expected 2H 2020 Topline results expected 1H 2021 Daytime treatment for Major Depressive Disorder
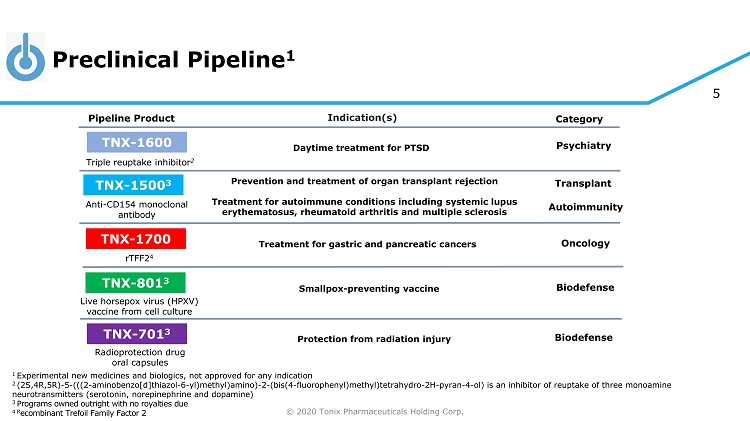
© 2020 Tonix Pharmaceuticals Holding Corp. 5 Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) 3 Programs owned outright with no royalties due 4 R ecombinant Trefoil Family Factor 2 Category Pipeline Product Indication(s) TNX - 801 3 Smallpox - preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture TNX - 701 3 Protection from radiation injury Radioprotection drug oral capsules Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for PTSD Psychiatry TNX - 1500 3 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Treatment for autoimmune conditions including systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Biodefense Biodefense

© 2020 Tonix Pharmaceuticals Holding Corp. 6 TNX - 102 SL Intellectual Property – U.S. Protection expected until 2035 • United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 9636408 in May 2017, U.S. Patent No. 9956188 in May 2018, U.S. Patent No. 10117936 in Nov 2018, and U.S. Patent No. 10,357,465 in July 2019 • European Patent Office (EPO) issued Patent No. 2968992 in December 2019 • China National Intellectual Property Administration issued Chinese Patent No. ZL 201480024011.1 in April 2019 • Indonesian Patent Office issued Indonesian Patent No. IDP000055516 in January 2019 • Saudi Arabian Patent Office issued Saudi Patent No. 6088 in September 2018 • Japanese Patent Office (JPO) issued Japanese Patent No. 6310542 in March 2018 • New Zealand Intellectual Property Office (NZIPO) issued New Zealand Patent No. 631152 in August 2017 • 35 patent applications pending (5 being allowed in U.S, Australia, Europe, Taiwan, South Africa) Composition of matter (eutectic): Protection expected to 2034/2035 • NZIPO issued New Zealand Patent No. 631144 in March 2017 and Patent No. 726488 in January 2019 • Taiwanese Intellectual Property Office issued Taiwanese Patent No. I590820 in July 2017 and Patent No. I642429 in December 2018 • Australian Patent Office issued Australian Patent No. 2013274003 in October 2018 • JPO issued Japanese Patent No. 6259452 in Dec 2017 • 21 patent applications pending Composition of matter (sublingual): Protection expected to 2033 • Hong Kong Patent Office issued Hong Kong Patent No. HK1176235 in September 2018 • USPTO issued U.S. Patent No. 9918948 in March 2018 • European Patent Office (EPO) issued European Patent No. 2501234B1 in Sept 2017 (validated in 37 countries). In response to an opposition filed in June 2018, EPO’s Opposition Division determined in October 2019 that it will uphold this patent. • 1 patent application pending Method of use (PTSD) for cyclobenzaprine: Protection expected to 2030

© 2020 Tonix Pharmaceuticals Holding Corp. 7 Overview of Posttraumatic Stress Disorder (PTSD) PSTD is a chronic disabling disorder in response to experiencing traumatic event(s) Symptoms of PTSD fall into four clusters: 1. Intrusion (aversive memories, nightmares, flashbacks) 2. Avoidance (avoiding persons, places or situations) 3. Mood/cognitions (memory block, emotional numbing, detachment from others) 4. Hyperarousal (anxiety, agitation & sleep disturbance) Diagnosis, symptom severity, as well as treatment effect, is determined by CAPS - 5* • Recognized as the standard for rating PTSD severity in clinical trials • Takes into account all four symptom clusters • Higher Total CAPS - 5 score reflects more severe PTSD symptoms * Clinician - administered PTSD scale for Diagnostic Statistical Manual version 5 (DSM - 5)

© 2020 Tonix Pharmaceuticals Holding Corp. 8 Impact of PTSD on People Consequences: • Impaired daily function and substantial interference with work and social interactions • Reckless or destructive behavior • Increased health care utilization and greater medical morbidity PTSD as a risk factor for: • Depression • Alcohol or substance abuse • Absenteeism/unemployment • Homelessness • Violent acts • Suicidal thoughts and suicide
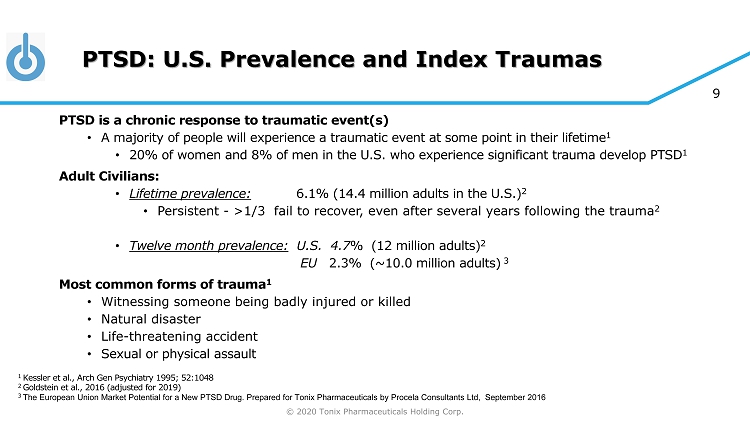
© 2020 Tonix Pharmaceuticals Holding Corp. 9 PTSD: U.S. Prevalence and Index Traumas PTSD is a chronic response to traumatic event(s) • A majority of people will experience a traumatic event at some point in their lifetime 1 • 20% of women and 8% of men in the U.S. who experience significant trauma develop PTSD 1 Adult Civilians: • Lifetime prevalence: 6.1% (14.4 million adults in the U.S.) 2 • Persistent - >1/3 fail to recover, even after several years following the trauma 2 • Twelve month prevalence: U.S. 4.7 % (12 million adults) 2 EU 2.3% (~10.0 million adults) 3 Most common forms of trauma 1 • Witnessing someone being badly injured or killed • Natural disaster • Life - threatening accident • Sexual or physical assault 1 Kessler et al., Arch Gen Psychiatry 1995; 52:1048 2 Goldstein et al., 2016 (adjusted for 2019) 3 The European Union Market Potential for a New PTSD Drug. Prepared for Tonix Pharmaceuticals by Procela Consultants Ltd, Sept emb er 2016
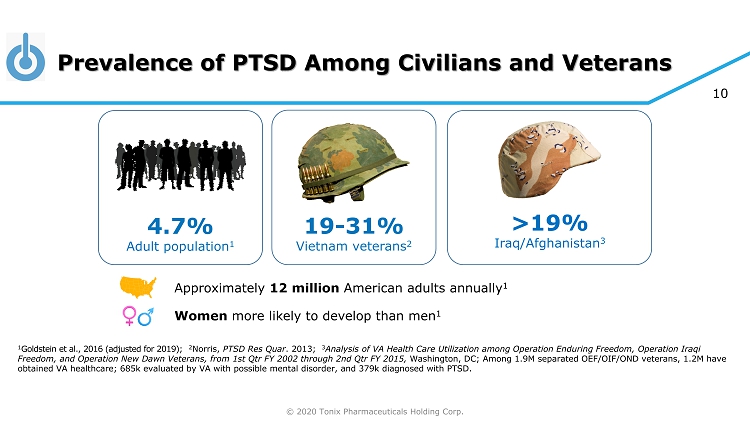
© 2020 Tonix Pharmaceuticals Holding Corp. 10 Prevalence of PTSD Among Civilians and Veterans 1 Goldstein et al., 2016 (adjusted for 2019) ; 2 Norris, PTSD Res Quar . 2013; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; Among 1.9M separated OEF/OIF/OND veterans, 1.2M have obtained VA healthcare; 685k evaluated by VA with possible mental disorder, and 379k diagnosed with PTSD. >19% Iraq/Afghanistan 3 4.7% Adult population 1 19 - 31% Vietnam veterans 2 Approximately 12 million American adults annually 1 Women more likely to develop than men 1
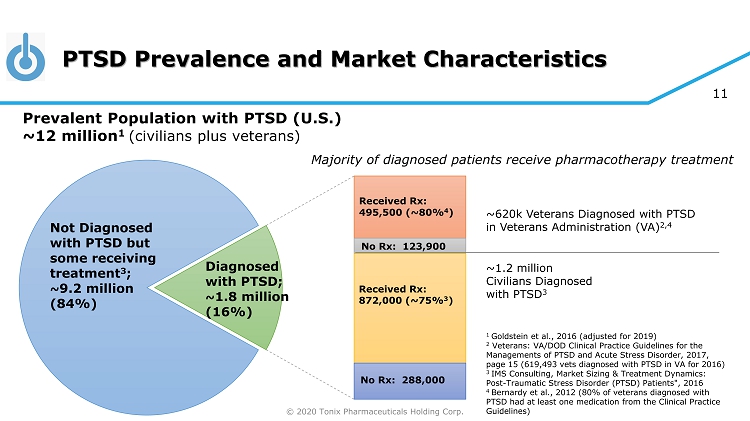
© 2020 Tonix Pharmaceuticals Holding Corp. 11 PTSD Prevalence and Market Characteristics 1 Goldstein et al., 2016 (adjusted for 2019) 2 Veterans: VA/DOD Clinical Practice Guidelines for the Managements of PTSD and Acute Stress Disorder, 2017, page 15 (619,493 vets diagnosed with PTSD in VA for 2016) 3 IMS Consulting, Market Sizing & Treatment Dynamics: Post - Traumatic Stress Disorder (PTSD) Patients", 2016 4 Bernardy et al., 2012 (80% of veterans diagnosed with PTSD had at least one medication from the Clinical Practice Guidelines) ~620k Veterans Diagnosed with PTSD in Veterans Administration (VA) 2,4 ~1.2 million Civilians Diagnosed with PTSD 3 Not Diagnosed with PTSD but some receiving treatment 3 ; ~ 9.2 million ( 84% ) Received Rx : 495,500 (~80% 4 ) No Rx : 123,900 Received Rx : 872,000 (~75% 3 ) No Rx : 288,000 Diagnosed with PTSD; ~ 1.8 million ( 16% ) Prevalent Population with PTSD (U.S.) ~12 million 1 (civilians plus veterans) Majority of diagnosed patients receive pharmacotherapy treatment
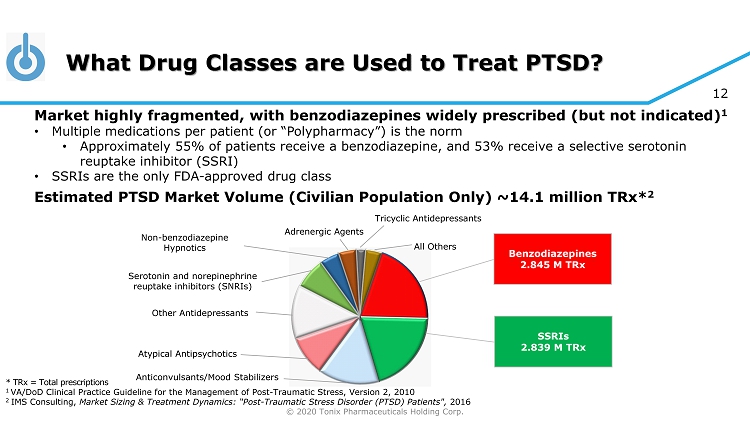
© 2020 Tonix Pharmaceuticals Holding Corp. 12 What Drug Classes are Used to Treat PTSD? * TRx = Total prescriptions 1 VA/DoD Clinical Practice Guideline for the Management of Post - Traumatic Stress, Version 2, 2010 2 IMS Consulting, Market Sizing & Treatment Dynamics: “Post - Traumatic Stress Disorder (PTSD) Patients", 2016 Benzodiazepines 2.845 M TRx SSRIs 2.839 M TRx Other Antidepressants Non - benzodiazepine Hypnotics Serotonin and norepinephrine reuptake inhibitors (SNRIs) Tricyclic Antidepressants Adrenergic Agents All Others Atypical Antipsychotics Anticonvulsants/Mood Stabilizers Market highly fragmented, with benzodiazepines widely prescribed (but not indicated) 1 • Multiple medications per patient (or “Polypharmacy”) is the norm • Approximately 55% of patients receive a benzodiazepine, and 53% receive a selective serotonin reuptake inhibitor (SSRI) • SSRIs are the only FDA - approved drug class Estimated PTSD Market Volume (Civilian Population Only) ~14.1 million TRx * 2

© 2020 Tonix Pharmaceuticals Holding Corp. 13 PTSD: Not Well - Served by Approved Treatments FDA - approved SSRIs, paroxetine and sertraline, are indicated as a treatment for PTSD • Neither drug has shown efficacy in military - related PTSD • Majority of male PTSD patients unresponsive or intolerant to current treatments • Side effects relating to sexual dysfunction, sleep disturbance and weight gain are commonly reported Characteristics of an ideal drug therapy that would be compatible and complementary with behavioral therapy • Lack of retrograde amnesia (e.g., unlike off - label use of benzodiazepines and non - benzodiazepines) • Lack of interference on sleep (e.g., unlike approved SSRIs) TNX - 102 SL is being investigated in both military and civilian PTSD and is expected to be indicated as a “treatment for PTSD”
© 2020 Tonix Pharmaceuticals Holding Corp. 14 Why Initially Targeted Military - Related PTSD? 1 Friedman et al., J Clin Psychiatry 2007; 68:711 2 Zoloft Package Insert, August, 2014 3 Paxil Package Insert, June, 2014 4 Fava et al., Psychother Psychosom 84:72 - 81, 2015 Military - related PTSD not well - served by existing FDA - approved therapies • No clear treatment response observed in U.S. military population Sertraline: failed to show efficacy in a large multicenter trial in U.S. military (placebo numerically better) 1 Paroxetine: no large trials conducted with predominantly military trauma • Inconsistent treatment response observed in males Sertraline: FDA - conducted post - hoc analysis concluded no effect for male civilian subgroup 2 Paroxetine: no sex - related difference in treatment outcomes 3 • Important tolerability issues with SSRIs in this population Sexual dysfunction 2,3 Insomnia 2,3 SSRI withdrawal syndrome 4
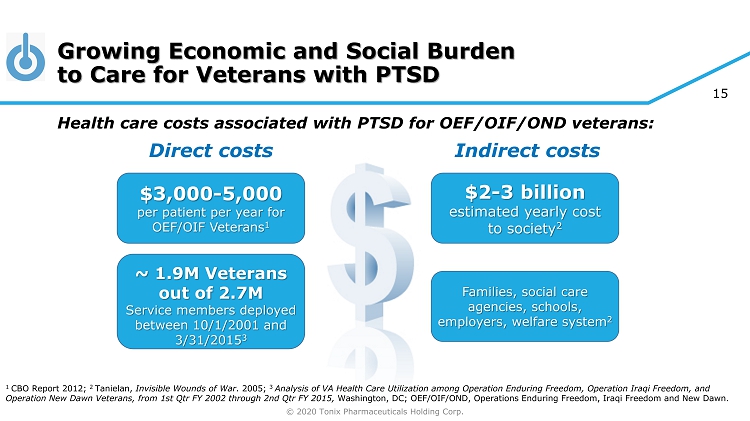
© 2020 Tonix Pharmaceuticals Holding Corp. 15 Growing Economic and Social Burden to Care for Veterans with PTSD Health care costs associated with PTSD for OEF/OIF/OND veterans: Direct costs Indirect costs 1 CBO Report 2012; 2 Tanielan , Invisible Wounds of War . 2005; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; OEF/OIF/OND, Operations Enduring Freedom, Iraqi Freedom and New Dawn. $3,000 - 5,000 per patient per year for OEF/OIF Veterans 1 ~ 1.9M Veterans out of 2.7M Service members deployed between 10/1/2001 and 3/31/2015 3 $2 - 3 billion estimated yearly cost to society 2 Families, social care agencies, schools, employers, welfare system 2
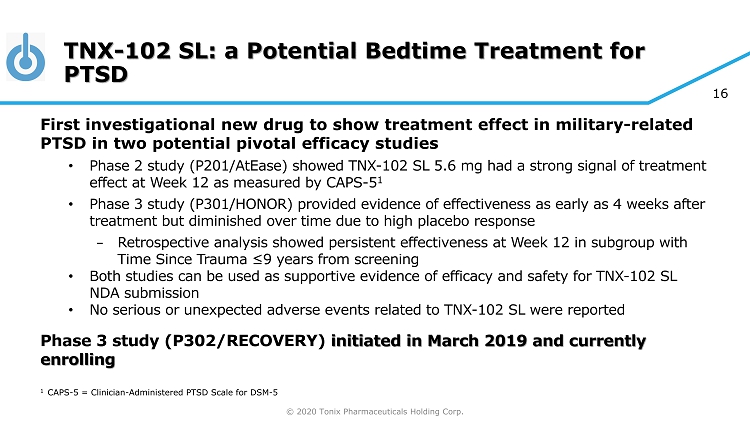
© 2020 Tonix Pharmaceuticals Holding Corp. 16 TNX - 102 SL: a Potential Bedtime Treatment for PTSD First investigational new drug to show treatment effect in military - related PTSD in two potential pivotal efficacy studies • Phase 2 study (P201/ AtEase ) showed TNX - 102 SL 5.6 mg had a strong signal of treatment effect at Week 12 as measured by CAPS - 5 1 • Phase 3 study (P301/HONOR) provided evidence of effectiveness as early as 4 weeks after treatment but diminished over time due to high placebo response − Retrospective analysis showed persistent effectiveness at Week 12 in subgroup with Time Since Trauma ≤9 years from screening • Both studies can be used as supportive evidence of efficacy and safety for TNX - 102 SL NDA submission • No serious or unexpected adverse events related to TNX - 102 SL were reported Phase 3 study (P302/RECOVERY) initiated in March 2019 and currently enrolling 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5

© 2020 Tonix Pharmaceuticals Holding Corp. 17 No Recognized Abuse Potential in Clinical Studies Active ingredient is cyclobenzaprine, which is structurally related to tricyclic antidepressants • Cyclobenzaprine interacts with receptors that regulate sleep quality: 5 - HT 2A, a 1 - adrenergic and histamine H 1 receptors • Cyclobenzaprine does NOT interact with the same receptors as traditional hypnotic sleep drugs, benzodiazepines or non - benzodiazepines that are associated with retrograde amnesia • Cyclobenzaprine - containing product was approved 40 years ago and current labeling (May 2016) indicates no abuse or dependence concern TNX - 102 SL NDA can be filed without drug abuse and dependency assessment studies • April 2017 m eeting minutes from the March 2017 FDA meeting

© 2020 Tonix Pharmaceuticals Holding Corp. 18 TNX - 102 SL: Sublingual Formulation is Designed for Bedtime Administration TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption • Innovation by design with patent protected CBP/mannitol eutectic • Rapid systemic exposure • Increases bioavailability during sleep • Avoids first - pass metabolism • Lowers exposure to long - lived active major metabolite, norcyclobenzaprine ( norCBP ) CBP undergoes extensive first - pass hepatic metabolism when orally ingested • Active major metabolite, norCBP 1 • Long half - life (~72 hours) • Less selective for target receptors ( 5 - HT 2A, a 1 - adrenergic, histamine H 1 ) • More selective for norepinephrine transporter and muscarinic M 1 TNX - 102 SL 505(b)(2) NDA approval can rely on the safety of the reference listed drug (AMRIX ® ) 2 1 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015, Toronto Ont ario, Canada 2 FDA Minutes (November 26, 2018)
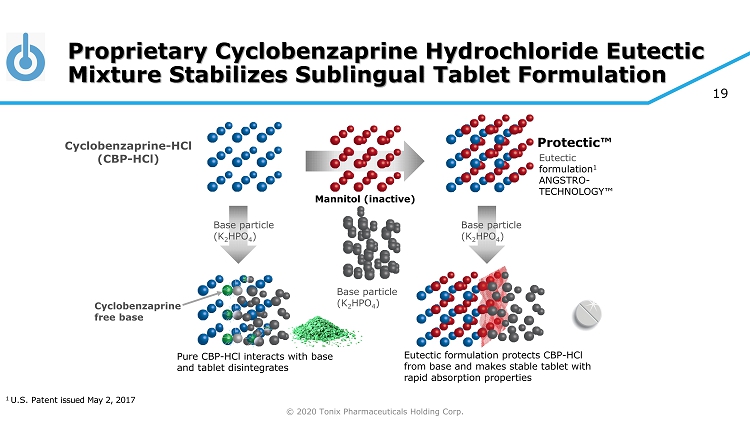
© 2020 Tonix Pharmaceuticals Holding Corp. 19 Proprietary Cyclobenzaprine Hydrochloride Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Eutectic formulation 1 ANGSTRO - TECHNOLOGY™ Cyclobenzaprine - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cyclobenzaprine free base Protectic™ 1 U.S. Patent issued May 2, 2017 Mannitol (inactive)

© 2020 Tonix Pharmaceuticals Holding Corp. 20 TNX - 102 SL: Hypothesized Novel Mechanism Targets Sleep Quality for Recovery from PTSD PTSD is a disorder of recovery • Most people exposed to extreme trauma recover over a few weeks • In PTSD, recovery process impeded due to insufficient sleep - dependent memory processing 1,2 Memory processing is essential to recovery • Vulnerability to memory intrusions and trauma triggers remains if no consolidation of new learning (extinction) TNX - 102 SL targets sleep quality 3 • The active ingredient in TNX - 102 SL, cyclobenzaprine, interacts with receptors that regulate sleep quality: strongly binds and potently blocks 5 - HT 2A , a 1 - adrenergic and histamine H 1 receptors, permissive to sleep - dependent recovery processes 1 Straus LD, Acheson DT, Risbrough VB, Drummond SPA. Sleep Deprivation Disrupts Recall of Conditioned Fear Extinction. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017; 2(2):123 - 129. 2 Murkar ALA, De Koninck J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep Med Rev. 2018; 41:173 - 184. 3 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015, Toronto Ont ario, Canada
© 2020 Tonix Pharmaceuticals Holding Corp. 21 • Generally, serotonin (5 - HT) activity promotes the awake state and inhibits REM sleep; whereas once in REM sleep, the 5 - HT system is normally quiescent • Extinction learning is critical to recovery from trauma, and such new learning is consolidated (moving from labile short term to established long term memory) during particular stages of sleep 1,2 • Recent rodent research shows how particular brain wave patterns during REM sleep, known as “P - waves” are critical to extinction consolidation 3 • 5 - HT activation of pontine brainstem region richly expressing 5 - HT 2A receptors inhibits P - wave generation during REM 4 • Nocturnal blockage of 5 - HT 2A receptors may restore extinction consolidation by inhibition of errant 5 - HT stimulation during REM (see model in next 2 slides) Proposed Mechanism of Action of TNX - 102 SL in the Treatment of PTSD: Focus on Nocturnal 5 - HT 2A Receptor Blockade in REM 1. Pace - Schott, et al. Biology of Mood & Anxiety Disorders . 2015;5(3):1 - 19. 2. Straus et al. Biol Psych: CNNI. 2017;2(2):123 - 129. 3. Datta S, et al. J Neurosci . 2013;33(10):4561 - 4569. 4. Datta S, et al. Sleep . 2003;26(5):513 - 520.
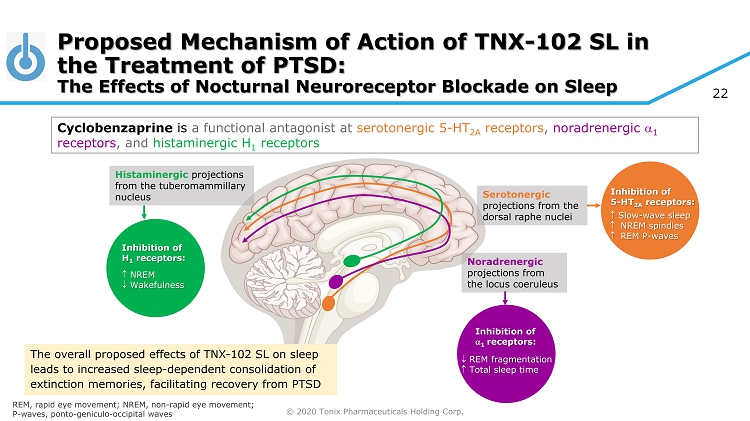
© 2020 Tonix Pharmaceuticals Holding Corp. 22 Proposed Mechanism of Action of TNX - 102 SL in the Treatment of PTSD: The Effects of Nocturnal Neuroreceptor Blockade on Sleep Cyclobenzaprine is a functional antagonist at serotonergic 5 - HT 2A receptors , noradrenergic 1 receptors , and histaminergic H 1 receptors REM, rapid eye movement; NREM, non - rapid eye movement; P - waves, ponto - geniculo - occipital waves Serotonergic projections from the dorsal raphe nuclei Inhibition of 5 - HT 2A receptors: Slow - wave sleep NREM spindles REM P - waves Noradrenergic projections from the locus coeruleus Inhibition of 1 receptors: REM fragmentation Total sleep time Histaminergic projections from the tuberomammillary nucleus Inhibition of H 1 receptors: NREM Wakefulness The overall proposed effects of TNX - 102 SL on sleep leads to increased sleep - dependent consolidation of extinction memories, facilitating recovery from PTSD
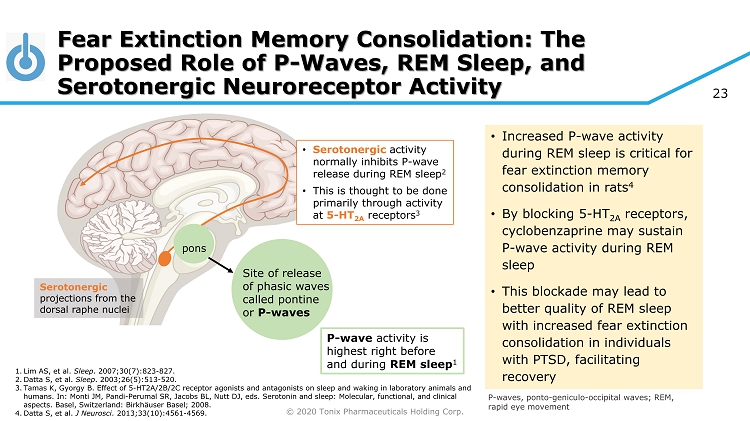
© 2020 Tonix Pharmaceuticals Holding Corp. 23 Fear Extinction Memory Consolidation: The Proposed Role of P - Waves, REM Sleep, and Serotonergic Neuroreceptor Activity Serotonergic projections from the dorsal raphe nuclei pons Site of release of phasic waves called pontine or P - waves P - wave activity is highest right before and during REM sleep 1 • Serotonergic activity normally inhibits P - wave release during REM sleep 2 • This is thought to be done primarily through activity at 5 - HT 2A receptors 3 • Increased P - wave activity during REM sleep is critical for fear extinction memory consolidation in rats 4 • By blocking 5 - HT 2A receptors, cyclobenzaprine may sustain P - wave activity during REM sleep • This blockade may lead to better quality of REM sleep with increased fear extinction consolidation in individuals with PTSD, facilitating recovery P - waves, ponto - geniculo - occipital waves; REM, rapid eye movement 1. Lim AS, et al. Sleep . 2007;30(7):823 - 827. 2. Datta S, et al. Sleep . 2003;26(5):513 - 520. 3. Tamas K, Gyorgy B. Effect of 5 - HT2A/2B/2C receptor agonists and antagonists on sleep and waking in laboratory animals and humans. In: Monti JM, Pandi - Perumal SR, Jacobs BL, Nutt DJ, eds. Serotonin and sleep: Molecular, functional, and clinical aspects. Basel, Switzerland: Birkhäuser Basel; 2008. 4. Datta S, et al. J Neurosci . 2013;33(10):4561 - 4569.
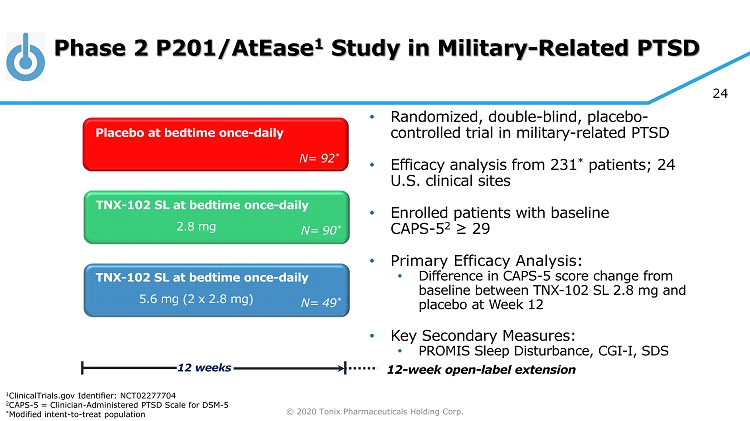
© 2020 Tonix Pharmaceuticals Holding Corp. 24 Phase 2 P201/AtEase 1 Study in Military - Related PTSD • Randomized, double - blind, placebo - controlled trial in military - related PTSD • Efficacy analysis from 231 * patients; 24 U.S. clinical sites • Enrolled patients with baseline CAPS - 5 2 ≥ 29 • Primary Efficacy Analysis: • Difference in CAPS - 5 score change from baseline between TNX - 102 SL 2.8 mg and placebo at Week 12 • Key Secondary Measures: • PROMIS Sleep Disturbance, CGI - I, SDS TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 90 * TNX - 102 SL at bedtime once - daily N= 92 * N= 49 * 2.8 mg 5.6 mg (2 x 2.8 mg) 12 - week open - label extension 1 ClinicalTrials.gov Identifier: NCT02277704 2 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 * Modified intent - to - treat population
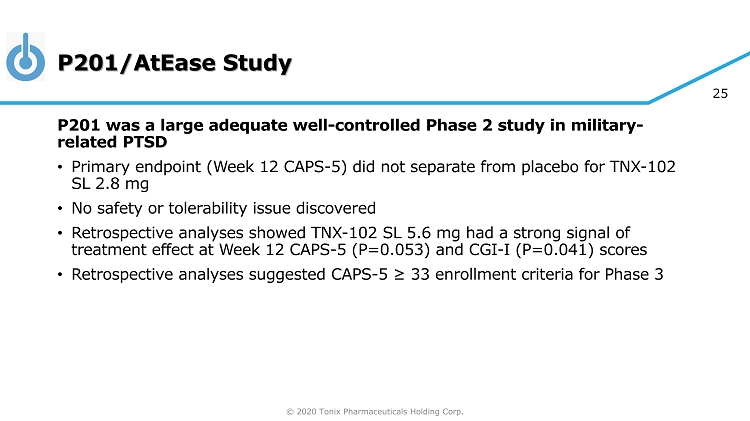
© 2020 Tonix Pharmaceuticals Holding Corp. 25 P201/AtEase Study P201 was a large adequate well - controlled Phase 2 study in military - related PTSD • Primary endpoint (Week 12 CAPS - 5) did not separate from placebo for TNX - 102 SL 2.8 mg • No safety or tolerability issue discovered • Retrospective analyses showed TNX - 102 SL 5.6 mg had a strong signal of treatment effect at Week 12 CAPS - 5 (P=0.053) and CGI - I (P=0.041) scores • Retrospective analyses suggested CAPS - 5 ≥ 33 enrollment criteria for Phase 3
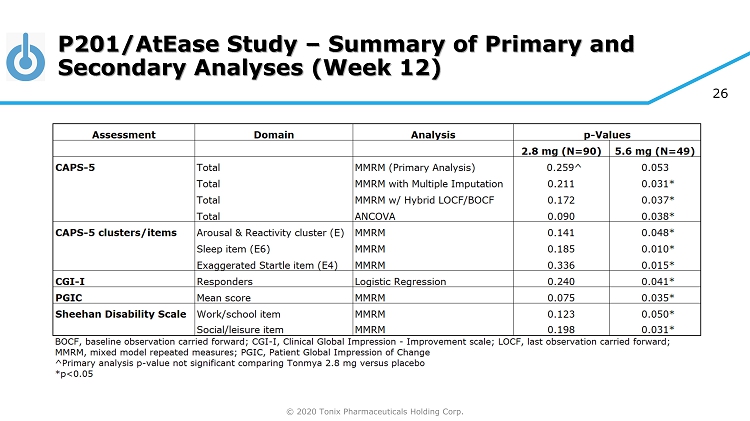
© 2020 Tonix Pharmaceuticals Holding Corp. 26 P201/AtEase Study – Summary of Primary and Secondary Analyses (Week 12)
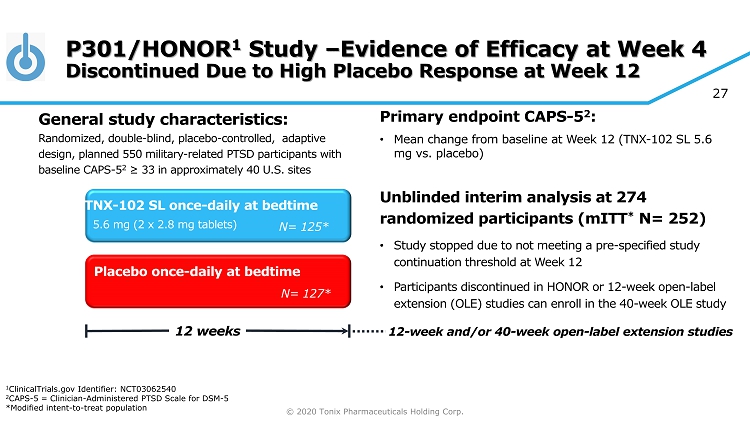
© 2020 Tonix Pharmaceuticals Holding Corp. 27 P301/HONOR 1 Study – Evidence of Efficacy at Week 4 Discontinued Due to High Placebo Response at Week 12 Primary e ndpoint CAPS - 5 2 : • Mean change from baseline at W eek 12 (TNX - 102 SL 5.6 mg vs. placebo ) Unblinded interim analysis at 274 randomized participants ( mITT * N= 252) • Study stopped due to not meeting a pre - specified study continuation threshold at Week 12 • Participants discontinued in HONOR or 12 - week open - label extension (OLE) studies can enroll in the 40 - week OLE study Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime N= 127* N= 125* 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: Randomized, double - blind, placebo - controlled , adaptive design, planned 550 military - related PTSD participants with baseline CAPS - 5 2 ≥ 33 in approximately 40 U.S. sites 12 - week and/or 40 - week open - label extension studies 1 ClinicalTrials.gov Identifier: NCT03062540 2 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 *Modified intent - to - treat population
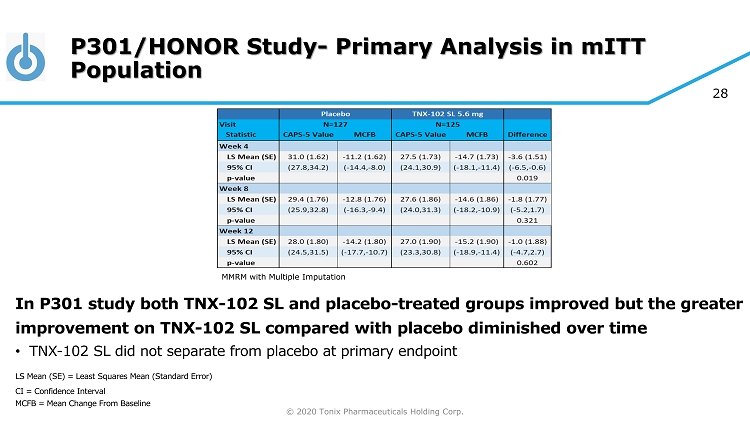
© 2020 Tonix Pharmaceuticals Holding Corp. 28 P301/HONOR Study - Primary Analysis in mITT Population In P301 study both TNX - 102 SL and placebo - treated groups improved but the greater improvement on TNX - 102 SL compared with placebo diminished over time • TNX - 102 SL did not separate from placebo at primary endpoint LS Mean (SE) = Least Squares Mean (Standard Error) CI = Confidence Interval MCFB = Mean Change From Baseline MMRM with Multiple Imputation
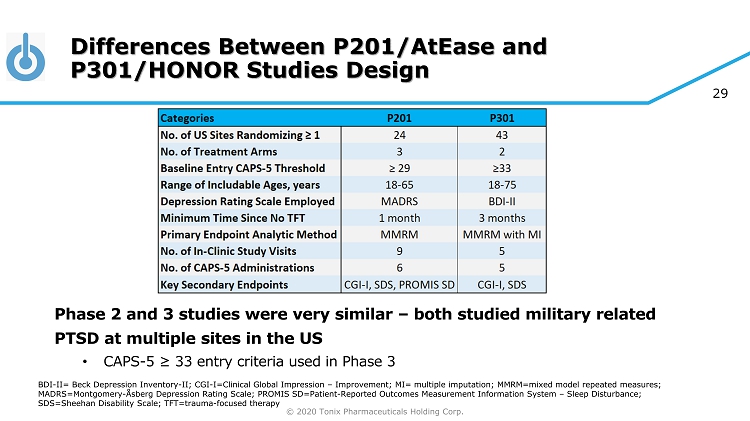
© 2020 Tonix Pharmaceuticals Holding Corp. 29 Differences Between P201/AtEase and P301/HONOR Studies Design Phase 2 and 3 studies were very similar – both studied military related PTSD at multiple sites in the US • CAPS - 5 ≥ 33 entry criteria used in Phase 3 BDI - II= Beck Depression Inventory - II; CGI - I=Clinical Global Impression – Improvement; MI= multiple imputation; MMRM=mixed model repeated measures; MADRS=Montgomery - Åsberg Depression Rating Scale; PROMIS SD=Patient - Reported Outcomes Measurement Information System – Sleep Disturbance; SDS=Sheehan Disability Scale; TFT=trauma - focused therapy
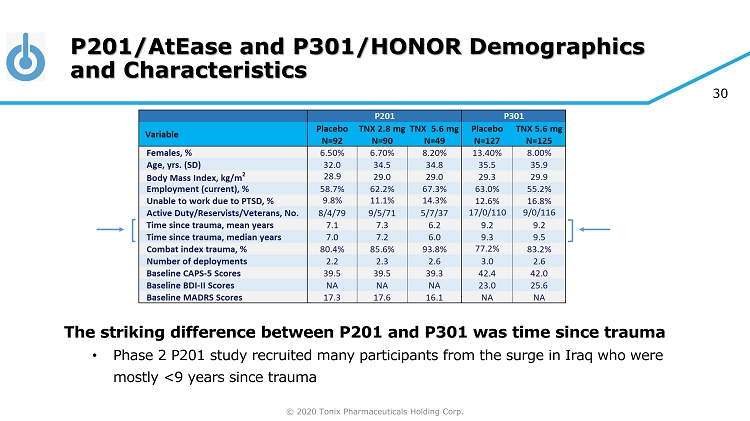
© 2020 Tonix Pharmaceuticals Holding Corp. 30 P201/AtEase and P301/HONOR Demographics and Characteristics The striking difference between P201 and P301 was time since trauma • Phase 2 P201 study recruited many participants from the surge in Iraq who were mostly <9 years since trauma
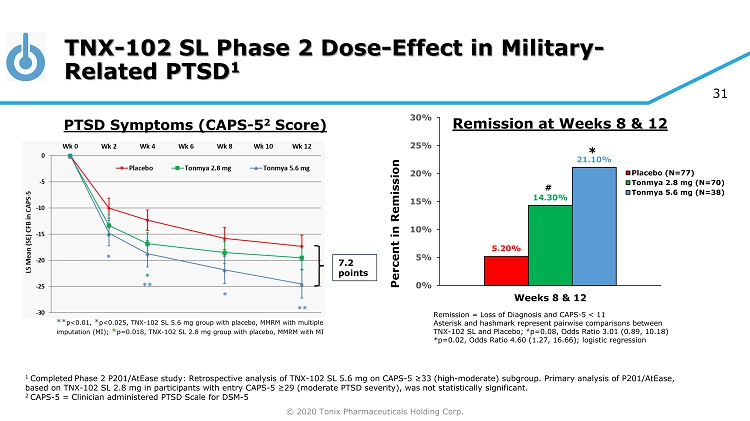
© 2020 Tonix Pharmaceuticals Holding Corp. 31 TNX - 102 SL Phase 2 Dose - Effect in Military - Related PTSD 1 1 Completed Phase 2 P201/AtEase study: Retrospective analysis of TNX - 102 SL 5.6 mg on CAPS - 5 ≥33 (high - moderate) subgroup. Primary analysis of P201/ AtEase , based on TNX - 102 SL 2.8 mg in participants with entry CAPS - 5 ≥29 ( moderate PTSD severity), was not statistically significant. 2 CAPS - 5 = Clinician administered PTSD Scale for DSM - 5 7.2 points ** p<0.01, * p<0.025, TNX - 102 SL 5.6 mg group with placebo, MMRM with multiple imputation (MI); * p=0.018, TNX - 102 SL 2.8 mg group with placebo, MMRM with MI PTSD Symptoms (CAPS - 5 2 Score) Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between TNX - 102 SL and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # * Remission at Weeks 8 & 12
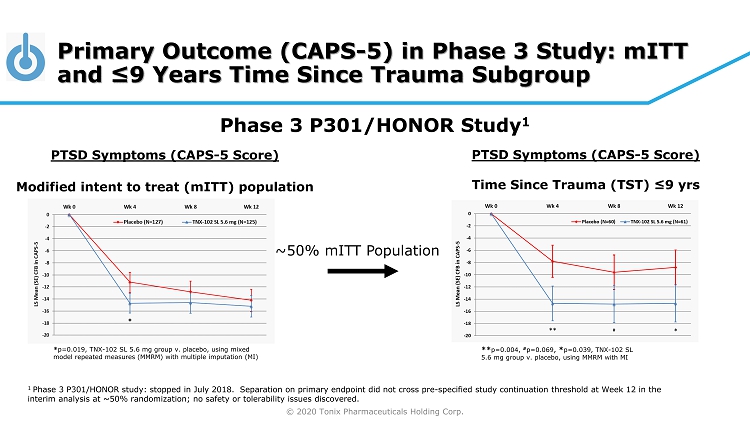
© 2020 Tonix Pharmaceuticals Holding Corp. Primary Outcome (CAPS - 5) in Phase 3 Study: mITT and ≤9 Years Time Since Trauma Subgroup PTSD Symptoms (CAPS - 5 Score) Modified intent to treat ( mITT ) population Phase 3 P301/HONOR Study 1 ** p=0.004, # p=0.069 , * p=0.039, TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI ~50% mITT Population * p=0.019, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI) PTSD Symptoms (CAPS - 5 Score) Time Since Trauma (TST) ≤9 yrs 1 Phase 3 P301/HONOR study: stopped in July 2018. Separation on primary endpoint did not cross pre - specified study continuation t hreshold at Week 12 in the interim analysis at ~50% randomization; no safety or tolerability issues discovered.
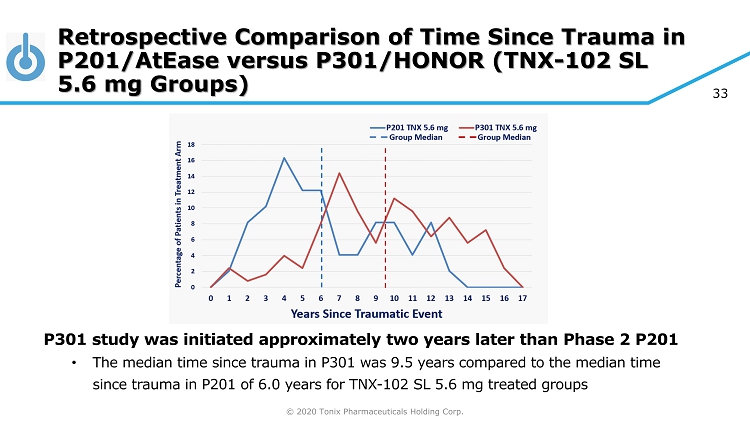
© 2020 Tonix Pharmaceuticals Holding Corp. 33 Retrospective Comparison of Time Since Trauma in P201/AtEase versus P301/HONOR (TNX - 102 SL 5.6 mg Groups) P301 study was initiated approximately two years later than Phase 2 P201 • The median time since trauma in P301 was 9.5 years compared to the median time since trauma in P201 of 6.0 years for TNX - 102 SL 5.6 mg treated groups
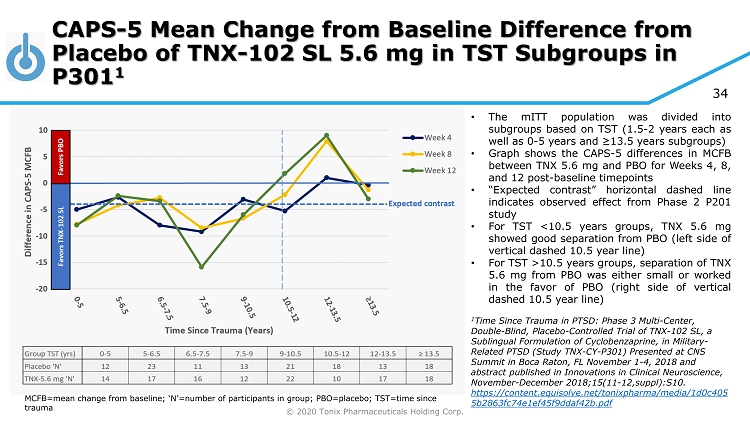
© 2020 Tonix Pharmaceuticals Holding Corp. 34 CAPS - 5 Mean Change from Baseline Difference from Placebo of TNX - 102 SL 5.6 mg in TST Subgroups in P301 1 MCFB=mean change from baseline; ‘N’=number of participants in group; PBO=placebo; TST=time since trauma • The mITT population was divided into subgroups based on TST ( 1 . 5 - 2 years each as well as 0 - 5 years and ≥ 13 . 5 years subgroups) • Graph shows the CAPS - 5 differences in MCFB between TNX 5 . 6 mg and PBO for Weeks 4 , 8 , and 12 post - baseline timepoints • “Expected contrast” horizontal dashed line indicates observed effect from Phase 2 P 201 study • For TST < 10 . 5 years groups, TNX 5 . 6 mg showed good separation from PBO (left side of vertical dashed 10 . 5 year line) • For TST > 10 . 5 years groups, separation of TNX 5 . 6 mg from PBO was either small or worked in the favor of PBO (right side of vertical dashed 10 . 5 year line) 1 Time Since Trauma in PTSD: Phase 3 Multi - Center, Double - Blind, Placebo - Controlled Trial of TNX - 102 SL, a Sublingual Formulation of Cyclobenzaprine, in Military - Related PTSD (Study TNX - CY - P301) Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018 and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12,suppl):S10. https://content.equisolve.net/tonixpharma/media/1d0c405 5b2863fc74e1ef45f9ddaf42b.pdf
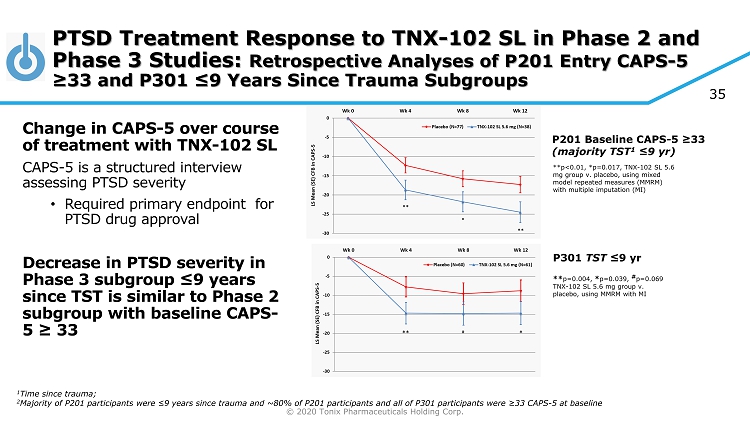
© 2020 Tonix Pharmaceuticals Holding Corp. P201 Baseline CAPS - 5 ≥33 (majority TST 1 ≤9 yr ) **p<0.01, *p=0.017, TNX - 102 SL 5.6 mg group v. placebo, using mixed model repeated measures (MMRM) with multiple imputation (MI) P301 TST ≤9 yr ** p=0.004, * p=0.039, # p=0.069 TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI PTSD Treatment Response to TNX - 102 SL in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Change in CAPS - 5 over course of treatment with TNX - 102 SL CAPS - 5 is a structured interview assessing PTSD severity • Required primary endpoint for PTSD drug approval Decrease in PTSD severity in Phase 3 subgroup ≤9 years since TST is similar to Phase 2 subgroup with baseline CAPS - 5 ≥ 33 1 Time since trauma; 2 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥33 CAPS - 5 at baseline 35
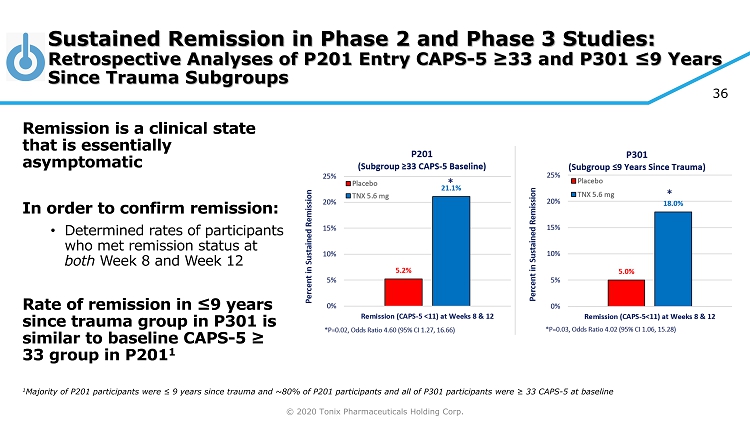
© 2020 Tonix Pharmaceuticals Holding Corp. 36 Sustained Remission in Phase 2 and Phase 3 Studies: Retrospective Analyses of P201 Entry CAPS - 5 ≥33 and P301 ≤9 Years Since Trauma Subgroups Remission is a clinical state that is essentially asymptomatic In order to confirm remission: • Determined rates of participants who met remission status at both Week 8 and Week 12 Rate of remission in ≤9 years since trauma group in P301 is similar to baseline CAPS - 5 ≥ 33 group in P201 1 1 Majority of P201 participants were ≤ 9 years since trauma and ~80% of P201 participants and all of P301 participants were ≥ 33 CAPS - 5 at baseline
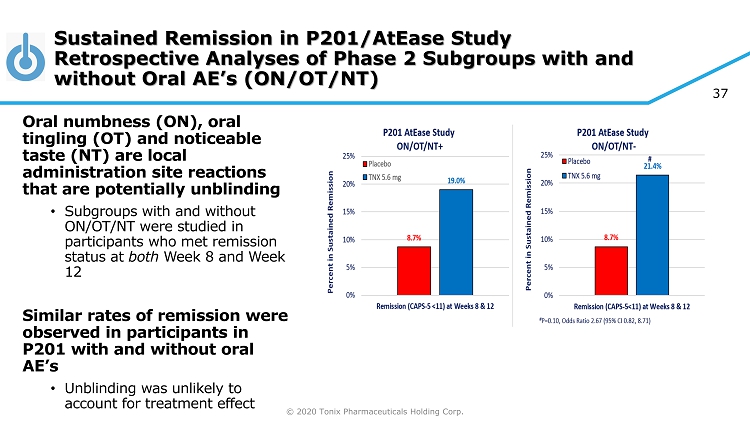
© 2020 Tonix Pharmaceuticals Holding Corp. 37 Sustained Remission in P201/ AtEase Study Retrospective Analyses of Phase 2 Subgroups with and without Oral AE’s (ON/OT/NT) Oral numbness (ON), oral tingling (OT) and noticeable taste (NT) are local administration site reactions that are potentially unblinding • Subgroups with and without ON/OT/NT were studied in participants who met remission status at both Week 8 and Week 12 Similar rates of remission were observed in participants in P201 with and without oral AE’s • Unblinding was unlikely to account for treatment effect
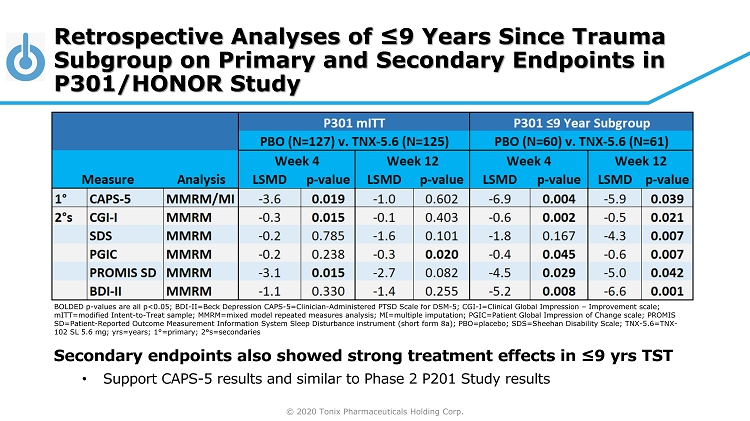
© 2020 Tonix Pharmaceuticals Holding Corp. BOLDED p - values are all p<0.05; BDI - II=Beck Depression CAPS - 5=Clinician - Administered PTSD Scale for DSM - 5; CGI - I=Clinical Global Impression – Improvement scale; mITT =modified Intent - to - Treat sample; MMRM=mixed model repeated measures analysis; MI=multiple imputation; PGIC=Patient Global Impre ssion of Change scale; PROMIS SD=Patient - Reported Outcome Measurement Information System Sleep Disturbance instrument (short form 8a); PBO=placebo; SDS=Sheeha n Disability Scale; TNX - 5.6=TNX - 102 SL 5.6 mg; yrs =years; 1 ° =primary; 2 ° s=secondaries Retrospective Analyses of ≤9 Years Since Trauma Subgroup on Primary and Secondary Endpoints in P301/HONOR Study Secondary endpoints also showed strong treatment effects in ≤9 yrs TST • Support CAPS - 5 results and similar to Phase 2 P201 Study results
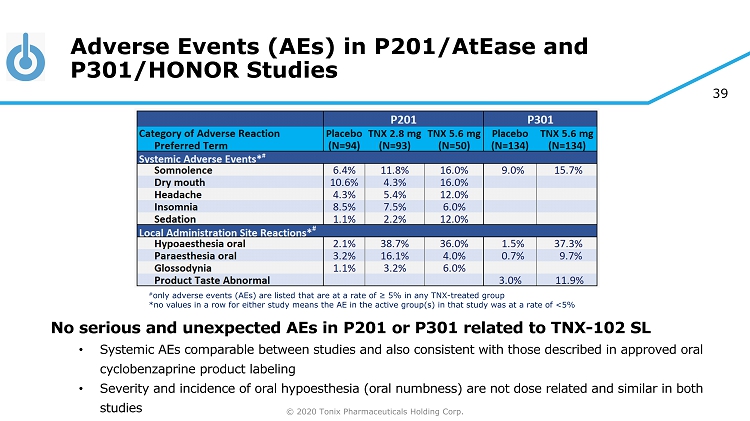
© 2020 Tonix Pharmaceuticals Holding Corp. 39 Adverse Events (AEs) in P201/AtEase and P301/HONOR Studies No serious and unexpected AEs in P201 or P301 related to TNX - 102 SL • Systemic AEs comparable between studies and also consistent with those described in approved oral cyclobenzaprine product labeling • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related and similar in both studies # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%
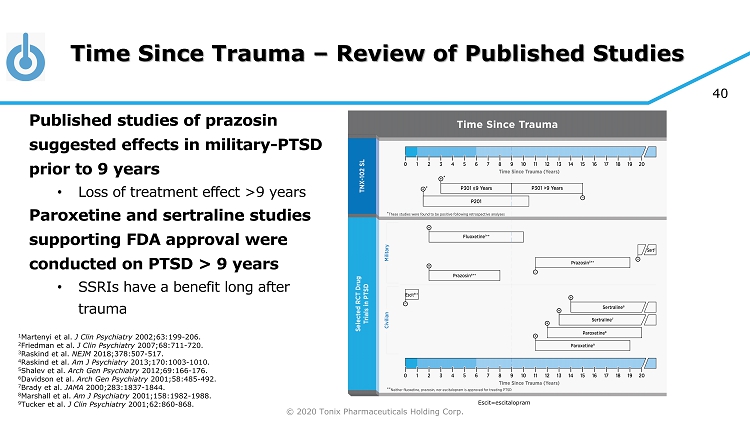
© 2020 Tonix Pharmaceuticals Holding Corp. 40 Time Since Trauma – Review of Published Studies Published studies of prazosin suggested effects in military - PTSD prior to 9 years • Loss of treatment effect >9 years Paroxetine and sertraline studies supporting FDA approval were conducted on PTSD > 9 years • SSRIs have a benefit long after trauma 1 Martenyi et al. J Clin Psychiatry 2002;63:199 - 206. 2 Friedman et al. J Clin Psychiatry 2007;68:711 - 720. 3 Raskind et al. NEJM 2018;378:507 - 517. 4 Raskind et al. Am J Psychiatry 2013;170:1003 - 1010. 5 Shalev et al. Arch Gen Psychiatry 2012;69:166 - 176. 6 Davidson et al. Arch Gen Psychiatry 2001;58:485 - 492. 7 Brady et al. JAMA 2000;283:1837 - 1844. 8 Marshall et al. Am J Psychiatry 2001;158:1982 - 1988. 9 Tucker et al. J Clin Psychiatry 2001;62:860 - 868. Escit =escitalopram
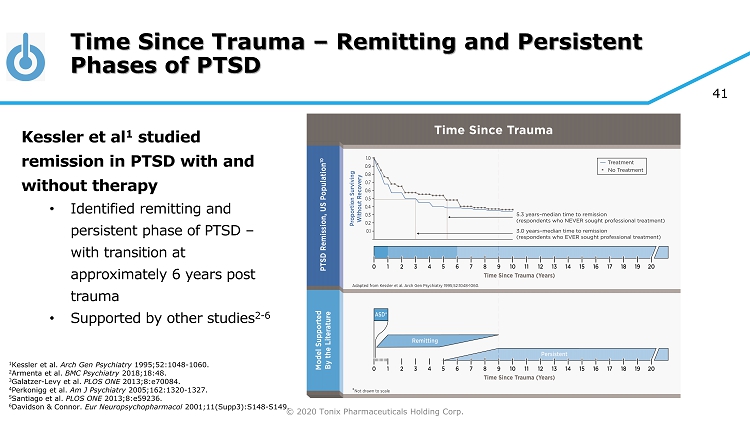
© 2020 Tonix Pharmaceuticals Holding Corp. 41 Time Since Trauma – Remitting and Persistent Phases of PTSD Kessler et al 1 studied remission in PTSD with and without therapy • Identified remitting and persistent phase of PTSD – with transition at approximately 6 years post trauma • Supported by other studies 2 - 6 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327. 5 Santiago et al. PLOS ONE 2013;8:e59236. 6 Davidson & Connor. Eur Neuropsychopharmacol 2001;11(Supp3):S148 - S149.
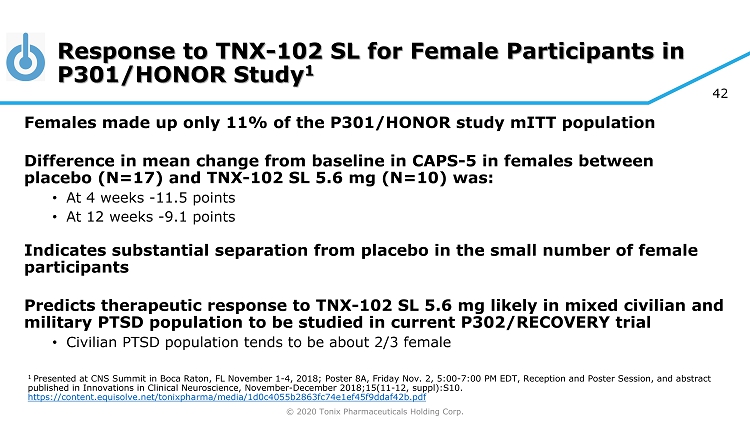
© 2020 Tonix Pharmaceuticals Holding Corp. 42 Response to TNX - 102 SL for Female Participants in P301/HONOR Study 1 Females made up only 11% of the P301/HONOR study mITT population Difference in mean change from baseline in CAPS - 5 in females between placebo (N=17) and TNX - 102 SL 5.6 mg (N=10) was: • At 4 weeks - 11.5 points • At 12 weeks - 9.1 points Indicates substantial separation from placebo in the small number of female participants Predicts therapeutic response to TNX - 102 SL 5.6 mg likely in mixed civilian and military PTSD population to be studied in current P302/RECOVERY trial • Civilian PTSD population tends to be about 2/3 female 1 Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018; Poster 8A, Friday Nov. 2, 5:00 - 7:00 PM EDT, Reception and Poster S ession, and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12, suppl):S10. https://content.equisolve.net/tonixpharma/media/1d0c4055b2863fc74e1ef45f9ddaf42b.pdf
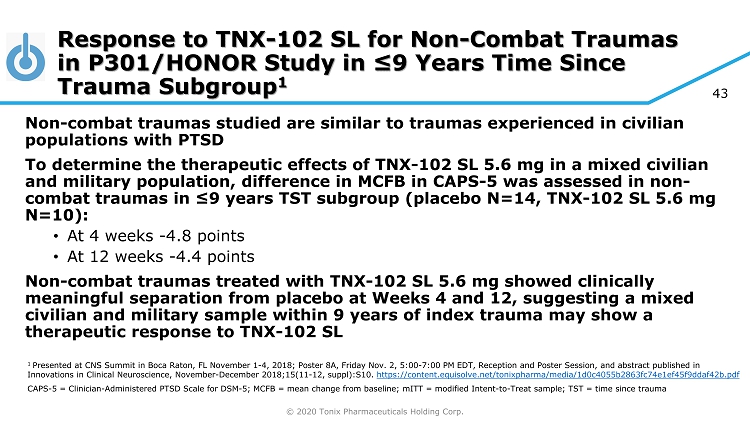
© 2020 Tonix Pharmaceuticals Holding Corp. 43 Non - combat traumas studied are similar to traumas experienced in civilian populations with PTSD To determine the therapeutic effects of TNX - 102 SL 5.6 mg in a mixed civilian and military population, difference in MCFB in CAPS - 5 was assessed in non - combat traumas in ≤9 years TST subgroup (placebo N=14, TNX - 102 SL 5.6 mg N=10): • At 4 weeks - 4.8 points • At 12 weeks - 4.4 points Non - combat traumas treated with TNX - 102 SL 5.6 mg showed clinically meaningful separation from placebo at Weeks 4 and 12, suggesting a mixed civilian and military sample within 9 years of index trauma may show a therapeutic response to TNX - 102 SL Response to TNX - 102 SL for Non - Combat Traumas in P301/HONOR Study in ≤9 Years Time Since Trauma Subgroup 1 1 Presented at CNS Summit in Boca Raton, FL November 1 - 4, 2018; Poster 8A, Friday Nov. 2, 5:00 - 7:00 PM EDT, Reception and Poster S ession, and abstract published in Innovations in Clinical Neuroscience, November - December 2018;15(11 - 12, suppl):S10. https://content.equisolve.net/tonixpharma/media/1d0c4055b2863fc74e1ef45f9ddaf42b.pdf CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5; MCFB = mean change from baseline; mITT = modified Intent - to - Treat sample; TST = time since trauma
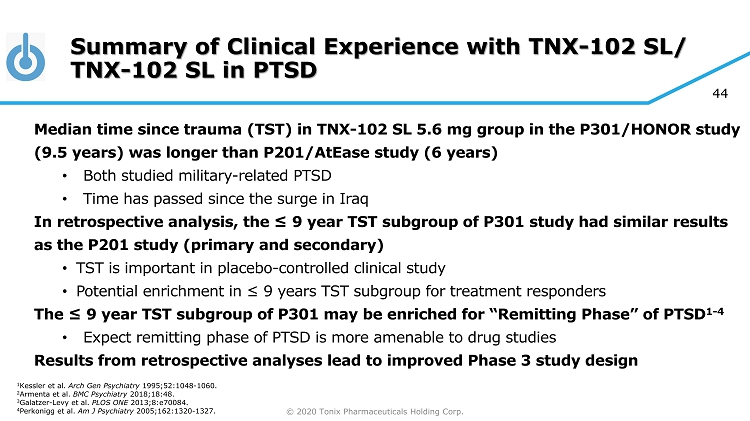
© 2020 Tonix Pharmaceuticals Holding Corp. 44 Summary of Clinical Experience with TNX - 102 SL/ TNX - 102 SL in PTSD Median time since trauma (TST) in TNX - 102 SL 5.6 mg group in the P301/HONOR study (9.5 years) was longer than P201/AtEase study (6 years) • Both studied military - related PTSD • Time has passed since the surge in Iraq In retrospective analysis, the ≤ 9 year TST subgroup of P301 study had similar results as the P201 study (primary and secondary) • TST is important in placebo - controlled clinical study • Potential enrichment in ≤ 9 years TST subgroup for treatment responders The ≤ 9 year TST subgroup of P301 may be enriched for “Remitting Phase” of PTSD 1 - 4 • Expect remitting phase of PTSD is more amenable to drug studies Results from retrospective analyses lead to improved Phase 3 study design 1 Kessler et al. Arch Gen Psychiatry 1995;52:1048 - 1060. 2 Armenta et al. BMC Psychiatry 2018;18:48. 3 Galatzer - Levy et al. PLOS ONE 2013;8:e70084. 4 Perkonigg et al. Am J Psychiatry 2005;162:1320 - 1327.
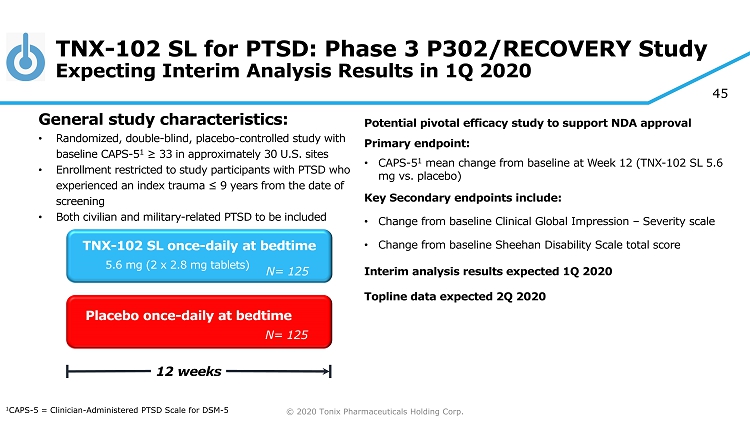
© 2020 Tonix Pharmaceuticals Holding Corp. 45 TNX - 102 SL for PTSD: Phase 3 P302/RECOVERY Study Expecting Interim Analysis Results in 1Q 2020 Potential pivotal efficacy study to support NDA approval Primary e ndpoint: • CAPS - 5 1 mean change from baseline at Week 12 (TNX - 102 SL 5.6 mg vs. placebo) Key Secondary e ndpoint s include: • Change from baseline Clinical Global Impression – Severity scale • Change from baseline Sheehan Disability Scale total score Interim analysis results expected 1Q 2020 Topline data expected 2Q 2020 Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study with baseline CAPS - 5 1 ≥ 33 in approximately 30 U.S. sites • Enrollment restricted to study participants with PTSD who experienced an index trauma ≤ 9 years from the date of screening • Both civilian and military - related PTSD to be included 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 N= 125 N= 125
© 2020 Tonix Pharmaceuticals Holding Corp. 46 Commercialization Options Tonix is exploring a variety of options to commercialize TNX - 102 SL, including commercializing on our own or partnering all or some indications in specific regions of the world Tonix has participated in numerous partnering meetings Commercial Considerations: • Primary physician audience is well defined: psychiatrists (~30,000 in U.S.) • Small specialty sales force sufficient for coverage • Primary market research with psychiatrists indicate strong interest in new therapeutic options
© 2020 Tonix Pharmaceuticals Holding Corp. 47 TNX - 102 SL – Multi - Functional Mechanism Involves Antagonism at 3 Neuronal Receptors Active ingredient, cyclobenzaprine, interacts with 3 receptors • Antagonist at 5 - HT 2A receptors • Similar activity to trazodone and Nuplazid ® ( pimivanserin ) • Antagonist at a 1 - adrenergic receptor • Similar activity to prazosin • Antagonist at histamine H 1 receptors • Similar activity to Benadryl ® (diphenhydramine) and hydroxyzine Multi - functional activity suggests potential for other indications • TNX - 102 SL was developed for the management of fibromyalgia (Phase 3) • Sleep quality is a problem in other conditions
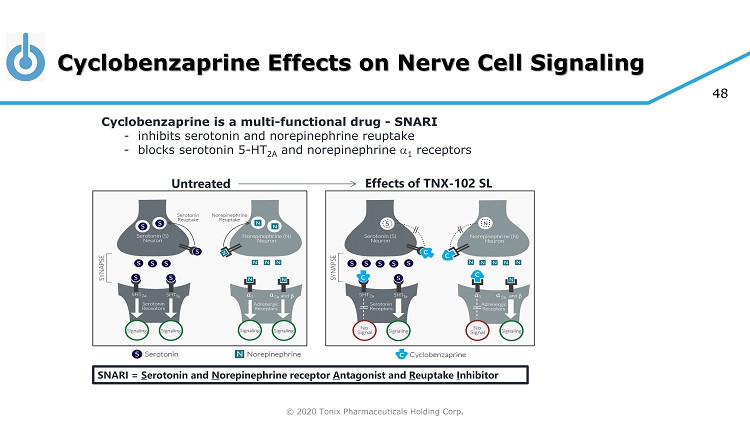
© 2020 Tonix Pharmaceuticals Holding Corp. 48 Cyclobenzaprine Effects on Nerve Cell Signaling Untreated Effects of TNX - 102 SL Cyclobenzaprine is a multi - functional drug - SNARI - inhibits serotonin and norepinephrine reuptake - blocks serotonin 5 - HT 2A and norepinephrine a 1 receptors SNARI = S erotonin and N orepinephrine receptor A ntagonist and R euptake I nhibitor
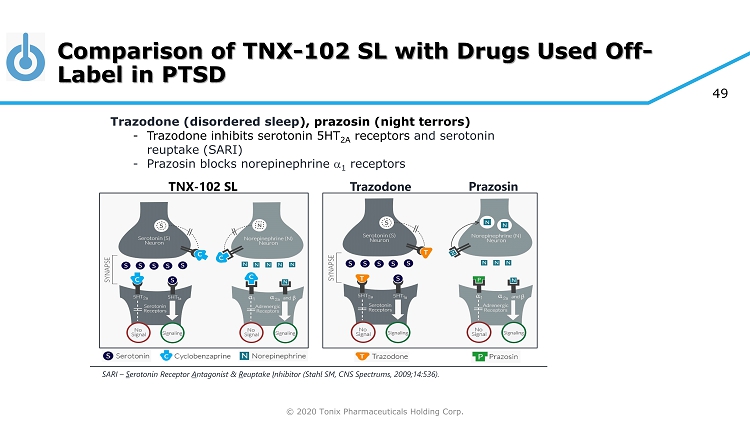
© 2020 Tonix Pharmaceuticals Holding Corp. 49 Comparison of TNX - 102 SL with Drugs Used Off - Label in PTSD TNX - 102 SL Trazodone Trazodone (disordered sleep ), prazosin (night terrors) - Trazodone inhibits serotonin 5HT 2A receptors and serotonin reuptake (SARI) - Prazosin blocks norepinephrine a 1 receptors Prazosin SARI – S erotonin Receptor A ntagonist & R euptake I nhibitor (Stahl SM, CNS Spectrums, 2009;14:536).
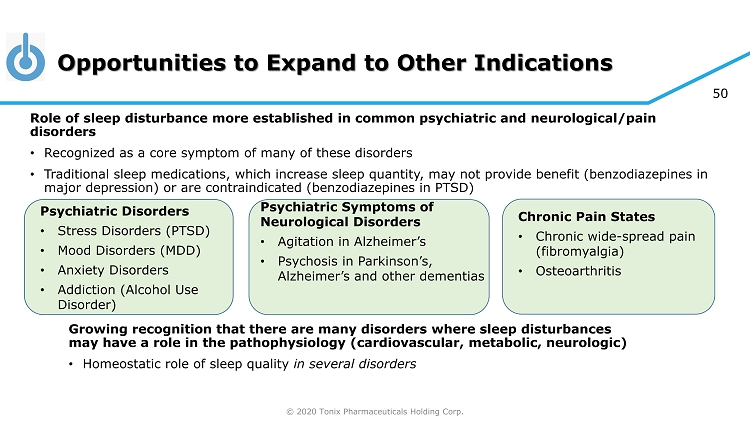
© 2020 Tonix Pharmaceuticals Holding Corp. 50 Opportunities to Expand to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Homeostatic role of sleep quality in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (MDD) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated (benzodiazepines in PTSD) Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias
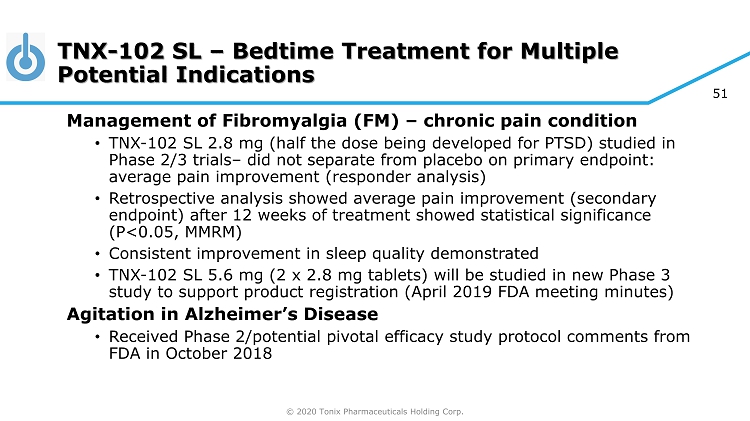
© 2020 Tonix Pharmaceuticals Holding Corp. 51 TNX - 102 SL – Bedtime Treatment for Multiple Potential Indications Ma nagement of Fibromyalgia (FM) – chronic pain condition • TNX - 102 SL 2.8 mg (half the dose being developed for PTSD) studied in Phase 2/3 trials – did not separate from placebo on primary endpoint: average pain improvement (responder analysis) • Retrospective analysis showed average pain improvement (secondary endpoint) after 12 weeks of treatment showed statistical significance (P<0.05, MMRM) • Consistent improvement in sleep quality demonstrated • TNX - 102 SL 5.6 mg (2 x 2.8 mg tablets) will be studied in new Phase 3 study to support product registration (April 2019 FDA meeting minutes) Agitation in Alzheimer’s Disease • Received Phase 2/potential pivotal efficacy study protocol comments from FDA in October 2018

© 2020 Tonix Pharmaceuticals Holding Corp. 52 Volkswagen Check Engine [Photograph]. (2011, October 14). Wikipedia When the check engine light malfunctions, the light is on even though the car is not malfunctioning • Fibromyalgia is considered a neurobiological disorder characterized by 1 : chronic widespread pain, non - restorative sleep, fatigue, diminished cognition • Believed to result from inappropriate pain signaling in central nervous system in the absence of peripheral injury 1 • An estimated 6 - 12 million adults in the U.S. have fibromyalgia 2 • Causes significant impairment in all areas of life 3 • Lower levels of health - related quality of life – reduced daily functioning • Interference with work (loss of productivity, disability) • Fewer than half of those treated for fibromyalgia receive complete relief from the three FDA - approved drugs 4 • Inflicts substantial strain on the healthcare system • Average patient has 20 physician office visits per year 5 • Annual direct medical costs are twice those of non - fibromyalgia individuals 6 TNX - 102 SL: Potential Treatment for Fibromyalgia 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141. 2 American Chronic Pain Association (www.theacpa.org, 2019) 3 Schaefer et al., Pain Pract , 2015. 4 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 5 Robinson et al, Pain Medicine 2013;14:1400. 6 White et al, J Occupational Environ Med 2008;50:13.
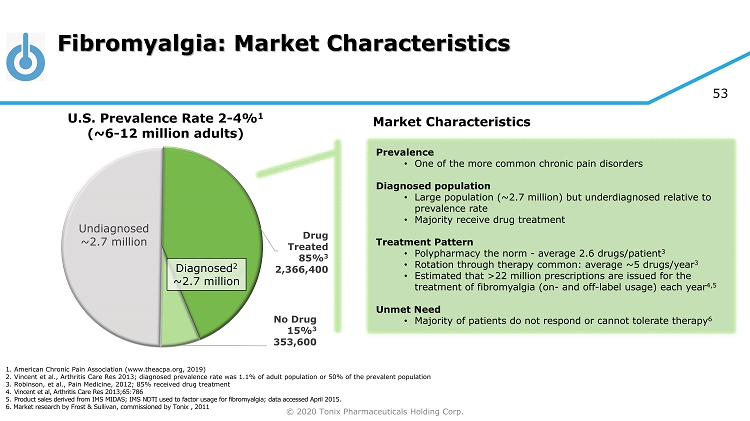
© 2020 Tonix Pharmaceuticals Holding Corp. 53 Fibromyalgia: Market Characteristics Undiagnosed ~2.7 million Drug Treated 85% 3 2,366,400 No Drug 15% 3 353,600 U.S. Prevalence Rate 2 - 4% 1 (~6 - 12 million adults) Diagnosed 2 ~2.7 million Market Characteristics Prevalence • One of the more common chronic pain disorders Diagnosed population • Large population (~2.7 million) but underdiagnosed relative to prevalence rate • Majority receive drug treatment Treatment Pattern • Polypharmacy the norm - average 2.6 drugs/patient 3 • Rotation through therapy common: average ~5 drugs/year 3 • Estimated that >22 million prescriptions are issued for the treatment of fibromyalgia (on - and off - label usage) each year 4,5 Unmet Need • Majority of patients do not respond or cannot tolerate therapy 6 1. American Chronic Pain Association (www.theacpa.org, 2019) 2. Vincent et al., Arthritis Care Res 2013; diagnosed prevalence rate was 1.1% of adult population or 50% of the prevalent po pul ation 3. Robinson, et al., Pain Medicine, 2012; 85% received drug treatment 4. Vincent et al, Arthritis Care Res 2013;65:786 5. Product sales derived from IMS MIDAS; IMS NDTI used to factor usage for fibromyalgia; data accessed April 2015. 6. Market research by Frost & Sullivan, commissioned by Tonix , 2011
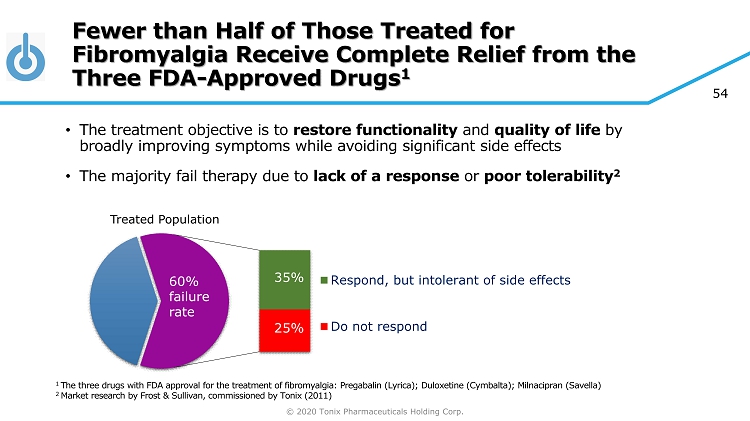
© 2020 Tonix Pharmaceuticals Holding Corp. 54 Fewer than Half of Those Treated for Fibromyalgia Receive Complete Relief from the Three FDA - Approved Drugs 1 • The treatment objective is to restore functionality and quality of life by broadly improving symptoms while avoiding significant side effects • The majority fail therapy due to lack of a response or poor tolerability 2 Respond, but intolerant of side effects Do not respond 25% 35% 60% failure rate 1 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 2 Market research by Frost & Sullivan, commissioned by Tonix (2011) Treated Population
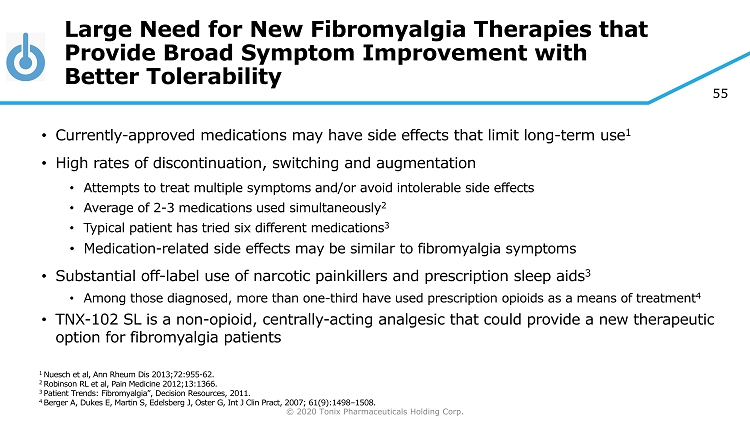
© 2020 Tonix Pharmaceuticals Holding Corp. 55 Large Need for New Fibromyalgia Therapies that Provide Broad Symptom Improvement with Better Tolerability • Currently - approved medications may have side effects that limit long - term use 1 • High rates of discontinuation, switching and augmentation • A ttempts to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications used simultaneously 2 • Typical patient has tried six different medications 3 • Medication - related side effects may be similar to fibromyalgia symptoms • Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 • TNX - 102 SL is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources, 2011. 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J Clin Pract , 2007; 61(9):1498 – 1508.
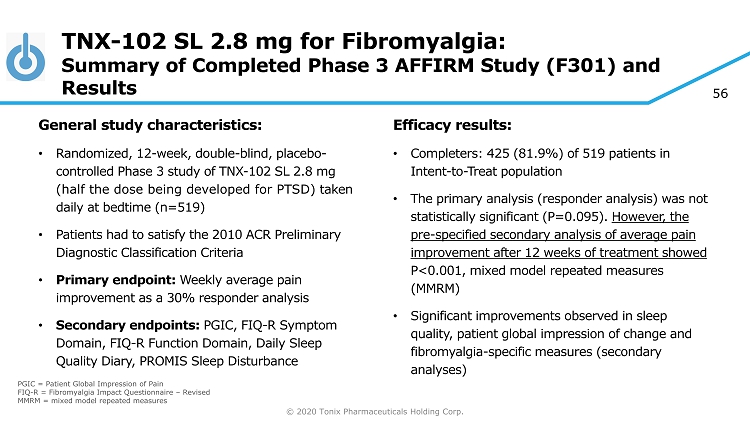
© 2020 Tonix Pharmaceuticals Holding Corp. 56 TNX - 102 SL 2.8 mg for Fibromyalgia: Summary of Completed Phase 3 AFFIRM Study (F301) and Results General s tudy c haracteristics: • Randomized, 12 - week, double - blind, placebo - controlle d Phase 3 study of TNX - 102 SL 2.8 mg (half the dose being developed for PTSD) taken daily at bedtime (n=519) • Patients had to satisfy the 2010 ACR Preliminary Diagnostic Classification Criteria • Primary endpoint: Weekly average pain improvement as a 30% responder analysis • Secondary endpoints: PGIC, FIQ - R Symptom Domain, FIQ - R Function Domain, Daily Sleep Quality Diary, PROMIS Sleep Disturbance Efficacy results: • Completers: 425 (81.9%) of 519 patients in Intent - to - Treat population • The primary analysis (responder analysis) was not statistically significant (P=0.095). However, the pre - specified secondary analysis of average pain improvement after 12 weeks of treatment showed P<0.001, mixed model repeated measures (MMRM) • Significant improvements observed in sleep quality, patient global impression of change and fibromyalgia - specific measures (secondary analyses) PGIC = Patient Global Impression of Pain FIQ - R = Fibromyalgia Impact Questionnaire – Revised MMRM = mixed model repeated measures
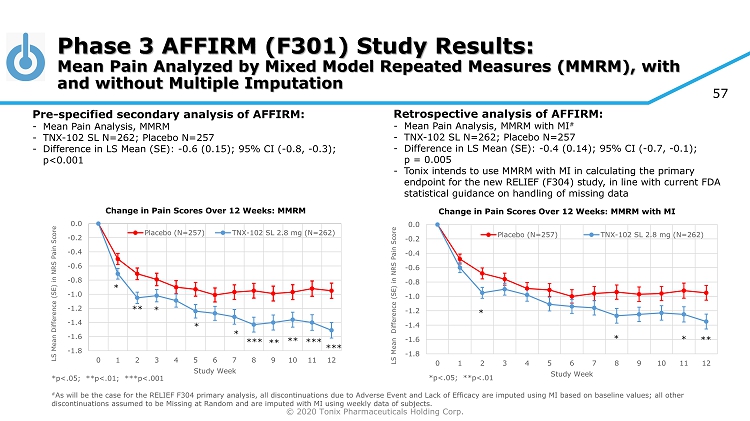
© 2020 Tonix Pharmaceuticals Holding Corp. 57 *p<.05; **p<.01; ***p<.001 # As will be the case for the RELIEF F304 primary analysis, all discontinuations due to Adverse Event and Lack of Efficacy are imp uted using MI based on baseline values; all other discontinuations assumed to be Missing at Random and are imputed with MI using weekly data of subjects. Phase 3 AFFIRM (F301) Study Results: Mean Pain Analyzed by Mixed Model Repeated Measures (MMRM), with and without Multiple Imputation Pre - specified secondary analysis of AFFIRM: - Mean Pain Analysis, MMRM - TNX - 102 SL N=262; Placebo N=257 - Difference in LS Mean (SE): - 0.6 (0.15); 95% CI ( - 0.8, - 0.3); p<0.001 Retrospective analysis of AFFIRM: - Mean Pain Analysis, MMRM with MI # - TNX - 102 SL N=262; Placebo N=257 - Difference in LS Mean (SE): - 0.4 (0.14); 95% CI ( - 0.7, - 0.1); p = 0.005 - Tonix intends to use MMRM with MI in calculating the primary endpoint for the new RELIEF (F304) study, in line with current FDA statistical guidance on handling of missing data -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM with MI Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * * * ** *p<.05; **p<.01 -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * ** *** * * * *** *** ** **
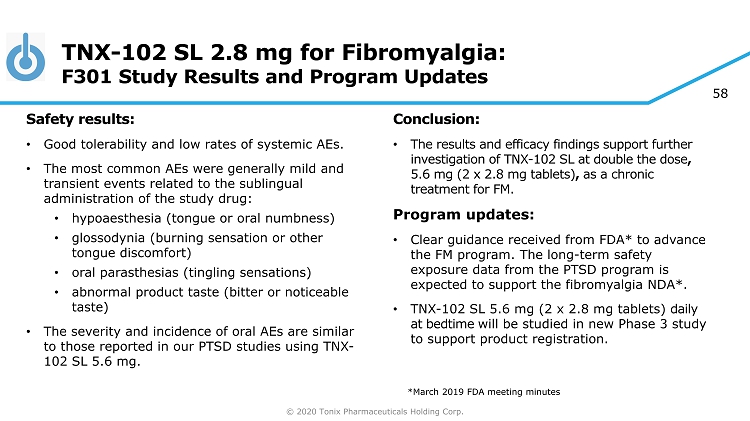
© 2020 Tonix Pharmaceuticals Holding Corp. 58 TNX - 102 SL 2.8 mg for Fibromyalgia: F301 Study Results and Program Updates Safety results: • Good tolerability and low rates of systemic AEs. • The most common AEs were generally mild and transient events related to the sublingual administration of the study drug: • hypoaesthesia (tongue or oral numbness) • glossodynia (burning sensation or other tongue discomfort) • oral parasthesias (tingling sensations) • abnormal product taste (bitter or noticeable taste) • The severity and incidence of oral AEs are similar to those reported in our PTSD studies using TNX - 102 SL 5.6 mg. Conclusion: • The results and efficacy findings support further investigation of TNX - 102 SL at double the dose , 5.6 mg (2 x 2.8 mg tablets) , as a chronic treatment for FM. Program updates: • Clear guidance received from FDA* to advance the FM program. The long - term safety exposure data from the PTSD program is expected to support the fibromyalgia NDA*. • TNX - 102 SL 5.6 mg (2 x 2.8 mg tablets) daily at bedtime will be studied in new Phase 3 study to support product registration. *March 2019 FDA meeting minutes
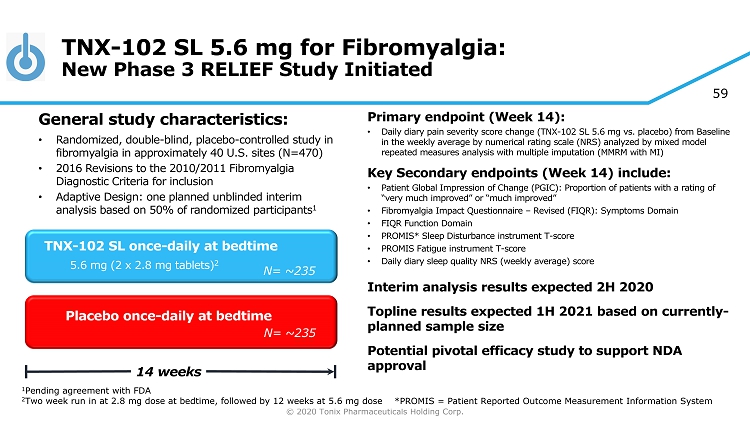
© 2020 Tonix Pharmaceuticals Holding Corp. 59 TNX - 102 SL 5.6 mg for Fibromyalgia: New Phase 3 RELIEF Study Initiated Primary e ndpoint (Week 14) : • Daily diary pain severity score change (TNX - 102 SL 5.6 mg vs. placebo) from Baseline in the weekly average by numerical rating scale (NRS) analyzed by mixed model repeated measures analysis with multiple imputation (MMRM with MI) Key Secondary e ndpoint s (Week 14) include: • Patient Global Impression of Change (PGIC): Proportion of patients with a rating of “very much improved” or “much improved” • Fibromyalgia Impact Questionnaire – Revised (FIQR): Symptoms Domain • FIQR Function Domain • PROMIS* Sleep Disturbance instrument T - score • PROMIS Fatigue instrument T - score • Daily diary sleep quality NRS (weekly average) score Interim analysis results expected 2H 2020 Topline results expected 1H 2021 based on currently - planned sample size Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) 2 General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia in approximately 40 U.S. sites (N=470) • 2016 Revisions to the 2010/2011 Fibromyalgia Diagnostic Criteria for inclusion • Adaptive Design: one planned unblinded interim analysis based on 50% of randomized participants 1 1 Pending agreement with FDA 2 Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose N= ~235 N= 250 N= ~235 *PROMIS = Patient Reported Outcome Measurement Information System

© 2020 Tonix Pharmaceuticals Holding Corp. 60 What is Agitation in Alzheimer’s Disease? Agitation is one of the most distressing and debilitating of the behavioral complications of Alzheimer’s disease • Includes emotional lability, restlessness, irritability and aggression 1 Link between disturbed sleep and agitation in Alzheimer’s 1 - 3 • Agitation is commonly diurnal (“ sundowning ”) Prevalence • Agitation is likely to affect more than half of the 5.3 million Americans who currently suffer from moderate to severe Alzheimer’s disease, and this number is expected to nearly triple by 2050 4 1 Rose, K.et al. (2015). American Journal of Alzheimer's Disease & Other Dementias , 30 :78 2 Shih, Y. H., et al. (2017). Journal of the American Medical Directors Association , 18 , 396. 3 Canevelli, M., et al. (2016). Frontiers in medicine , 3 . 4 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/

© 2020 Tonix Pharmaceuticals Holding Corp. 61 Consequences of Agitation in Alzheimer’s Disease Outcomes • Agitation is associated with significant poor outcomes for Alzheimer’s patients and challenges for their caregivers Common reason for institutionalization • Development of agitation, or its worsening, is one of the most common reasons for patients having to transition from lower - to higher levels of care (nursing homes and other long - term care settings) 1 Cost • The presence of agitation nearly doubles the cost of caring for patients with Alzheimer’s disease, and agitation is estimated to account for more than 12% of the healthcare and societal cost of Alzheimer’s disease, which is currently estimated to be $256 Billion for the year 2017 in the United States 1 1 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/
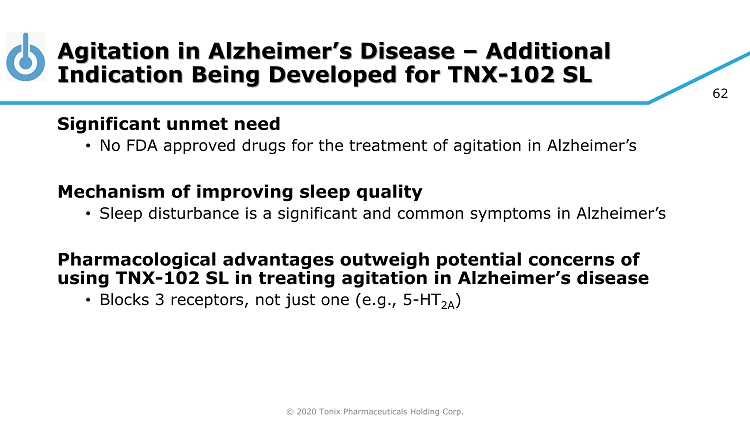
© 2020 Tonix Pharmaceuticals Holding Corp. 62 Agitation in Alzheimer’s Disease – Additional Indication Being Developed for TNX - 102 SL Significant unmet need • No FDA approved drugs for the treatment of agitation in Alzheimer’s Mechanism of improving sleep quality • Sleep disturbance is a significant and common symptoms in Alzheimer’s Pharmacological advantages outweigh potential concerns of using TNX - 102 SL in treating agitation in Alzheimer’s disease • Blocks 3 receptors, not just one (e.g., 5 - HT 2A )

© 2020 Tonix Pharmaceuticals Holding Corp. 63 TNX - 102 SL for Agitation in Alzheimer’s – Regulatory Status and Registration Strategy Proposed Phase 2 IND study can potentially serve as a pivotal efficacy study to support NDA approval • FDA comments on final protocol received October 2018 Registration Strategy of TNX - 102 SL for agitation in Alzheimer’s disease • Efficacy Supplement (sNDA 1 ) may be leveraged from the PTSD/FM development program and supported by Initial NDA approval for PTSD/FM 1 Supplemental New Drug Application
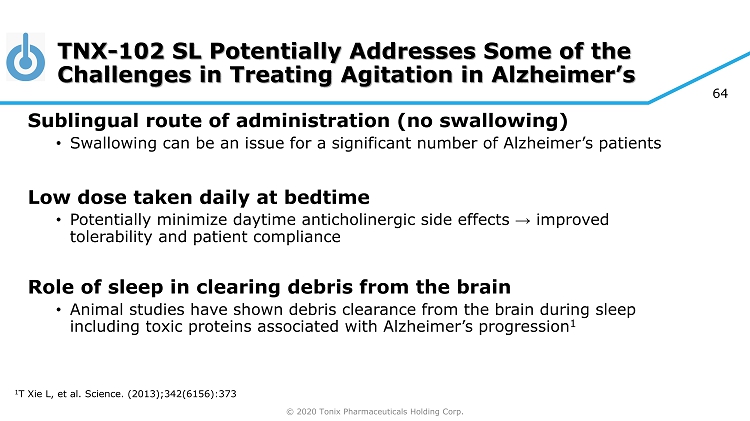
© 2020 Tonix Pharmaceuticals Holding Corp. 64 TNX - 102 SL Potentially Addresses Some of the Challenges in Treating Agitation in Alzheimer’s Sublingual route of administration (no swallowing) • Swallowing can be an issue for a significant number of Alzheimer’s patients Low dose taken daily at bedtime • Potentially minimize daytime anticholinergic side effects → improved tolerability and patient compliance Role of sleep in clearing debris from the brain • Animal studies have shown debris clearance from the brain during sleep including toxic proteins associated with Alzheimer’s progression 1 1 T Xie L, et al. Science. (2013);342(6156):373
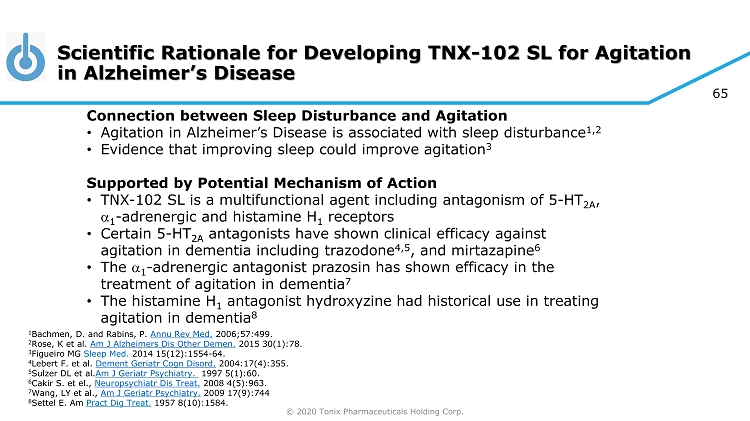
© 2020 Tonix Pharmaceuticals Holding Corp. 65 Scientific Rationale for Developing TNX - 102 SL for Agitation in Alzheimer’s Disease Connection between Sleep Disturbance and Agitation • Agitation in Alzheimer’s Disease is associated with sleep disturbance 1,2 • Evidence that improving sleep could improve agitation 3 Supported by Potential Mechanism of Action • TNX - 102 SL is a multifunctional agent including antagonism of 5 - HT 2A , a 1 - adrenergic and histamine H 1 receptors • Certain 5 - HT 2A antagonists have shown clinical efficacy against agitation in dementia including trazodone 4,5 , and mirtazapine 6 • The a 1 - adrenergic antagonist prazosin has shown efficacy in the treatment of agitation in dementia 7 • The histamine H 1 antagonist hydroxyzine had historical use in treating agitation in dementia 8 1 Bachmen, D. and Rabins , P. Annu Rev Med. 2006;57:499. 2 Rose, K et al. Am J Alzheimers Dis Other Demen . 2015 30(1):78. 3 Figueiro MG Sleep Med. 2014 15(12):1554 - 64. 4 Lebert F. et al. Dement Geriatr Cogn Disord . 2004:17(4):355. 5 Sulzer DL et al. Am J Geriatr Psychiatry. 1997 5(1):60. 6 Cakir S. et el., Neuropsychiatr Dis Treat. 2008 4(5):963. 7 Wang, LY et al., Am J Geriatr Psychiatry. 2009 17(9):744 8 Settel E. Am Pract Dig Treat. 1957 8(10):1584.
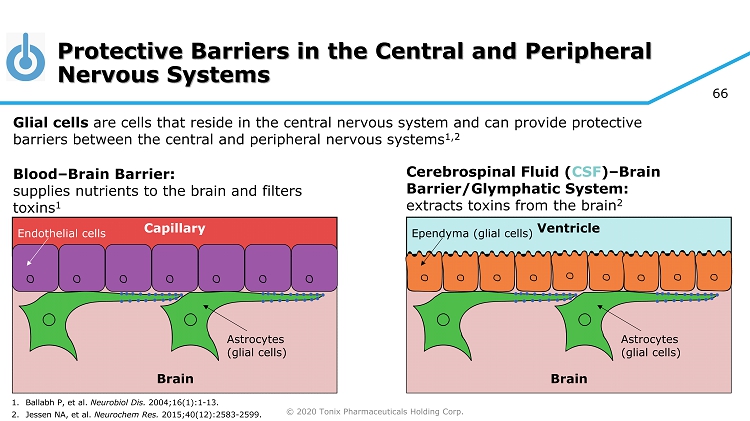
© 2020 Tonix Pharmaceuticals Holding Corp. 66 Protective Barriers in the Central and Peripheral Nervous Systems Glial cells are cells that reside in the central nervous system and can provide protective barriers between the central and peripheral nervous systems 1,2 Blood – Brain Barrier: supplies nutrients to the brain and filters toxins 1 Cerebrospinal Fluid ( CSF ) – Brain Barrier/Glymphatic System: extracts toxins from the brain 2 Ventricle Brain Ependyma (glial cells) Astrocytes (glial cells) Capillary Brain Endothelial cells Astrocytes (glial cells) 1. Ballabh P, et al. Neurobiol Dis. 2004;16(1):1 - 13. 2. Jessen NA, et al. Neurochem Res. 2015;40(12):2583 - 2599.
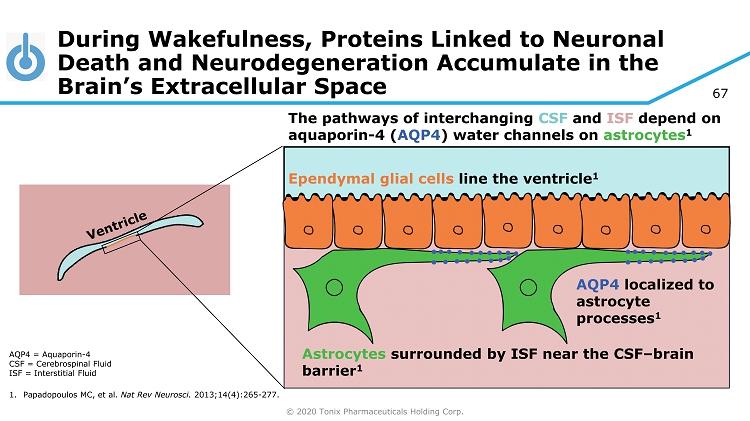
© 2020 Tonix Pharmaceuticals Holding Corp. 67 During Wakefulness, Proteins Linked to Neuronal Death and Neurodegeneration Accumulate in the Brain’s Extracellular Space AQP4 = A quaporin - 4 CSF = Cerebrospinal Fluid ISF = Interstitial Fluid 1. Papadopoulos MC, et al. Nat Rev Neurosci . 2013;14(4):265 - 277. Ependymal glial cells line the ventricle 1 AQP4 localized to astrocyte processes 1 Astrocytes surrounded by ISF near the CSF – brain barrier 1 The pathways of interchanging CSF and ISF depend on aquaporin - 4 ( AQP4 ) water channels on astrocytes 1
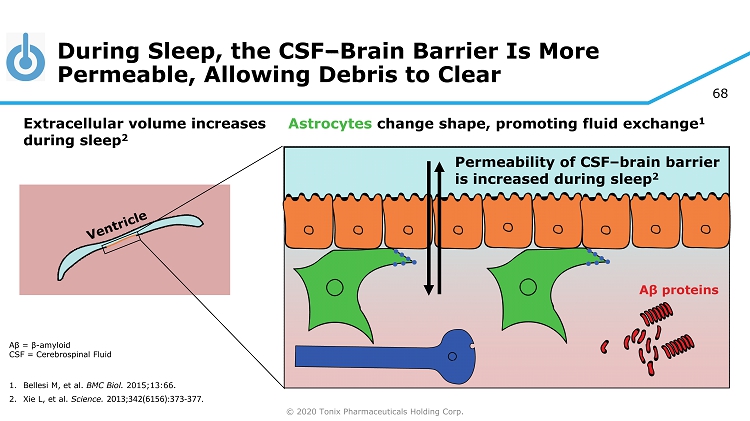
© 2020 Tonix Pharmaceuticals Holding Corp. 68 During Sleep, the CSF – Brain Barrier Is More Permeable, Allowing Debris to Clear Aβ = β - amyloid CSF = Cerebrospinal Fluid 1. Bellesi M, et al. BMC Biol. 2015;13:66. 2. Xie L, et al. Science. 2013;342(6156):373 - 377. Permeability of CSF – brain barrier is increased during sleep 2 Astrocytes change shape, promoting fluid exchange 1 Extracellular volume increases during sleep 2
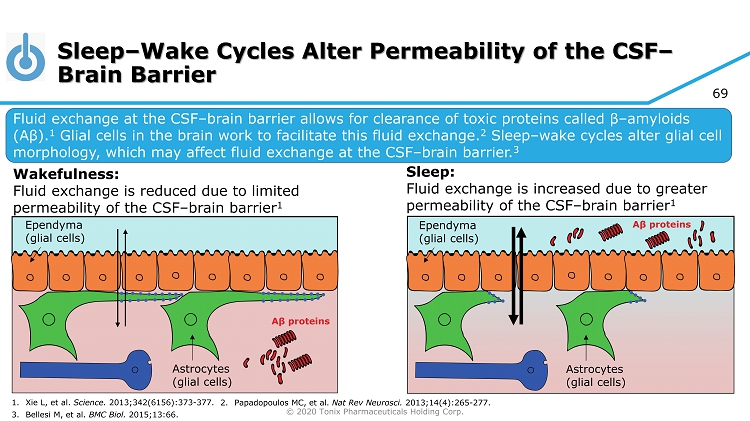
© 2020 Tonix Pharmaceuticals Holding Corp. 69 Sleep – Wake Cycles Alter Permeability of the CSF – Brain Barrier Fluid exchange at the CSF – brain barrier allows for clearance of toxic proteins called β – amyloids (A β ). 1 Glial cells in the brain work to facilitate this fluid exchange. 2 Sleep – wake cycles alter glial cell morphology, which may affect fluid exchange at the CSF – brain barrier. 3 Wakefulness: Fluid exchange is reduced due to limited permeability of the CSF – brain barrier 1 Sleep: Fluid exchange is increased due to greater permeability of the CSF – brain barrier 1 1. Xie L, et al. Science. 2013;342(6156):373 - 377. 3. Bellesi M, et al. BMC Biol. 2015;13:66. 2. Papadopoulos MC, et al. Nat Rev Neurosci. 2013;14(4):265 - 277. Ependyma (glial cells) Astrocytes (glial cells) Ependyma (glial cells) Astrocytes (glial cells)
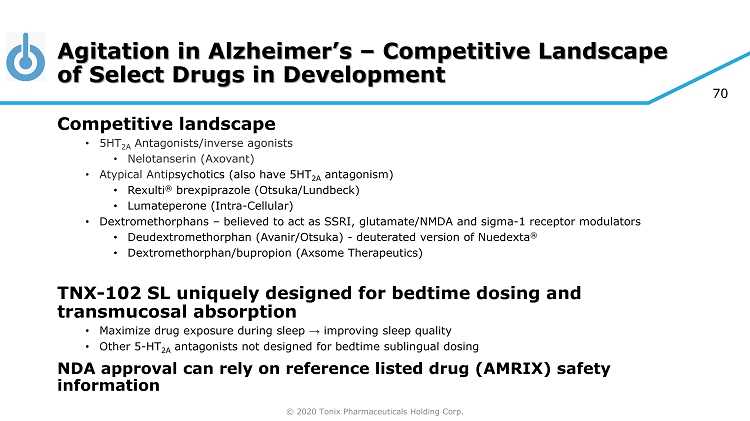
© 2020 Tonix Pharmaceuticals Holding Corp. 70 Agitation in Alzheimer’s – Competitive Landscape of Select Drugs in Development Competitive landscape • 5HT 2A Antagonists/inverse agonists • Nelotanserin ( Axovant ) • Atypical Antip sychotics (also have 5HT 2A antagonism) • Rexulti ® brexpiprazole (Otsuka/ Lundbeck ) • Lumateperone (Intra - Cellular ) • Dextromethorphans – believed to act as SSRI, glutamate/NMDA and sigma - 1 receptor modulators • Deudextromethorphan ( Avanir /Otsuka) - deuterated version of Nuedexta ® • Dextromethorphan/bupropion ( Axsome Therapeutics) TNX - 102 SL uniquely designed for bedtime dosing and transmucosal absorption • Maximize drug exposure during sleep → improving sleep quality • Other 5 - HT 2A antagonists not designed for bedtime sublingual dosing NDA approval can rely on reference listed drug (AMRIX) safety information

© 2020 Tonix Pharmaceuticals Holding Corp. 71 TNX - 102 SL: Potential Treatment for Alcohol Use Disorder (AUD) AUD is a chronic relapsing brain disease • Characterized by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not using Sleep disturbance is extremely common in alcohol recovery 1 • Significantly impacts daytime cognition, mood, and ability to participate in alcohol treatment, and is associated with increased risk of relapse Prevalence • An estimated 36 million adults in the U.S. have AUD 2 Three FDA - approved medications • Remains an unmet need due to compliance and safety issues Pre - IND meeting with the FDA completed in October 2019 • Discussed 505(b)(2) development plan for TNX - 102 SL as a treatment for AUD • FDA official meeting minutes confirm plan to submit IND application in 1Q 2020 for a Phase 2 Proof of Concept Study 1 Arnedt et al, J Addict Dis. 2007 ; 26(4): 41 – 54 2 Grant et al, JAMA Psychiatry 2015; 72(8): 757 - 766; www.census.gov
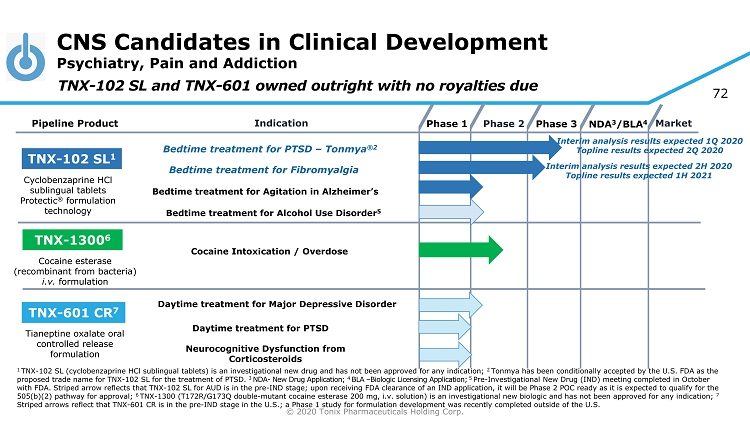
© 2020 Tonix Pharmaceuticals Holding Corp. 72 CNS Candidates in Clinical Development Psychiatry, Pain and Addiction Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime treatment for PTSD – Tonmya ®2 Daytime treatment for PTSD TNX - 601 CR 7 TNX - 1300 6 Cocaine Intoxication / Overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral controlled release formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 Bedtime t reatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due Bedtime t reatment for Agitation in Alzheimer’s Neurocognitive Dysfunction from Corticosteroids Bedtime treatment for Alcohol Use Disorder 5 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 Pre - Investigational New Drug (IND) meeting completed in October with FDA. Striped arrow reflects that TNX - 102 SL for AUD is in the pre - IND stage; upon receiving FDA clearance of an IND applica tion, it will be Phase 2 POC ready as it is expected to qualify for the 505(b)(2) pathway for approval; 6 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 7 Striped arrows reflect that TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 study for formulation development was recently completed outside of th e U.S. Interim analysis results expected 1Q 2020 Topline results expected 2Q 2020 Interim analysis results expected 2H 2020 Topline results expected 1H 2021 Daytime treatment for Major Depressive Disorder
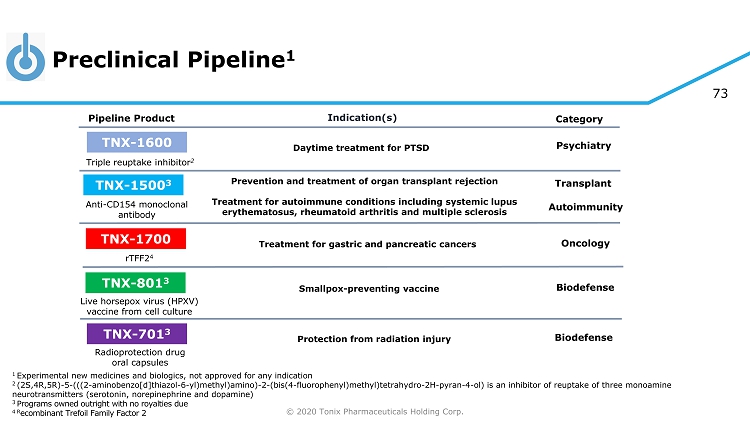
© 2020 Tonix Pharmaceuticals Holding Corp. 73 Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) 3 Programs owned outright with no royalties due 4 R ecombinant Trefoil Family Factor 2 Category Pipeline Product Indication(s) TNX - 801 3 Smallpox - preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture TNX - 701 3 Protection from radiation injury Radioprotection drug oral capsules Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for PTSD Psychiatry TNX - 1500 3 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Treatment for autoimmune conditions including systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Biodefense Biodefense

© 2020 Tonix Pharmaceuticals Holding Corp. TNX - 1300* for the Treatment of Cocaine Intoxication Recombinant protein that degrades cocaine in the bloodstream 1 • Double - mutant cocaine esterase Phase 2 study completed by Rickett Benckiser (TNX - 1300 was formerly RBP - 8000) 2 • Volunteer cocaine abusers received cocaine 50 mg i.v. infusion over 10 minutes • TNX - 1300 given one minute after completion of cocaine infusion • Rapidly reversed the physiologic effects of cocaine; cocaine plasma exposures dropped by 90% within two minutes • Well tolerated with the most frequently reported adverse events being gastrointestinal disorders ( including dry mouth, nausea); nervous systems disorders (including headache, dizziness) and skin and subcutaneous tissue disorders (including hyperhidrosis , dermatitis) *TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication. 1 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23. 2 Nasser AF et al, J Addict Dis . 2014;33(4):289 - 302.
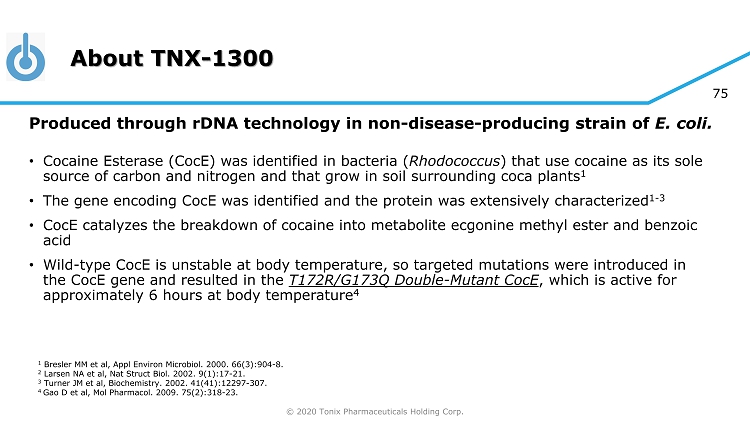
© 2020 Tonix Pharmaceuticals Holding Corp. 75 About TNX - 1300 Produced through rDNA technology in non - disease - producing strain of E. coli. • Cocaine Esterase ( CocE ) was identified in bacteria ( Rhodococcus ) that use cocaine as its sole source of carbon and nitrogen and that grow in soil surrounding coca plants 1 • The gene encoding CocE was identified and the protein was extensively characterized 1 - 3 • CocE catalyzes the breakdown of cocaine into metabolite ecgonine methyl ester and benzoic acid • Wild - type CocE is unstable at body temperature, so targeted mutations were introduced in the CocE gene and resulted in the T172R/G173Q Double - Mutant CocE , which is active for approximately 6 hours at body temperature 4 1 Bresler MM et al, Appl Environ Microbiol. 2000. 66(3):904 - 8. 2 Larsen NA et al, Nat Struct Biol. 2002. 9(1):17 - 21. 3 Turner JM et al, Biochemistry. 2002. 41(41):12297 - 307. 4 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23.
© 2020 Tonix Pharmaceuticals Holding Corp. 76 About Cocaine and Cocaine Intoxication Cocaine: an illegal recreational drug taken for its pleasurable effects and associated euphoria. • Cocaine blocks the reuptake of the neurotransmitter dopamine (DA) in the CNS • Results in accumulation of DA within the synapse and amplifies DA signaling • Creates positive feeling but with intense use of cocaine, results in cocaine craving • High potential for abuse/addiction (dependence), and risk of cocaine intoxication. Cocaine intoxication: deleterious effects on the body, especially cardiovascular system. • Common symptoms include tachyarrhythmias and elevated blood pressure, either of which can be life - threatening. • Known or suspected cocaine intoxication cases are sent immediately to the emergency department, preferably by ambulance in case cardiac arrest occurs during transit.
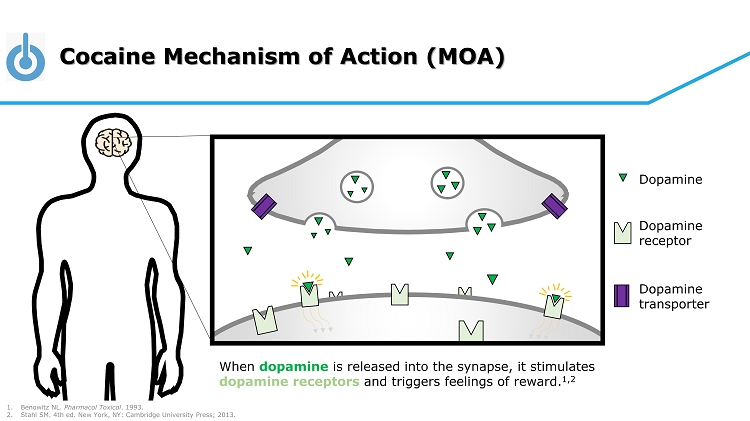
Cocaine Mechanism of Action (MOA) When dopamine is released into the synapse, it stimulates dopamine receptors and triggers feelings of reward. 1,2 Dopamine Dopamine receptor Dopamine transporter 1. Benowitz NL. Pharmacol Toxicol . 1993. 2. Stahl SM. 4th ed. New York, NY: Cambridge University Press; 2013.
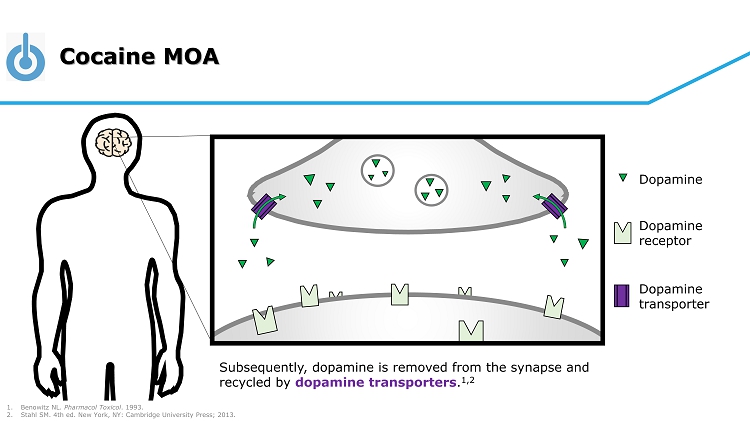
Cocaine MOA Dopamine Dopamine receptor Dopamine transporter Subsequently, dopamine is removed from the synapse and recycled by dopamine transporters . 1,2 1. Benowitz NL. Pharmacol Toxicol . 1993. 2. Stahl SM. 4th ed. New York, NY: Cambridge University Press; 2013.
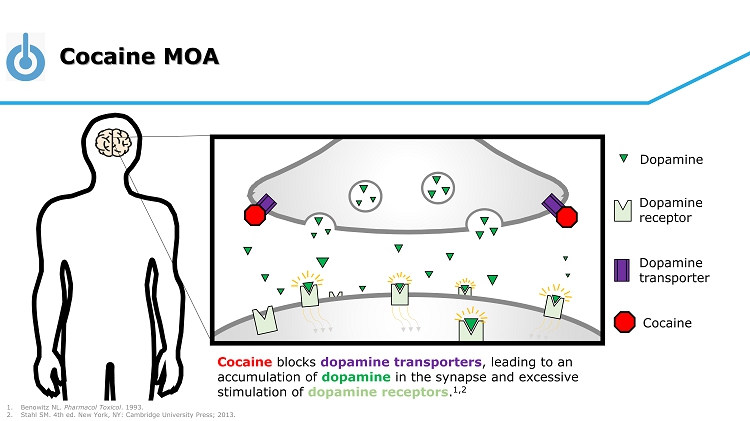
Cocaine MOA Cocaine blocks dopamine transporters , leading to an accumulation of dopamine in the synapse and excessive stimulation of dopamine receptors . 1,2 Cocaine Dopamine Dopamine receptor Dopamine transporter 1. Benowitz NL. Pharmacol Toxicol . 1993. 2. Stahl SM. 4th ed. New York, NY: Cambridge University Press; 2013.
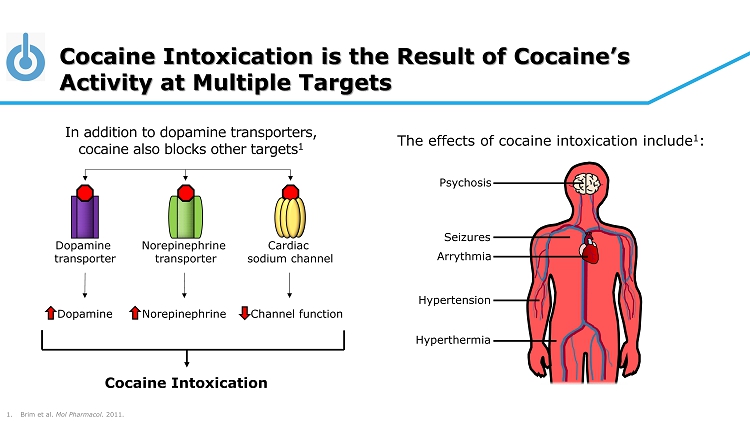
Cocaine Intoxication is the Result of Cocaine’s Activity at Multiple Targets 1. Brim et al. Mol Pharmacol . 2011. Psychosis Seizures Arrythmia Hypertension Hyperthermia The effects of cocaine intoxication include 1 : In addition to dopamine transporters, cocaine also blocks other targets 1 Dopamine transporter Norepinephrine transporter Cardiac sodium channel Cocaine Intoxication Dopamine Norepinephrine Channel function
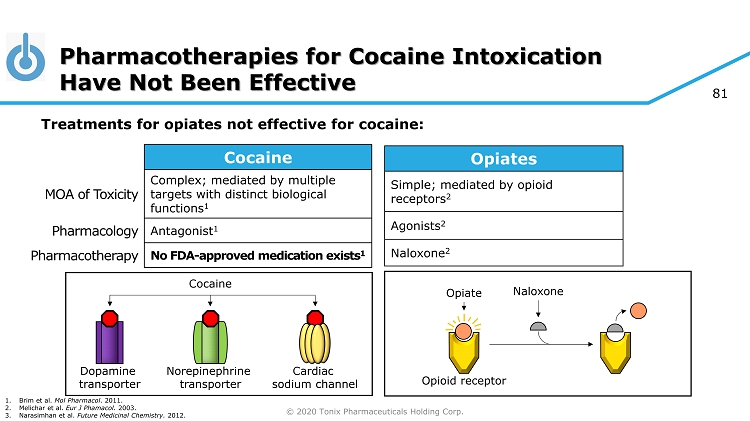
© 2020 Tonix Pharmaceuticals Holding Corp. 81 Pharmacotherapies for Cocaine Intoxication Have Not Been Effective 1. Brim et al. Mol Pharmacol . 2011. 2. Melichar et al. Eur J Phamacol . 2003. 3. Narasimhan et al. Future Medicinal Chemistry. 2012. Opiates Simple; mediated by opioid receptors 2 Agonists 2 Naloxone 2 Cocaine MOA of Toxicity Complex; mediated by multiple targets with distinct biological functions 1 Pharmacology Antagonist 1 Pharmacotherapy No FDA - approved medication exists 1 Dopamine transporter Norepinephrine transporter Cardiac sodium channel Cocaine Opiate Opioid receptor Naloxone Treatments for opiates not effective for cocaine:
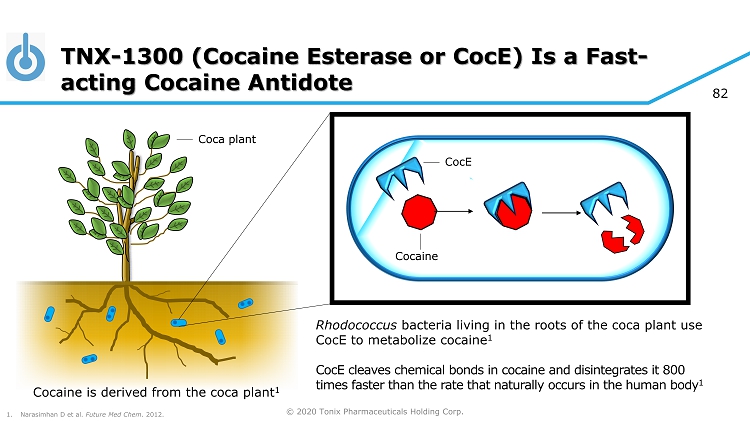
© 2020 Tonix Pharmaceuticals Holding Corp. 82 TNX - 1300 (Cocaine Esterase or CocE ) Is a Fast - acting Cocaine Antidote CocE Rhodococcus bacteria living in the roots of the coca plant use CocE to metabolize cocaine 1 CocE cleaves chemical bonds in cocaine and disintegrates it 800 times faster than the rate that naturally occurs in the human body 1 Cocaine Cocaine is derived from the coca plant 1 1. Narasimhan D et al. Future Med Chem . 2012. Coca plant

Pharmacotherapies for Cocaine Intoxication Have Not Been Effective • While simple pharmacological agents such as naloxone (Narcan ® ) are effective for the treatment of opiate intoxication 1 , a similar approach to treat cocaine intoxication is hampered by cocaine’s complex mechanism of action, or MOA 2 • Another key difference between opiates and cocaine is that opiates are agonists at opiate receptors 1 , while cocaine acts as an antagonist at its key targets. 2 Compounds that compete with an inhibitor such as cocaine are likely to be inhibitors themselves. 3 • Despite years of research, pharmacotherapies designed to prevent cocaine from binding to its target molecules have not been effective 2,3 References 1. Melichar JK, Nutt DJ, Malizia AL. Naloxone displacement at opioid receptor sites measured in vivo in the human brain. European Journal of Pharmacology. 2003; 459(2 - 3):217 - 219. 2. Brim RL, Noon KR, Collins GT, Nichols J, Narasimhan D, Sunahara RK, Woods JH. The ability of bacterial cocaine esterase to hydrolyze cocaine metabolites and their simultaneous quantification using high - performance liquid chromatography - tandem mass spectrometry. Molecular Pharmacology . 2011; 80:1119 - 1127. 3. Narasimhan D, Woods JH, Sunahara RK. Bacterial cocaine esterase: a protein - based therapy for cocaine overdose and addiction. Future Medicinal Chemistry. 2012; 4(2):137 - 150.
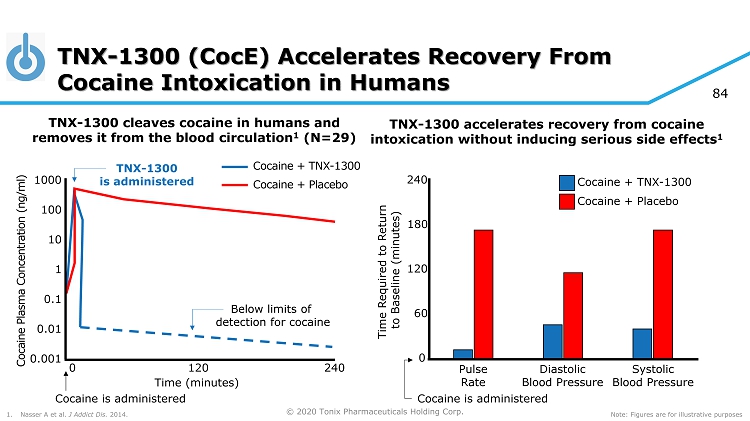
© 2020 Tonix Pharmaceuticals Holding Corp. 84 TNX - 1300 cleaves cocaine in humans and removes it from the blood circulation 1 (N=29) Time Required to Return to Baseline (minutes) 120 0 240 Pulse Rate Diastolic Blood Pressure Systolic Blood Pressure 60 180 Time (minutes) 120 240 0 Cocaine Plasma Concentration (ng/ml) TNX - 1300 accelerates recovery from cocaine intoxication without inducing serious side effects 1 TNX - 1300 (CocE) Accelerates Recovery From Cocaine Intoxication in Humans Cocaine + TNX - 1300 Cocaine + Placebo Cocaine + Placebo Cocaine + TNX - 1300 Cocaine is administered TNX - 1300 is administered Note: Figures are for illustrative purposes 1. Nasser A et al. J Addict Dis. 2014. Cocaine is administered 0.1 0.001 10 0.01 1 100 1000 Below limits of detection for cocaine
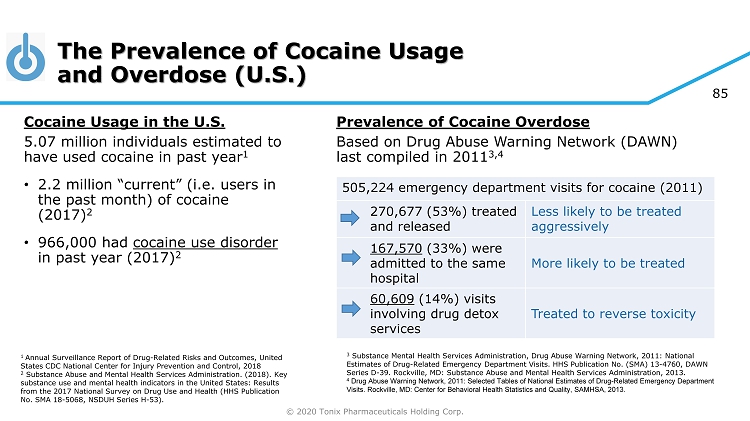
© 2020 Tonix Pharmaceuticals Holding Corp. 85 The Prevalence of Cocaine Usage and Overdose (U.S.) Cocaine Usage in the U.S. 5.07 million individuals estimated to have used cocaine in past year 1 • 2.2 million “current” (i.e. users in the past month) of cocaine (2017) 2 • 966,000 had cocaine use disorder in past year (2017) 2 1 Annual Surveillance Report of Drug - Related Risks and Outcomes, United States CDC National Center for Injury Prevention and Control, 2018 2 Substance Abuse and Mental Health Services Administration. (2018). Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18 - 5068, NSDUH Series H - 53). Prevalence of Cocaine Overdose Based on Drug Abuse Warning Network (DAWN) last compiled in 2011 3,4 505,224 emergency department visits for cocaine (2011) 270,677 (53%) treated and released Less likely to be treated aggressively 167,570 (33%) were admitted to the same hospital More likely to be treated 60,609 (14%) visits involving drug detox services Treated to reverse toxicity 3 Substance Mental Health Services Administration, Drug Abuse Warning Network, 2011: National Estimates of Drug - Related Emergency Department Visits. HHS Publication No. (SMA) 13 - 4760, DAWN Series D - 39. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. 4 Drug Abuse Warning Network, 2011: Selected Tables of National Estimates of Drug - Related Emergency Department Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality, SAMHSA, 2013.
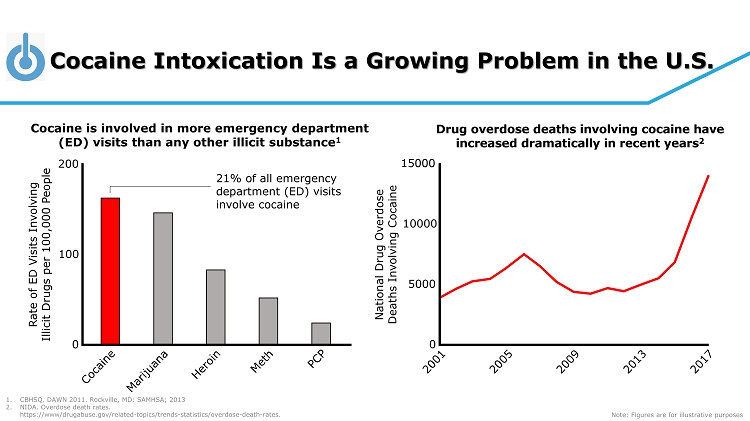
Cocaine Intoxication Is a Growing Problem in the U.S. National Drug Overdose Deaths Involving Cocaine 0 5000 10000 15000 Cocaine is involved in more emergency department (ED) visits than any other illicit substance 1 Drug overdose deaths involving cocaine have increased dramatically in recent years 2 1. CBHSQ. DAWN 2011. Rockville, MD: SAMHSA; 2013 2. NIDA. Overdose death rates. https://www/drugabuse.gov/related - topics/trends - statistics/overdose - death - rates. Note: Figures are for illustrative purposes Rate of ED Visits Involving Illicit Drugs per 100,000 People 0 100 200 21% of all emergency department (ED) visits involve cocaine
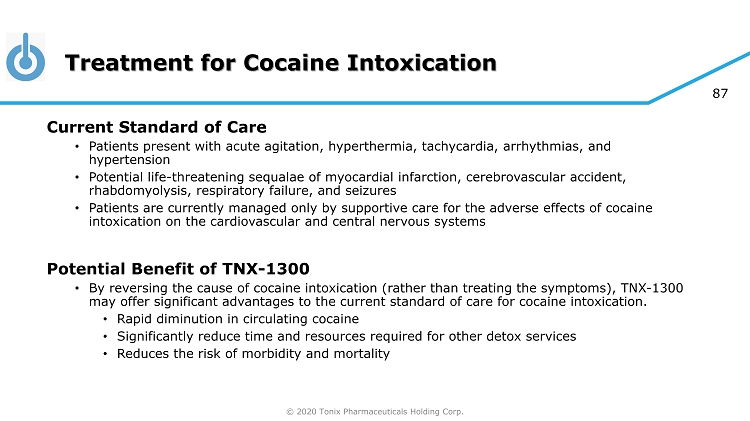
© 2020 Tonix Pharmaceuticals Holding Corp. 87 Treatment for Cocaine Intoxication Current Standard of Care • Patients present with acute agitation, hyperthermia, tachycardia, arrhythmias, and hypertension • Potential life - threatening sequalae of myocardial infarction, cerebrovascular accident, rhabdomyolysis, respiratory failure, and seizures • Patients are currently managed only by supportive care for the adverse effects of cocaine intoxication on the cardiovascular and central nervous systems Potential Benefit of TNX - 1300 • By reversing the cause of cocaine intoxication (rather than treating the symptoms), TNX - 1300 may offer significant advantages to the current standard of care for cocaine intoxication. • Rapid diminution in circulating cocaine • Significantly reduce time and resources required for other detox services • Reduces the risk of morbidity and mortality

© 2020 Tonix Pharmaceuticals Holding Corp. Value of TNX - 1300 to Tonix Features of the Acquired Asset: • Full rights to the IP and to develop and commercialize TNX - 1300 worldwide • An inventory of investigational drug product • Clinical trial results from previous Phase 2 study in which TNX - 1300 at 100 mg or 200 mg i.v. doses was well tolerated and interrupted cocaine effects after cocaine 50 mg i.v. challenge Development Plan: • Re - qualify the drug substance for Good Manufacturing Practice (GMP) purposes • Conduct non - clinical studies in reproductive toxicology • Initiate a Phase 2 study in Emergency Room cocaine intoxication Exclusivity: • Expected patent protection through 2029 • As a biologic and new molecular entity, TNX - 1300 is eligible for 12 years of U.S. market exclusivity upon approval by the FDA. Pipeline Diversification: • Brings Tonix into an additional therapeutic area: Addiction Medicine
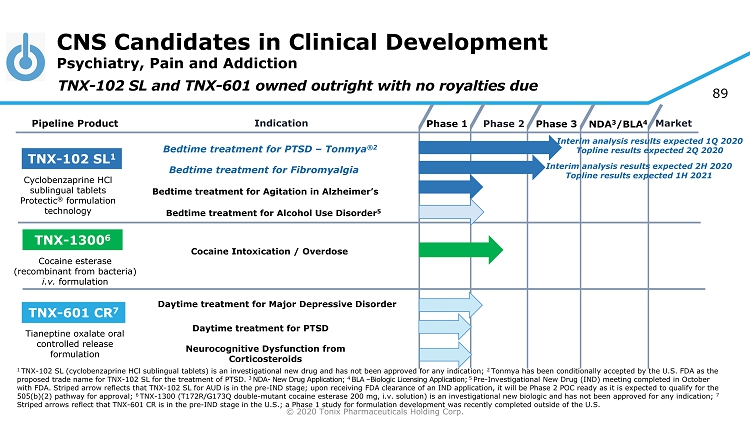
© 2020 Tonix Pharmaceuticals Holding Corp. 89 CNS Candidates in Clinical Development Psychiatry, Pain and Addiction Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime treatment for PTSD – Tonmya ®2 Daytime treatment for PTSD TNX - 601 CR 7 TNX - 1300 6 Cocaine Intoxication / Overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral controlled release formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 Bedtime t reatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due Bedtime t reatment for Agitation in Alzheimer’s Neurocognitive Dysfunction from Corticosteroids Bedtime treatment for Alcohol Use Disorder 5 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 Pre - Investigational New Drug (IND) meeting completed in October with FDA. Striped arrow reflects that TNX - 102 SL for AUD is in the pre - IND stage; upon receiving FDA clearance of an IND applica tion, it will be Phase 2 POC ready as it is expected to qualify for the 505(b)(2) pathway for approval; 6 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 7 Striped arrows reflect that TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 study for formulation development was recently completed outside of th e U.S. Interim analysis results expected 1Q 2020 Topline results expected 2Q 2020 Interim analysis results expected 2H 2020 Topline results expected 1H 2021 Daytime treatment for Major Depressive Disorder
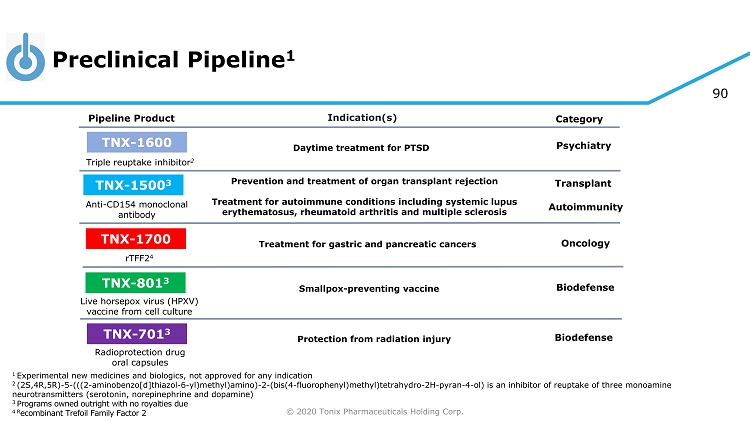
© 2020 Tonix Pharmaceuticals Holding Corp. 90 Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) 3 Programs owned outright with no royalties due 4 R ecombinant Trefoil Family Factor 2 Category Pipeline Product Indication(s) TNX - 801 3 Smallpox - preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture TNX - 701 3 Protection from radiation injury Radioprotection drug oral capsules Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for PTSD Psychiatry TNX - 1500 3 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Treatment for autoimmune conditions including systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Biodefense Biodefense
© 2020 Tonix Pharmaceuticals Holding Corp. 91 TNX - 601 CR* (Tianeptine Oxalate Controlled Release) Tablets Proprietary new controlled release formulation for once - daily dosing • Suitability for once - daily dosing established in Phase 1 pharmacokinetic study, completed outside of the U.S. • Well tolerated in study and side effects were consistent with the known safety profile of tianeptine sodium • Tianeptine sodium immediate release is approved and marketed outside of the U.S. for three times a day dosing for the treatment of depression • Once - daily dosing for TNX - 601 CR believed to have an adherence advantage over three times a day dosing with tianeptine sodium • Plan to request pre - IND meeting with FDA in first half 2020 • Plan for Phase 2 study in depression, ex - U.S., in second half 2020 Proprietary new oxalate salt with improved pharmaceutical properties • Tianeptine oxalate is crystalline, while tianeptine sodium is amorphous Issued patents directed to tianeptine and tianeptine oxalate • Composition of Matter: Issued US patent directed to oxalate salt, U.S. Patent No. 10,449,203 • Method of Use: Issued U.S. and European patents directed to methods of treating cognitive impairment associated with corticosteroid treatment (U.S. Patent No. 9,314,469; European Patent No. 3246031) *TNX - 601 (tianeptine oxalate CR tablets) is in the pre - IND stage in the U.S. and has not been approved for any indication.
© 2020 Tonix Pharmaceuticals Holding Corp. 92 TNX - 601 CR : A Potential Daytime Treatment for Depression and PTSD Depression: majority suffering from depression do not have an adequate response to initial antidepressant therapy • Tianeptine sodium immediate release (IR) tablets for three times a day dosing is approved as an antidepressant in the EU, Russia, Asia and Latin America; first marketed for depression in France in 1989 • Tianeptine sodium is reported to have prominent anti - anxiety effects in depression with a low incidence of sexual side effects • TNX - 601 CR leverages the established efficacy and safety of tianeptine sodium IR as a treatment for depression outside of the U.S. • Despite multiple approved products for depression in the U.S., there remains significant interest and need for new treatments, particularly for medicines that modulate the glutamatergic system PTSD: heterogeneous condition, so not all patients are expected to respond to a single medicine • Distinct mechanism of action from TNX - 102 SL – TNX - 601 CR modulates the glutamatergic system • Published studies show tianeptine is active in the treatment of PTSD 1 - 4 • Leverages Tonix expertise in PTSD (clinical and regulatory, market analysis, etc.) 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747
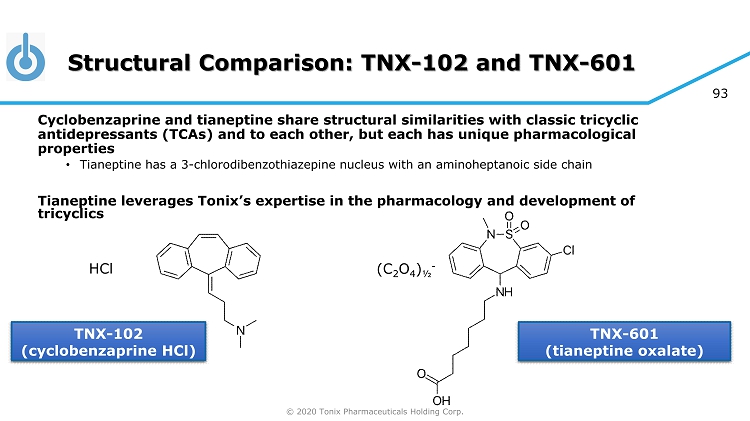
© 2020 Tonix Pharmaceuticals Holding Corp. 93 Structural Comparison: TNX - 102 and TNX - 601 Cyclobenzaprine and tianeptine share structural similarities with classic tricyclic antidepressants (TCAs) and to each other, but each has unique pharmacological properties • Tianeptine has a 3 - chlorodibenzothiazepine nucleus with an aminoheptanoic side chain Tianeptine leverages Tonix’s expertise in the pharmacology and development of tricyclics TNX - 102 (cyclobenzaprine HCl) TNX - 601 (tianeptine oxalate) HCl (C 2 O 4 ) ½ -
© 2020 Tonix Pharmaceuticals Holding Corp. 94 TNX - 1600 1 (Triple Reuptake Inhibitor): A Potential Daytime Treatment for PTSD Pre - IND Candidate Targeted as a 1 st line monotherapy for PTSD: oral formulation for daytime dosing x Leverages internal expertise in PTSD (clinical and regulatory experience, market analysis, etc.) x Mechanism of Action (MOA) is different from TNX - 102 SL or TNX - 601 TNX - 1600 is a New Chemical Entity, triple - reuptake inhibitor • Inhibits reuptake of serotonin, norepinephrine and dopamine Patents and patent applications • Issued patent directed to composition of matter • Worldwide exclusive license from Wayne State University Targeting a Condition with Significant Unmet Need Preclinical evidence for treating PTSD in animal model • Pre - clinical studies have shown TNX - 1600 to be active in an animal model of PTSD 2 1 T NX - 1600, f.k.a . D - 578 or (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) 2 Dutta, AK, et al., Eur J. Pharmacol . 2019 862:172632
© 2020 Tonix Pharmaceuticals Holding Corp. 95 TNX - 1500 (monoclonal antibody anti - CD154): A Potential Treatment for Autoimmune Conditions and Organ Transplant Rejection Pre - IND Candidate Targeted as a 1 st line monotherapy for autoimmunity and add - on therapy for preventing and treating organ transplant rejection x Mechanism of Action (MOA) is distinct • TNX - 1500 blocks T cell helper function New Molecular Entity, biologic • US Patient Protection and Affordable Care Act provides 12 years of exclusivity for biologics Patent applications directed to composition of matter • Expected patent protection through 2039 Targeting a Condition with Significant Unmet Need Clinical evidence for anti - CD154 mAbs in Systemic Lupus (SLE) and allogeneic kidney transplant • Several studies have shown TNX - 1500 to be active in the treatment of human SLE 1 - 3 and transplant 4,5 1 Huang W, et al . Arthritis Rheum. 46(6):1554 – 62 (2002) 2 Boumpas DT, et al , Arthritis Rheum. 48:719 – 27. (2003) 3 Grammer AC, et al . J Clin Invest. 112:1506 – 20. (2003) 4 Kawai T, et al. Nat Med. 2000;6:114. (2000); 5 Koyama I, et al ., Transplantation. 77(3):460 - 2. (2004)
© 2020 Tonix Pharmaceuticals Holding Corp. 96 About CD40L (CD154) Transiently expressed T cell surface molecule also known as CD40 - ligand 1 - 4 • Predominantly expressed by T cells • Interacts with CD40 on B cells and macrophages Mediates T cell helper function 1 - 4 • Activates B cells for humoral (antibody - mediated) immune response • Activates macrophages and dendritic cell • Provides T cell help to activated CD8+ T cells X - linked Hyper - IgM Syndrome – defective CD40L gene 5 - 6 • Lack of T helper function • Serum antibodies: only IgM, and no IgG or IgE because T cells are required for B cell isotype switching • If maintained on gamma globulin are otherwise healthy Member of the TNF α superfamily 4 • TNF α and RANKL are other family members – drug targets for approved products 1 Lederman, S., et al. J. Exp. Med. 175:1091 - 1101. 1992. PMID: 1348081. 2 Lederman, S., et al;. J. Immunol. 149:3817 - 3826. 1992. PMID: 1281189. 3 Lederman, S., et al. J. Immunol. 152:2163. 1994. PMID: 7907632. 4 Covey, L.R., et al. Mol. Immunol. 31:471 - 484. 1994. PMID: 7514269. 5 Ramesh, N., et al. 1993. Inter Immunology 5:769 - 773. PMID: 8103673. 6 Callard, R.E., et al., J. Immunol. 153:3295. 1994. PMID: 7916370.
© 2020 Tonix Pharmaceuticals Holding Corp. 97 TNF α Superfamily CD154 is a member of the Tumor Necrosis Factor (TNF α ) Super Family 1 • No mAb against CD154 has been licensed anywhere in the world Other TNF α Super Family members have proven to be targets for antagonist (blocking) mAbs 2 • anti - TNFα mAbs for the treatment of certain autoimmune conditions • infliximab (Remicade ® ) • adalimumab (Humira ® ) • certolizumab pegol ( Cimzia ® ) • golimumab (Simponi ® ) • TNFα antagonist receptor fusion protein • Etanercept (Enbrel ® ) • anti - RANKL (CD254) mAb for the treatment of osteoporosis, treatment - induced bone loss, metastases to bone, and giant cell tumor of bone • denosumab (Prolia ® or Xgeva ® ) 1 Covey, L.R., et al. Mol. Immunol. 31:471 - 484. 1994. PMID: 7514269. 2 Remicade ® and Simponi ® are trademarks of Janssen; Humira ® is a trademark of AbbVie; Cimzia ® is a trademark of UCB; Enbrel ® is a trademark of Amgen; and Prolia ® and Xgeva ® are trademarks of Amgen.
© 2020 Tonix Pharmaceuticals Holding Corp. 98 TNX - 1500 (anti - CD40L (CD154)) Transplantation/Autoimmune treatment development asset • 3 rd generation of monoclonal antibody ( mAb ) for a class that has had extensive animal and human testing • Effects on T cell function with lower potential for side effects (e.g. thrombosis via Fc γ RIIA (CD32A) - dependent pathway) 1 • Patent protection expected through 2039 Transplantation • Unique effects on facilitating tolerance • Potential to facilitate xeno - transplants (genetically engineered mini - swine) 2 Autoimmune Diseases • Unique effect at controlling autoimmune conditions 3 - 5 • Clinical data on related mAbs for systemic lupus erythematosus (SLE) 3 - 5 Allergy • Blocks immunoglobin E ( IgE ) production 1 Company data 2 Längin M, et al., Nature. 2018 564(7736):430 - 433 3 Huang W, et al . Arthritis Rheum. 46(6):1554 – 62 (2002) 4 Boumpas DT, et al , Arthritis Rheum. 48:719 – 27. (2003) 5 Grammer AC, et al . J Clin Invest. 112:1506 – 20. (2003)
© 2020 Tonix Pharmaceuticals Holding Corp. 99 TNX - 1500 – Potential Treatment for Organ Transplant Rejection Facilitates ‘transplant tolerance’ in multiple preclinical transplant models • anti - CD154 therapy has a unique activity in controlling the immune response to organ transplants 1 - 3 • Significant need for new treatments with improved activity and tolerability to prevent or treat organ transplant rejection Human trials of first generation anti - CD154 showed evidence of activity • Development halted because of increased risk of thrombosis 4 - 6 Potential to enable use of genetically modified, or humanized pig organs – “xenotransplantation.” 7,8 • Potential treatment for humans with advanced organ failure or diabetes 1 Ferrant JL et al., International Immunol . (11):1583 (2004) 2 O ʼ Neill NA, et al. Transplantation. 101(9): 2038 (2017) 3 Zhang T, et al. Immunotherapy . 7(8):899 (2015) 4 Kawai T, et al. Nat Med. 2000;6:114. (2000) 5 Koyama I, et al ., Transplantation. 77(3):460 - 2. (2004) 6 Law and Grewal Adv Exp Med Biol. 647:8 - 36 (2009) 7 Längin M, et al. Nature. 564(7736):430 (2018) 8 Pierson RN 3rd. J Thorac Cardiovasc Surg. pii : S0022 - 5223(19)31024 - 4. (2019)
© 2020 Tonix Pharmaceuticals Holding Corp. 100 TNX - 1500 – Potential Treatment for Autoimmune Disease Treats autoimmune conditions in multiple preclinical transplant models • anti - CD154 therapy has a unique activity in controlling the immune response in autoimmune models 1 - 3 • Significant need for new treatments with improved activity and tolerability to prevent or treat autoimmunity Human trials of first generation anti - CD154 showed activity • Clinical trials of hu5c8, in systemic lupus erythematosus (SLE) showed evidence of activity 1 - 3 • Development halted because of increased risk of thrombosis 1 - 3 1 Huang W, et al . Arthritis Rheum. 46(6):1554 – 62 (2002) 2 Boumpas DT, et al , Arthritis Rheum. 48:719 – 27. (2003) 3 Grammer AC, et al . J Clin Invest. 112:1506 – 20. (2003)
© 2020 Tonix Pharmaceuticals Holding Corp. 101 Third Generation anti - CD154: Engineered to Potentially Decrease Risk of Thrombosis First generation anti - CD154 mAbs • Constant fragment (Fc) domain interacted with FcγRIIA (CD32A), which suggested a mechanism for increased risk of thrombosis 1,2 Second generation anti - CD154 mAbs • Dramatically reduced binding to FcγRIIA 3,4 , but had other issues, including decreased efficacy 5,6 TNX - 1500 is a third generation anti - CD154 mAb 6 - 8 • Designed by protein engineering to target CD154 therapeutically, while decreasing FcγRIIA binding and the potential for thrombosis 1 Inwald DP et al., Circ Res. 92(9):1041 - 8 (2003)) 2 Robles - Carrillo L et al ., J Immunol. 185(3):1577 - 83. (2010)) 3 Shock A. et al., Arthritis Res Ther . 17:234 (2015) 4 Xie et al., Journal of Immunol. 192(9):4083 (2014)) 5 Waters J, Biocentury ; October 26, (2018) 6 Company data 7 NCT02273960; ClinicalTrials.gov ; “Study to Evaluate Safety and Efficacy in Adult Subjects With ITP (ITP)”; results posted April 1, 2019, accessed July 29, 2 019 ) 8 Ferrant JL et al., International Immunol . (11):1583 (2004)
© 2020 Tonix Pharmaceuticals Holding Corp. 102 TNX - 1700 (rTFF2): A Potential Treatment for Gastric and Pancreatic Cancers Pre - IND Candidate Targeted as a treatment for Cancer x Particularly for gastric and pancreatic cancer x Mechanism of Action (MOA) is different from checkpoint inhibitors x Potential synergy with anti - PD - 1 or anti - PD - L1 monoclonal antibodies Patents and patent applications directed to rTFF2 • Issued patent licensed from Columbia University Inventor: Dr. Timothy Wang, MD • Chief, Division of Digestive and Liver Diseases at Columbia University and Cancer Research Center and Silberberg Professor of Medicine • Investigated the molecular mechanisms of gastrointestinal carcinogenesis for decades • Leadership roles in gastroenterology and cancer biology fields Targeting a Condition with Significant Unmet Need Pre - clinical evidence for inhibiting growth of cancer cells • Several studies have shown rTFF2 to be active in the treatment of cancer 1 - 2 1 Dubeykovskaya Z, et al. Nat Commun . 2016 7:1 - 11 2 Dubeykovskaya ZA, et al, Cancer Gene Ther . 2019 26(1 - 2):48 - 57
© 2020 Tonix Pharmaceuticals Holding Corp. 103 TNX - 1700 (rTFF2) for Potential Cancer Treatment • Oncology development program • Recombinant trefoil family factor 2 (rTFF2) has effects on cancer cells and the tumor microenvironment 1,2 • Potential synergy with α nti - PD - 1/PD - L1 mAbs (Keytruda ® and Opdivo ® ) and/or anti - CTLA - 4 ( Yervoy ® ) “Checkpoint Inhibitors” • anti - PD - 1 and anti - PDL - 1 are breakthrough treatments, but not all patients respond • Increasing the response rate to checkpoint inhibitors is an active area of research • rTFF2 acts in the tumor microenvironment • Novel mechanism for suppressing myeloid - derived suppressor cells, and activating anti - cancer CD8+ T cells • Implications for both cancer prevention and treatment • Potential to synergize with other immunotherapy drugs 1 Dubeykovskaya Z, et al. Nat Commun . 2016 7:1 - 11 2 Dubeykovskaya ZA, et al, Cancer Gene Ther . 2019 26(1 - 2):48 - 57
© 2020 Tonix Pharmaceuticals Holding Corp. 104 Cancer: Toxic Tumor Microenvironment • Tumor microenvironment sabotages immune T cells • Made up of blood vessels, inflammatory cells, and structural proteins • Difficult for cancer - killing immune T cells to penetrate • T cells detect and destroy cancer cells • Cancer surrounds tumors with a hostile microenvironment • Tumors thrive, while the body’s immune forces are not capable of performing their anti - cancer functions • Although the tumor microenvironment is known to be highly immunosuppressive, it has not been known precisely how it specifically hampers the function of T cells
© 2020 Tonix Pharmaceuticals Holding Corp. 105 Trefoil Family Factor 2 (rTFF2) and Cancer Biology • TFF2 is a small secreted protein • Encoded by the TFF2 gene in humans • Expressed in gastrointestinal mucosa where it functions to protect and repair mucosa • TFF2 is also expressed at low levels in splenic memory T cells • Upregulated in chronic inflammation • Activates the chemokine receptor CXCR4 in cancer cells • Blocked by AMD3100 (CXCR4 antagonist) or anti - CXCR4 mAb • TFF2 is epigenetically silenced in gastric cancer • Postulated to protect against cancer development through multiple mechanisms • Has effects on cancer cells and tumor microenvironment • Knockout of the TFF2 gene leads to faster tumor growth
© 2020 Tonix Pharmaceuticals Holding Corp. 106 Published Research on TNX - 1700 (rTFF2) by Dr. Wang at Columbia • Either TFF2 overexpression or adenovirus - delivered rTFF2 markedly suppresses tumor growth 1,2 • Curtailed the proliferation and expansion of myeloid progenitors that give rise to myeloid derived suppressor cells (MDSCs) • Adenovirus over - expression decreased tumor growth in a wild - type mouse model • Knockout of the TFF2 gene leads to faster tumor growth • Novel mechanism for suppressing myeloid - derived suppressor cells, and activating anti - cancer CD8+ T cells • Implications for both cancer prevention and treatment • Potential to synergize with other immunotherapy drugs • Modified version of human TFF2 appears to show greater stability and efficacy 2 • Native TFF2 has a short half - life 1 Dubeykovskaya Z, et al. Nat Commun . 2016 7:1 - 11 2 Dubeykovskaya ZA, et al, Cancer Gene Ther . 2019 26(1 - 2):48 - 57

© 2020 Tonix Pharmaceuticals Holding Corp. 107 TNX - 801 (Synthesized Live Horsepox Virus): A Potential Smallpox - Preventing Vaccine Pre - IND Stage Potential improvement over current biodefense tools against smallpox ✓ Leverages Tonix’s government affairs effort ✓ Collaboration with Professor David Evans and Dr. Ryan Noyce at University of Alberta ✓ Demonstrated protective vaccine activity in mice ✓ Patent application on novel vaccine submitted Regulatory strategy • We intend to meet with FDA to discuss the most efficient and appropriate investigational plan to support the licensure, either: ✓ Application of the “Animal Rule”, or ✓ Conducting an active comparator study using ACAM2000 • Good Manufacturing Practice (GMP) viral production process in development Targeting a Potential Public Health Issue Material threat medical countermeasure under 21 st Century Cures Act • Qualifies for Priority Review Voucher (PRV) upon licensure * ✓ PRVs have no expiration date, are transferrable and have sold for ~$125 M *BLA/NDA priority 6 - month review is expected.

© 2020 Tonix Pharmaceuticals Holding Corp. 108 TNX - 801 (Synthesized Live Horsepox Virus): A Potential Smallpox - Preventing Vaccine Synthesis 1 from sequence of a 1976 Mongolian isolate 2 In mice, TNX - 801 behaved like attenuated vaccinia virus • Vaccinia is the term used to classify the live poxviruses that are used as smallpox vaccines, including ACAM2000, which is the latest smallpox vaccine licensed in the U.S. How is HPXV related to modern vaccines? • Multiple sources 3 - 5 indicate that the smallpox vaccine discovered by Dr. Edward Jenner in the early 19 th century was either HPXV or a very similar virus and that vaccinia vaccines are derived from this ancestral strain • A 1902 U.S. smallpox vaccine was found to be highly similar (99.7% similarity in core genome 6 ) to HPXV sequence from the 1976 Mongolian isolate • Horsepox is now believed to be extinct 5 1 Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453 2 Tulman et al., Journal of Virology, 2006; 80(18): 9244 - 9258 3 Qin et al., Journal of Virology, 2011; 85(24):13049 - 13060 4 Medaglia et al., Journal of Virology, 2015; 89(23):11909 - 11925 5 Esparza J. Veterinary Record. 2013; 173: 272 - 273 6 Schrick , L. et al. , N Engl J Med 2017; 377:1491 - 1492, http://www.nejm.org/doi/full/10.1056/NEJMc1707600

© 2020 Tonix Pharmaceuticals Holding Corp. 109 ACAM2000 is sold to the U.S. Strategic National Stockpiles 1 • Sold by Emergent BioSolutions • Sanofi divested ACAM2000 to Emergent BioSolutions in 2017 for $97.5 M upfront plus milestones • ACAM2000 was developed by Acambis which was acquired by Sanofi in 2008 for $513 M Vaccinia (VACV) strains have demonstrated potential for zoonotic infections and re - infection of humans 2 - 5 • No known evidence for zoonosis of ACAM2000, but it has not been widely administered Modern VACV smallpox vaccines are associated with cardiotoxicity 6 The Currently Licensed Smallpox Vaccine ACAM2000 is a Live Vaccinia Virus (VACV) Vaccine 1 Nalca, A et al. Drug design, development and Therapy. (2010) 4:71 - 79 2 Medaglia MLG, et al. J Virol . (2015) 89:11909 – 11925. doi:10.1128/JVI.01833 - 15. 3 Trindade,GS. et al. Clinical Infectious Diseases. (2009) 48:e37 – 40 4 Leite,JA, et al. Emerging Infectious Diseases. (2005) www.cdc.gov/eid • Vol. 11, No. 12 5 Medaglia MLG, et al. Emerging Infectious Diseases (2009) www.cdc.gov/eid • Vol. 15, No. 7 6 Engler RJM et al., PloS ONE (2015) 10(3): e0118283. doi:10.1371/journal.pone.0118283
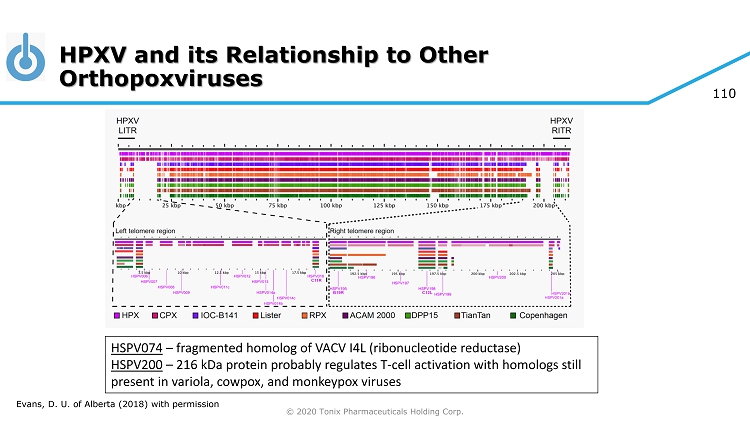
110 © 2020 Tonix Pharmaceuticals Holding Corp. HSPV074 – fragmented homolog of VACV I4L (ribonucleotide reductase) HSPV200 – 216 kDa protein probably regulates T - cell activation with homologs still present in variola , cowpox, and monkeypox viruses HPXV and its Relationship to Other Orthopoxviruses Evans, D. U. of Alberta (2018) with permission
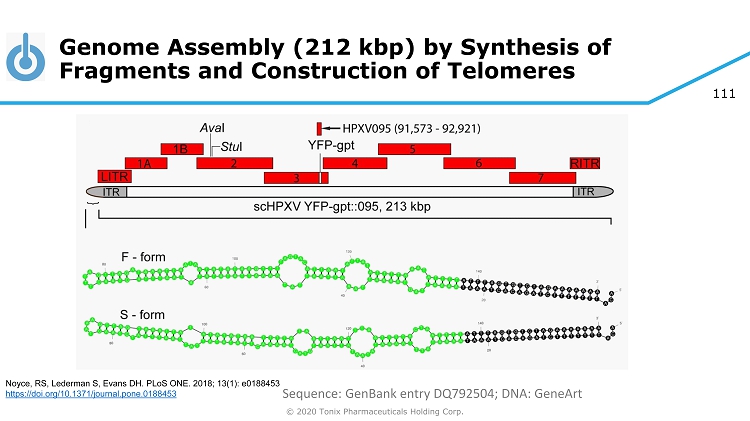
111 © 2020 Tonix Pharmaceuticals Holding Corp. Sequence: GenBank entry DQ792504; DNA: GeneArt Genome Assembly (212 kbp ) by Synthesis of Fragments and Construction of Telomeres Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
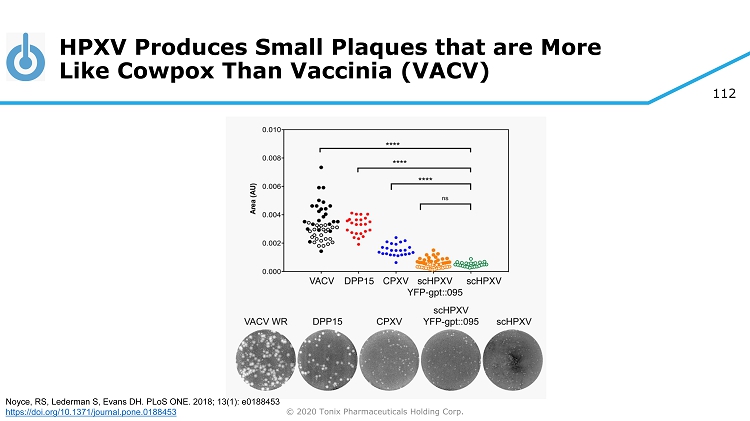
112 © 2020 Tonix Pharmaceuticals Holding Corp. HPXV Produces Small Plaques that are More Like Cowpox Than Vaccinia (VACV) Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
113 © 2020 Tonix Pharmaceuticals Holding Corp. Cell - associated virus Virus in the media Production of Cell - Associated and Extracellular Virus Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
114 © 2020 Tonix Pharmaceuticals Holding Corp. HPXV Growth Characteristics Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
115 © 2020 Tonix Pharmaceuticals Holding Corp. Testing Vaccine Protective Activity of HPXV in Mice Model Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
116 © 2020 Tonix Pharmaceuticals Holding Corp. PBS VACV WR (5x10 3 PFU) Dryvax clone (10 7 PFU) HPXV (10 7 PFU) Challenge (10 6 PFU VAC strain WR) Biological Properties of HPXV: Less Virulent than a Dryvax Clone, but Produces Protective Immunity Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
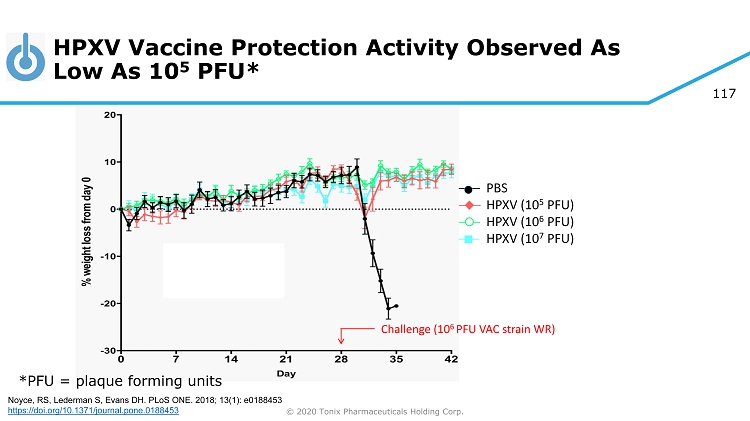
117 © 2020 Tonix Pharmaceuticals Holding Corp. PBS HPXV (10 5 PFU) HPXV (10 6 PFU) HPXV (10 7 PFU) Challenge (10 6 PFU VAC strain WR) HPXV Vaccine Protection Activity Observed As Low As 10 5 PFU* *PFU = plaque forming units Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
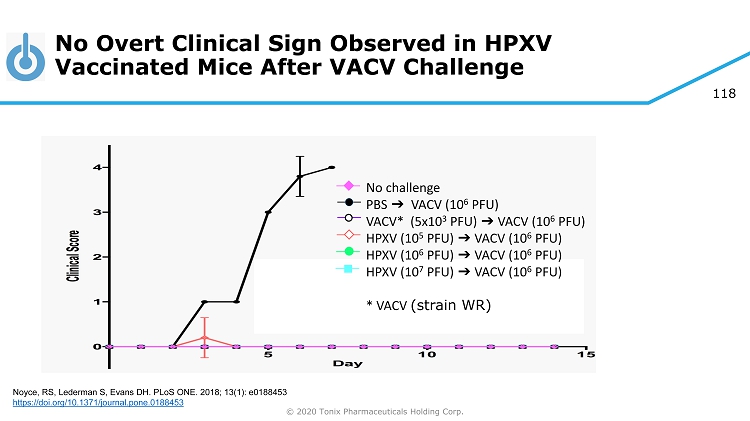
118 © 2020 Tonix Pharmaceuticals Holding Corp. No challenge PBS ➔ VACV (10 6 PFU) VACV* (5x10 3 PFU) ➔ VACV (10 6 PFU) HPXV (10 5 PFU) ➔ VACV (10 6 PFU) HPXV (10 6 PFU) ➔ VACV (10 6 PFU) HPXV (10 7 PFU) ➔ VACV (10 6 PFU) * VACV (strain WR) No Overt Clinical Sign Observed in HPXV Vaccinated Mice After VACV Challenge Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
© 2020 Tonix Pharmaceuticals Holding Corp. 119 HPXV or TNX - 801 – May Have an Improved Safety Profile as a Smallpox Preventing Vaccine Horsepox is caused by HPXV and is characterized by mouth and skin eruptions HXPV isolate from the 1976 outbreak later sequenced Modern smallpox vaccines are associated with cardiotoxicity 1 HPXV has potential for slower proliferation leading to possibly decreased toxicity 2 1 Engler RJM et al., PloS ONE 10(3): e0118283. doi:10.1371/journal.pone.0118283 (2015) 2 Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453

© 2020 Tonix Pharmaceuticals Holding Corp. 120 An Improved Smallpox - Preventing Vaccine is Important and Necessary for a Potential Public Health Issue Smallpox was eradicated as a result of global public health campaigns No cases of naturally - occurring smallpox have been reported since 1977 Accidental or intentional transmission of smallpox does not require a natural reservoir Stockpiles of smallpox - preventing vaccines are currently maintained and refreshed in case of need

© 2020 Tonix Pharmaceuticals Holding Corp. 121 Ongoing vaccination of U.S. troops • Troops in the Global Response Force Threat of smallpox re - introduction • Strategic National Stockpile & public health policy Re - emergence of monkey pox 1 • Believed to resurgent because of vaccinia - naïve populations in Africa • Multiple U.S. military operations ongoing in Africa Current Needs to Vaccinate Against Smallpox 1 Nda - Isaiah, J. Nigeria: Monkey Pox Scourge Spreads to Seven States. All Africa. 12 OCTOBER 2017, HTTP://ALLAFRICA.COM/STORIES/201710120177.HTML

© 2020 Tonix Pharmaceuticals Holding Corp. 122 TNX - 801: A Potential Medical Countermeasure 21st Century Cures Act (2016), Section 3086 • Encouraging treatments for agents that present a national security threat Medical countermeasures are drugs, biologics (vaccines) or devices intended to treat: • Biological, chemical, radiological, or nuclear agents that present a national security threat • Public health issues stemming from a naturally occurring emerging disease or a natural disaster New Priority Review Voucher program for “Material Threat Medical Countermeasures” • Priority Review Voucher may be transferred or sold

© 2020 Tonix Pharmaceuticals Holding Corp. 123 Live virus vaccines stimulate cross - reactive immunity • Protects from possible infection with smallpox virus • Renders recipient “immune” • Provides indirect protection to non - immunized population “herd immunity” Potential safety improvement over existing vaccines • Cardiotoxicity limits widespread smallpox vaccination in at - risk population Exclusivity • Patent application filed on novel virus composition • 12 years exclusivity can be anticipated Eligibility for Priority Review Voucher upon licensure if accepted as medical counter - measure Mechanism of Action Possible advantages of TNX - 801 TNX - 801 (HPVX) • Synthesized live horsepox virus • Shares structural characteristics with vaccinia - based smallpox vaccines • Unique properties that suggest lower toxicity TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate

© 2020 Tonix Pharmaceuticals Holding Corp. 124 Given that smallpox is eradicated the only evidence of effectiveness for modern vaccines is from historical use when smallpox was endemic • Stimulates interest in the evolution of vaccinia Vaccinia stocks around the world diverged from Jenner’s 1798 vaccine • Evolutionary argument that common progenitor was horsepox or a similar virus U.S. vaccine from 1902 was found to be 99.7% similar to horsepox in core viral sequence 1 • Strong evidence linking a horsepox - like virus as progenitor to modern vaccinia • Effectiveness of older vaccines support belief that HPXV will be protective against smallpox Evidence of Effectiveness for Smallpox Vaccine 1 Schrick, L. et al (2017) An Early American Smallpox Vaccine Based on Horsepox N Engl J Med 2017; 377:1491

© 2020 Tonix Pharmaceuticals Holding Corp. 125 Single clone picked from “swarm” of Dryvax ® 1 • Some rationale for selection 2 Growth in serum free Vero cells • Eliminates risk of Bovine Spongiform Encephalopathy (BSE)/prion contamination – safety concerns in Wyeth’s Dryvax (grown in calf lymph) In 2000, the evolutionary connection between vaccinia and horsepox was not understood • Tulman’s sequence of horsepox was published in 2006 3 ACAM2000 1 – Best Technology of its Time 1 US licensed smallpox preventing vaccine – ACAM2000 is currently marketed, Dryvax has been withdrawn from marketing 2 Monath, TP et al. Int. J. of Inf. Dis. (2004) 8S2:S31 3 Tulman, ER. Genome of Horsepox Virus J. Virol. (2006) 80(18) 9244
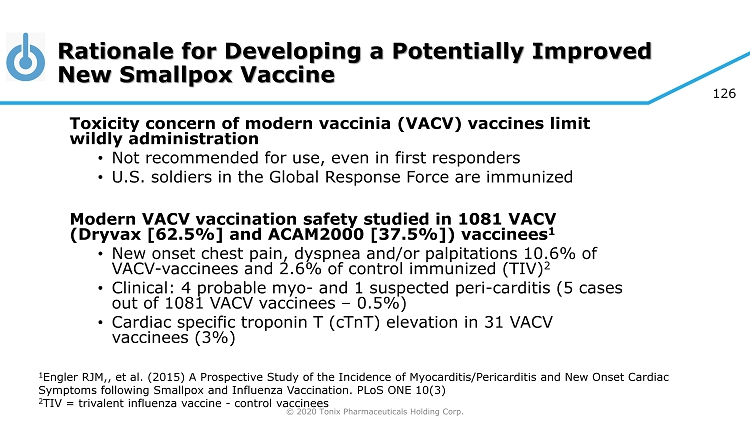
© 2020 Tonix Pharmaceuticals Holding Corp. 126 Toxicity concern of modern vaccinia (VACV) vaccines limit wildly administration • Not recommended for use, even in first responders • U.S. soldiers in the Global Response Force are immunized Modern VACV vaccination safety studied in 1081 VACV ( Dryvax [62.5%] and ACAM2000 [37.5%]) vaccinees 1 • New onset chest pain, dyspnea and/or palpitations 10.6% of VACV - vaccinees and 2.6% of control immunized (TIV) 2 • Clinical: 4 probable myo - and 1 suspected peri - carditis (5 cases out of 1081 VACV vaccinees – 0.5%) • Cardiac specific troponin T ( cTnT ) elevation in 31 VACV vaccinees (3%) Rationale for Developing a Potentially Improved New Smallpox Vaccine 1 Engler RJM,, et al. (2015) A Prospective Study of the Incidence of Myocarditis/Pericarditis and New Onset Cardiac Symptoms following Smallpox and Influenza Vaccination. PLoS ONE 10(3) 2 TIV = trivalent influenza vaccine - control vaccinees
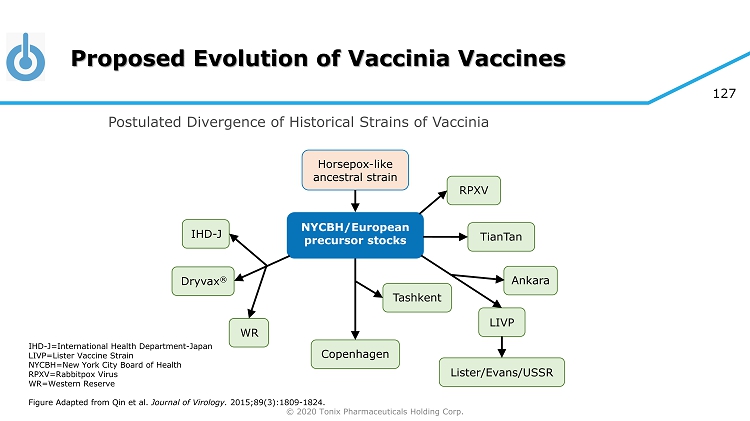
© 2020 Tonix Pharmaceuticals Holding Corp. 127 Postulated Divergence of Historical Strains of Vaccinia Proposed Evolution of Vaccinia Vaccines NYCBH/European precursor stocks Horsepox - like ancestral strain Lister/Evans/USSR Dryvax ® IHD - J WR Copenhagen Tashkent RPXV TianTan Ankara LIVP IHD - J=International Health Department - Japan LIVP=Lister Vaccine Strain NYCBH=New York City Board of Health RPXV=Rabbitpox Virus WR=Western Reserve Figure Adapted from Qin et al. Journal of Virology. 2015;89(3):1809 - 1824.
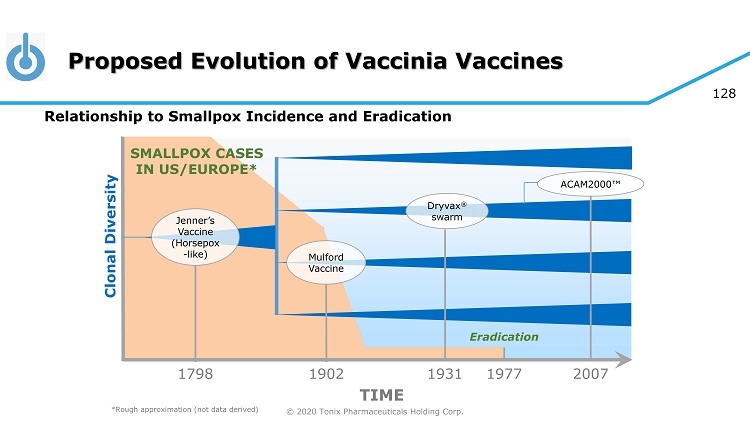
© 2020 Tonix Pharmaceuticals Holding Corp. 128 Relationship to Smallpox Incidence and Eradication Proposed Evolution of Vaccinia Vaccines TIME *Rough approximation (not data derived) 1977 Eradication SMALLPOX CASES IN US/EUROPE* ACAM2000™ 1798 1902 1931 2007 Dryvax ® swarm Mulford Vaccine Jenner’s Vaccine (Horsepox - like) Clonal Diversity

© 2020 Tonix Pharmaceuticals Holding Corp. 129 Theoretical effectiveness of modern vaccinia vaccines are based on extrapolation from older vaccines • Newer/modern vaccines were not widely used when smallpox was endemic MVA ( Modified Virus Ankara) which has large deletions also produces different T cell responses • In non - human primates, MVA is less effective than ACAM2000 in protecting against monkeypox 1 • MVA has fewer epitopes, and elicits different responses to existing epitopes 2 • MVA effectiveness argument is based on the immune response to intracellular mature virus (IMV) • Immunity to the other form of virus, extracellular enveloped virus (EEV), is weak because the immunodominant B5 gene is heavily mutated and deleted in MVA What’s the Evidence of Effectiveness of Smallpox Vaccines for Preventing Smallpox? 1 Golden JW, et al. (2012). PLoS ONE 7(7): e42353. doi:10.1371/journal.pone.0042353 2 Tscharke, DC et al., J. Exp. Med. 2005 201(1):95

© 2020 Tonix Pharmaceuticals Holding Corp. 130 Preventing Vaccine • Jenner’s vaccine, HPXV (upon licensure), Vaccinia Post - exposure vaccination 1 • Jenner’s vaccine Priming of the immune system • Imvamune ® (MVA) and DNA vaccines 2 Pharmacotherapy for infected or exposed individuals • Arestvyr ® /TPOXX ® ( tecovirimat , formerly ST - 246) Treatment of disseminated viremia in immunocompromised 3 • Arestvyr ® /TPOXX ® , Brincidofovir and vaccinia immune globulin Possible Smallpox Prevention and Treatment Strategies 1 Described by Jenner as one of his major discoveries 2 Hooper, JW et al. Smallpox DNA Vaccine Protects Nonhuman Primates Against Lethal Monkeypox . J. Virol . 2004. 78 (9) 4433 3 Lederman, ER et al, Progressive Vaccinia: Case Description and Laboratory - Guided Therapy With Vaccinia Immune Globulin, ST - 246, and CMX001 JID 2012. 206:1372
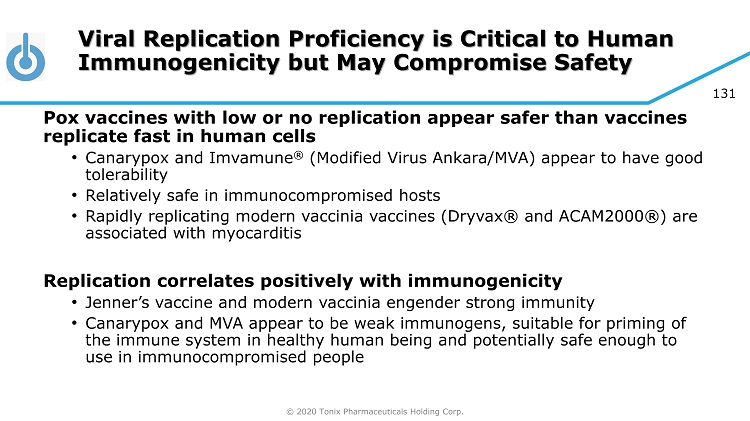
© 2020 Tonix Pharmaceuticals Holding Corp. 131 Pox vaccines with low or no replication appear safer than vaccines replicate fast in human cells • Canarypox and Imvamune ® (Modified Virus Ankara/MVA) appear to have good tolerability • Relatively safe in immunocompromised hosts • Rapidly replicating modern vaccinia vaccines ( Dryvax ® and ACAM2000®) are associated with myocarditis Replication correlates positively with immunogenicity • Jenner’s vaccine and modern vaccinia engender strong immunity • Canarypox and MVA appear to be weak immunogens, suitable for priming of the immune system in healthy human being and potentially safe enough to use in immunocompromised people Viral Replication Proficiency is Critical to Human Immunogenicity but May Compromise Safety

© 2020 Tonix Pharmaceuticals Holding Corp. 132 TNX - 801 (HPXV) is expected to have similar scalability for mass production as ACAM2000 • TNX - 801 grows well in cell lines – immunity is expected after single administration (immunization) • Only a small dose (replicating live virus) is required for immunization MVA is hard to scale up for commercial production • Requires high dose to engender an immune response (non - replicating virus) • Cumbersome immunization schedule – two doses, 4 weeks apart, are used typically to prime the immune system (slow growth) Antivirals • Relatively expensive to manufacture – requires repeated dosing • May provide logistical challenges to at risk population over the at risk period Manufacturing and Dosing Requirements

© 2020 Tonix Pharmaceuticals Holding Corp. 133 Vaccination protects against smallpox – both individuals and populations at risk • Use of Jenner’s vaccine resulted in eradication of smallpox Vaccination can protect AFTER smallpox infection • Vaccinia can be administered 1 - 3 days after infection Vaccination indirectly protects non - immunized people in a population • “Wetting the forest” or “herd immunity” Vaccination can be cost effective with safe/low - risk vaccines • Replication - efficient live virus vaccines can be manufactured and administered for broader use “The Time is Right” New synthetic biology technology and new understanding of vaccinia evolution provide an opportunity for a potentially safer vaccine using HPXV Rationale for Developing a Potentially Improved New Smallpox Vaccine Based on Jenner’s Vaccine
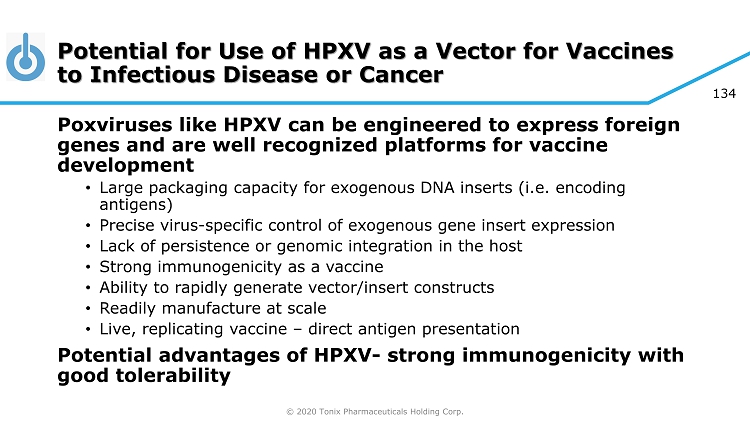
© 2020 Tonix Pharmaceuticals Holding Corp. 134 Potential for Use of HPXV as a Vector for Vaccines to Infectious Disease or Cancer Poxviruses like HPXV can be engineered to express foreign genes and are well recognized platforms for vaccine development • Large packaging capacity for exogenous DNA inserts (i.e. encoding antigens) • Precise virus - specific control of exogenous gene insert expression • Lack of persistence or genomic integration in the host • Strong immunogenicity as a vaccine • Ability to rapidly generate vector/insert constructs • Readily manufacture at scale • Live, replicating vaccine – direct antigen presentation Potential advantages of HPXV - strong immunogenicity with good tolerability
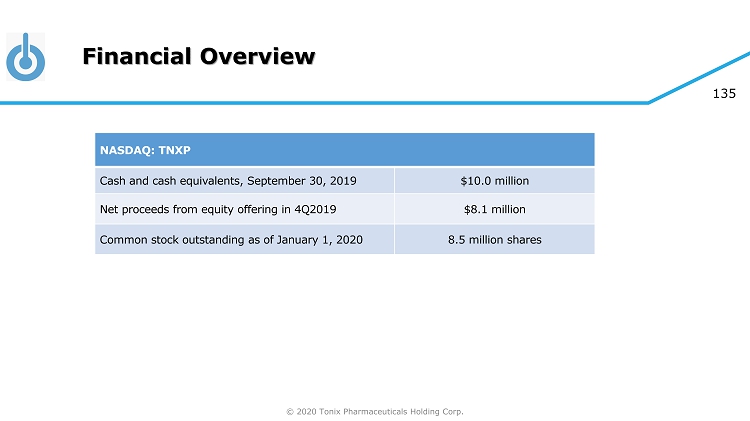
© 2020 Tonix Pharmaceuticals Holding Corp. 135 Financial Overview NASDAQ: TNXP Cash and cash equivalents, September 30, 2019 $10.0 million Net proceeds from equity offering in 4Q2019 $8.1 million Common stock outstanding as of January 1, 2020 8.5 million shares

© 2020 Tonix Pharmaceuticals Holding Corp. 136 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
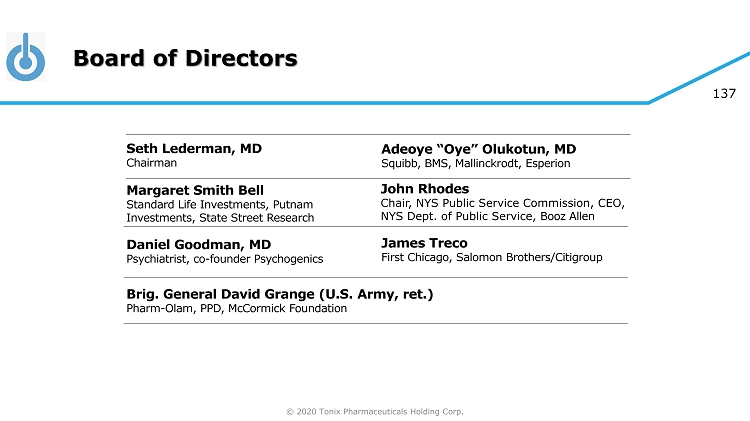
© 2020 Tonix Pharmaceuticals Holding Corp. 137 Board of Directors Seth Lederman, MD Chairman Adeoye “Oye” Olukotun, MD Squibb, BMS, Mallinckrodt, Esperion John Rhodes Chair, NYS Public Service Commission, CEO, NYS Dept. of Public Service, Booz Allen James Treco First Chicago, Salomon Brothers/Citigroup Brig. General David Grange (U.S. Army, ret.) Pharm - Olam, PPD, McCormick Foundation Margaret Smith Bell Standard Life Investments, Putnam Investments, State Street Research Daniel Goodman, MD Psychiatrist, co - founder Psychogenics

© 2020 Tonix Pharmaceuticals Holding Corp. 138 Milestones – Recently Completed and Upcoming □ May 2019 In - licensed TNX - 1300, in Phase 2 development for cocaine intoxication □ October 2019 Completed long - term exposure studies in PTSD to evaluate tolerability of TNX - 102 SL 5.6 mg □ October 2019 Met with FDA to discuss Phase 2 study for TNX - 102 SL to treat AUD □ 4 th Quarter 2019 Confirmed once - daily dosing for TNX - 601 CR in PK study □ 4 th Quarter 2019 Enrolled first patient in Phase 3 F304/RELIEF study for management of fibromyalgia □ 1 st Quarter 2020 Interim analysis results from Phase 3 P302/RECOVERY study in PTSD expected □ 1 st Quarter 2020 Expect to submit IND application to support Phase 2 POC study in AUD □ 2 nd Quarter 2020 Topline data from Phase 3 P302/RECOVERY study in PTSD expected □ 2 nd Half 2020 Interim analysis results from Phase 3 F304/RELIEF study in fibromyalgia expected □ 2 nd Half 2020 Expect to initiate Phase 2 study of TNX - 601 CR in depression, ex - U.S. □ 1 st Half 2021 Topline data from Phase 3 F304/RELIEF study in fibromyalgia expected x x x x x
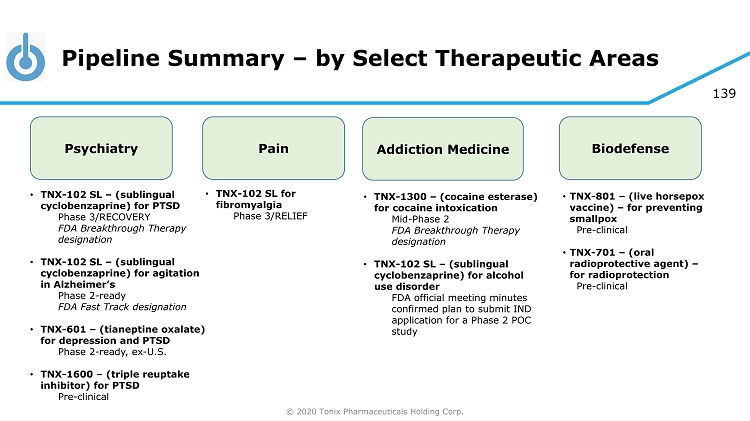
© 2020 Tonix Pharmaceuticals Holding Corp. 139 Pipeline Summary – by Select Therapeutic Areas Psychiatry Pain Addiction Medicine Biodefense • TNX - 102 SL – (sublingual cyclobenzaprine) for PTSD Phase 3/RECOVERY FDA Breakthrough Therapy designation • TNX - 102 SL – (sublingual cyclobenzaprine) for agitation in Alzheimer’s Phase 2 - ready FDA Fast Track designation • TNX - 601 – (tianeptine oxalate) for depression and PTSD Phase 2 - ready, ex - U.S. • TNX - 1600 – (triple reuptake inhibitor) for PTSD Pre - clinical • TNX - 102 SL for fibromyalgia Phase 3/RELIEF • TNX - 1300 – (cocaine esterase) for cocaine intoxication Mid - Phase 2 FDA Breakthrough Therapy designation • TNX - 102 SL – (sublingual cyclobenzaprine) for alcohol use disorder FDA official meeting minutes confirmed plan to submit IND application for a Phase 2 POC study • TNX - 801 – (live horsepox vaccine) – for preventing smallpox Pre - clinical • TNX - 701 – (oral radioprotective agent) – for radioprotection Pre - clinical
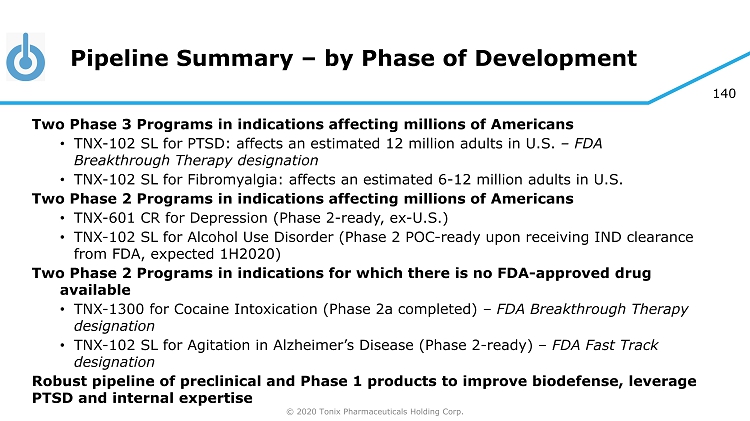
© 2020 Tonix Pharmaceuticals Holding Corp. 140 Pipeline Summary – by Phase of Development Two Phase 3 Programs in indications affecting millions of Americans • TNX - 102 SL for PTSD: affects an estimated 12 million adults in U.S. – FDA Breakthrough Therapy designation • TNX - 102 SL for Fibromyalgia: affects an estimated 6 - 12 million adults in U.S. Two Phase 2 Programs in indications affecting millions of Americans • TNX - 601 CR for Depression (Phase 2 - ready, ex - U.S.) • TNX - 102 SL for Alcohol Use Disorder (Phase 2 POC - ready upon receiving IND clearance from FDA, expected 1H2020) Two Phase 2 Programs in indications for which there is no FDA - approved drug available • TNX - 1300 for Cocaine Intoxication (Phase 2a completed) – FDA Breakthrough Therapy designation • TNX - 102 SL for Agitation in Alzheimer’s Disease (Phase 2 - ready) – FDA Fast Track designation Robust pipeline of preclinical and Phase 1 products to improve biodefense, leverage PTSD and internal expertise

© 2020 Tonix Pharmaceuticals Holding Corp. Thank you ! NASDAQ: TNXP