
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.01

© 2020 Tonix Pharmaceuticals Holding Corp. 1 Biotech Showcase 2020 Version P0217 1 - 13 - 20 (Doc 0584) Investor Presentation

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2018 , as filed with the Securities and Exchange Commission (the “SEC”) on March 18 , 2019 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
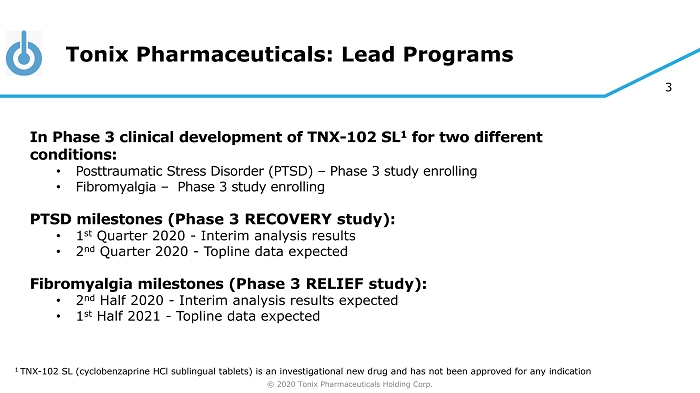
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals: Lead Programs In Phase 3 clinical development of TNX - 102 SL 1 for two different conditions: • Posttraumatic Stress Disorder (PTSD) – Phase 3 study enrolling • Fibromyalgia – Phase 3 study enrolling PTSD milestones ( Phase 3 RECOVERY study ): • 1 st Quarter 2020 - Interim analysis results • 2 nd Quarter 2020 - Topline data expected Fibromyalgia milestones ( Phase 3 RELIEF study ): • 2 nd Half 2020 - Interim analysis results expected • 1 st Half 2021 - Topline data expected 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication
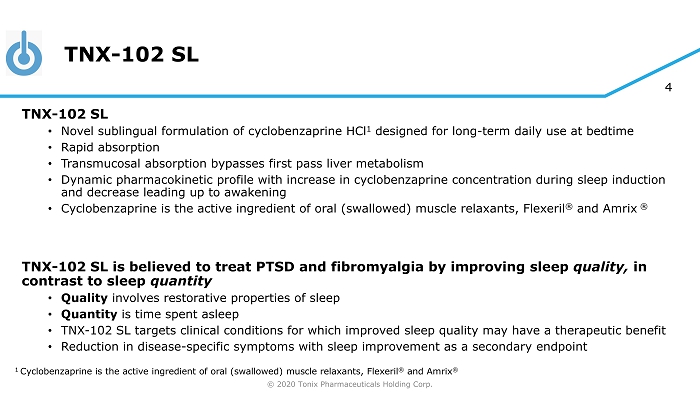
© 2020 Tonix Pharmaceuticals Holding Corp. 4 TNX - 102 SL TNX - 102 SL • Novel sublingual formulation of cyclobenzaprine HCl 1 designed for long - term daily use at bedtime • Rapid absorption • Transmucosal absorption bypasses first pass liver metabolism • Dynamic pharmacokinetic profile with increase in cyclobenzaprine concentration during sleep induction and decrease leading up to awakening • Cyclobenzaprine is the active ingredient of oral (swallowed) muscle relaxants, Flexeril ® and Amrix ® TNX - 102 SL is believed to treat PTSD and fibromyalgia by improving sleep quality, in contrast to sleep quantity • Quality involves restorative properties of sleep • Quantity is time spent asleep • TNX - 102 SL targets clinical conditions for which improved sleep quality may have a therapeutic benefit • Reduction in disease - specific symptoms with sleep improvement as a secondary endpoint 1 Cyclobenzaprine is the active ingredient of oral (swallowed) muscle relaxants, Flexeril ® and Amrix ®

© 2020 Tonix Pharmaceuticals Holding Corp. 5 Potential Therapeutic Advantages of TNX - 102 SL Cyclobenzaprine, active ingredient of TNX - 102 SL, is NEITHER a benzodiazepine nor a narcotic • Does NOT interact with the same receptors as traditional hypnotic sleep drugs associated with retrograde amnesia and is NOT an opiate TNX - 102 SL is non - addictive • Cyclobenzaprine is the active ingredient of an orally ingested immediate release tablet (Flexeril ® ), approved 40 years ago; Flexeril’s current labeling indicates no abuse and dependence concern at higher doses than TNX - 102 SL (15 - 30 mg/day v. 5.6 mg/day) • TNX - 102 SL NDA can be filed without drug abuse and dependency assessment studies Other desirable features of TNX - 102 SL include • Once - daily sublingual dose taken at bedtime is expected to enhance patient adherence • Rapid, transmucosal absorption aligns bioavailability of drug with sleep cycle • Sublingual formulation bypasses first - pass hepatic metabolism and reduces exposure to long - lived active metabolite of cyclobenzaprine associated with negative side effects
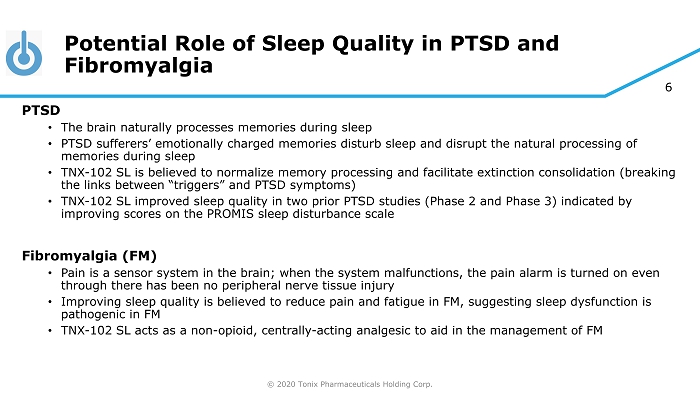
© 2020 Tonix Pharmaceuticals Holding Corp. 6 Potential Role of Sleep Quality in PTSD and Fibromyalgia PTSD • The brain naturally processes memories during sleep • PTSD sufferers’ emotionally charged memories disturb sleep and disrupt the natural processing of memories during sleep • TNX - 102 SL is believed to normalize memory processing and facilitate extinction consolidation (breaking the links between “triggers” and PTSD symptoms) • TNX - 102 SL improved sleep quality in two prior PTSD studies (Phase 2 and Phase 3) indicated by improving scores on the PROMIS sleep disturbance scale Fibromyalgia (FM) • Pain is a sensor system in the brain; when the system malfunctions, the pain alarm is turned on even through there has been no peripheral nerve tissue injury • Improving sleep quality is believed to reduce pain and fatigue in FM, suggesting sleep dysfunction is pathogenic in FM • TNX - 102 SL acts as a non - opioid, centrally - acting analgesic to aid in the management of FM
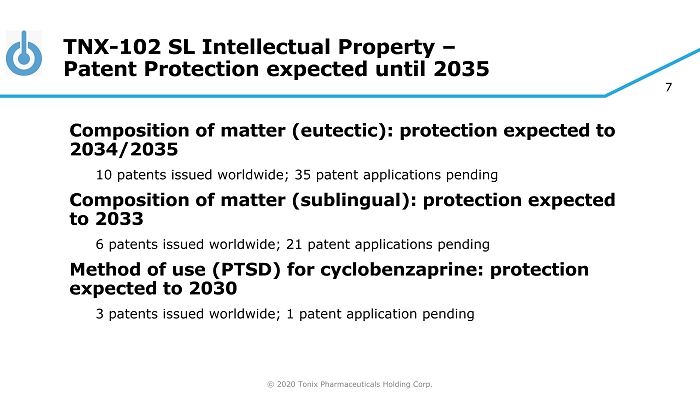
© 2020 Tonix Pharmaceuticals Holding Corp. 7 TNX - 102 SL Intellectual Property – Patent Protection expected until 2035 Composition of matter (eutectic): protection expected to 2034/2035 10 patents issued worldwide; 35 patent applications pending Composition of matter (sublingual): protection expected to 2033 6 patents issued worldwide; 21 patent applications pending Method of use (PTSD) for cyclobenzaprine: protection expected to 2030 3 patents issued worldwide; 1 patent application pending
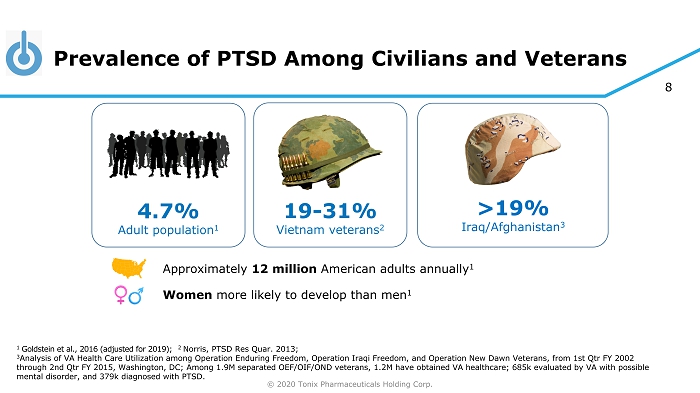
© 2020 Tonix Pharmaceuticals Holding Corp. 8 Prevalence of PTSD Among Civilians and Veterans 1 Goldstein et al., 2016 (adjusted for 2019) ; 2 Norris, PTSD Res Quar. 2013; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Vete ran s, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; Among 1.9M separated OEF/OIF/OND veterans, 1.2M have obtained VA healthcare; 685k evaluated by VA with possible mental disorder, and 379k diagnosed with PTSD. >19% Iraq/Afghanistan 3 4.7% Adult population 1 19 - 31% Vietnam veterans 2 Approximately 12 million American adults annually 1 Women more likely to develop than men 1

© 2020 Tonix Pharmaceuticals Holding Corp. 9 Unmet Need for Effective and Safe Therapies for Treatment of PTSD No FDA - approved products for PTSD since Pfizer’s Zoloft ® (sertraline) in 1999 and GSK’s Paxil ® (paroxetine) in 2001 • Neither has shown efficacy in military - related PTSD • Side effects relating to sexual dysfunction, sleep disruption and weight gain are commonly reported PTSD is signature wound of last 25 years of war • Affects servicemember health and performance, force readiness, and retention • Believed to be the underlying cause of suicide in many cases • Male PTSD patients often unresponsive or intolerant of current treatments Civilian PTSD is more prevalent than military • Results from physical and sexual assault trauma, vehicular accidents, natural disasters • Significant cause of morbidity
© 2020 Tonix Pharmaceuticals Holding Corp. 10 • The central feature of PTSD is the reliving of trauma through intrusive and vivid recollections, nightmares, or flashbacks 1 • Re - experiencing intrusive symptoms are believed to be caused by memory processing impairments 2 PTSD Is a Memory Processing Disorder 1. APA. https://www.psychiatry.org/patients - families/ptsd/what - is - ptsd. Accessed November 14, 2019. 2. van Marle, H. Eur J Psychotraumatol. 2015;6:27633.
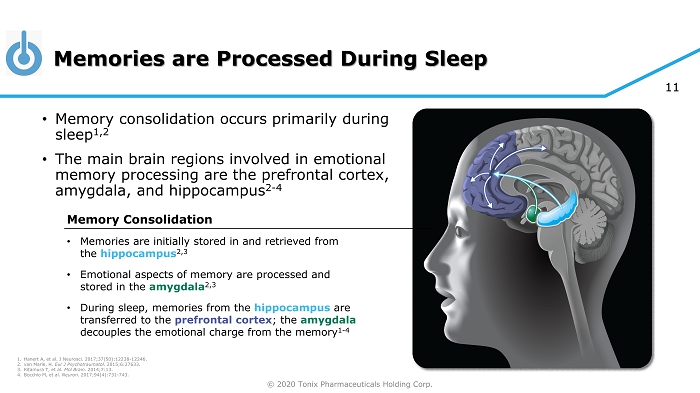
© 2020 Tonix Pharmaceuticals Holding Corp. 11 Memories are Processed During Sleep • Memory consolidation occurs primarily during sleep 1,2 • The main brain regions involved in emotional memory processing are the prefrontal cortex, amygdala, and hippocampus 2 - 4 1. Hanert A, et al. J Neurosci . 2017;37(50):12238 - 12246. 2. van Marle, H. Eur J Psychotraumatol. 2015;6:27633. 3. Kitamura T, et al. Mol Brain . 2014;7:13. 4. Bocchio M, et al. Neuron. 2017;94(4):731 - 743. Memory Consolidation • Memories are initially stored in and retrieved from the hippocampus 2,3 • Emotional aspects of memory are processed and stored in the amygdala 2,3 • During sleep, memories from the hippocampus are transferred to the prefrontal cortex ; the amygdala decouples the emotional charge from the memory 1 - 4
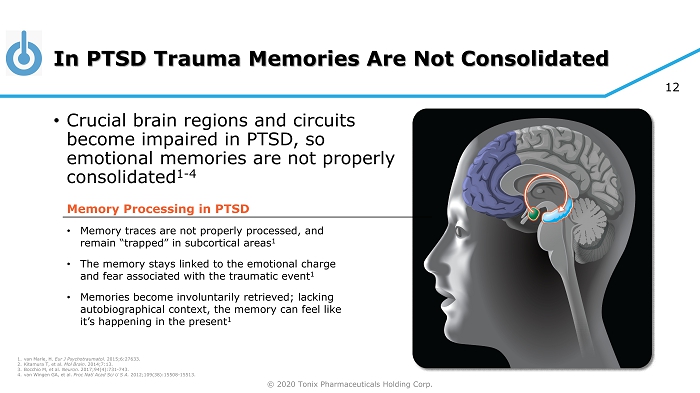
© 2020 Tonix Pharmaceuticals Holding Corp. 12 In PTSD Trauma Memories Are Not Consolidated • Crucial brain regions and circuits become impaired in PTSD, so emotional memories are not properly consolidated 1 - 4 Memory Processing in PTSD • Memory traces are not properly processed, and remain “trapped” in subcortical areas 1 • The memory stays linked to the emotional charge and fear associated with the traumatic event 1 • Memories become involuntarily retrieved; lacking autobiographical context, the memory can feel like it’s happening in the present 1 1. van Marle, H. Eur J Psychotraumatol. 2015;6:27633. 2. Kitamura T, et al. Mol Brain . 2014;7:13. 3. Bocchio M, et al. Neuron. 2017;94(4):731 - 743. 4. van Wingen GA, et al. Proc Natl Acad Sci U S A . 2012;109(38):15508 - 15513.
© 2020 Tonix Pharmaceuticals Holding Corp. 13 Sleep Disruption Is a Core Feature of PTSD • About 70% of individuals with PTSD report sleep problems 1 1. Brownlow JA, et al. Curr Psychiatry Rep. 2015;17(6):41. 2. Lipinksa M, et al. J Sleep Res. 2014;23(3):309 - 317. Sleep Problems in PTSD • Abnormalities in crucial sleep states have been observed in patients with PTSD 1,2 • At least 50% of patients with PTSD report experiencing frequent nightmares 1 • Sleep disruptions shortly after trauma are predictive of later development of PTSD 1,2
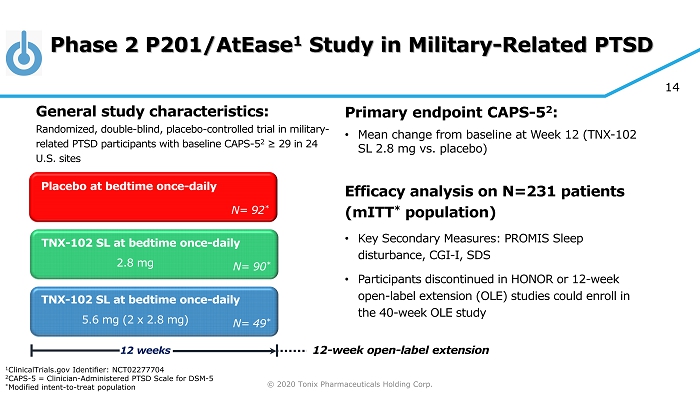
© 2020 Tonix Pharmaceuticals Holding Corp. 14 Phase 2 P201/AtEase 1 Study in Military - Related PTSD Primary e ndpoint CAPS - 5 2 : • Mean change from baseline at W eek 12 (TNX - 102 SL 2.8 mg vs. placebo ) Efficacy analysis on N=231 patients ( mITT * population) • Key Secondary Measures: PROMIS Sleep disturbance, CGI - I, SDS • Participants discontinued in HONOR or 12 - week open - label extension (OLE) studies could enroll in the 40 - week OLE study TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 90 * TNX - 102 SL at bedtime once - daily N= 92 * N= 49 * 2.8 mg 5.6 mg (2 x 2.8 mg) 12 - week open - label extension 1 ClinicalTrials.gov Identifier: NCT02277704 2 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 * Modified intent - to - treat population General s tudy c haracteristics: Randomized, double - blind, placebo - controlled trial in military - related PTSD participants with baseline CAPS - 5 2 ≥ 29 in 24 U.S. sites
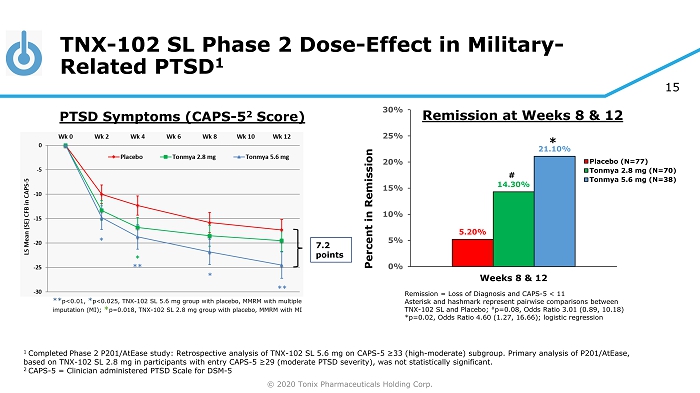
© 2020 Tonix Pharmaceuticals Holding Corp. 15 TNX - 102 SL Phase 2 Dose - Effect in Military - Related PTSD 1 1 Completed Phase 2 P201/AtEase study: Retrospective analysis of TNX - 102 SL 5.6 mg on CAPS - 5 ≥33 (high - moderate) subgroup. Primary analysis of P201/ AtEase , based on TNX - 102 SL 2.8 mg in participants with entry CAPS - 5 ≥29 ( moderate PTSD severity), was not statistically significant. 2 CAPS - 5 = Clinician administered PTSD Scale for DSM - 5 7.2 points ** p<0.01, * p<0.025, TNX - 102 SL 5.6 mg group with placebo, MMRM with multiple imputation (MI); * p=0.018, TNX - 102 SL 2.8 mg group with placebo, MMRM with MI PTSD Symptoms (CAPS - 5 2 Score) Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between TNX - 102 SL and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # * Remission at Weeks 8 & 12
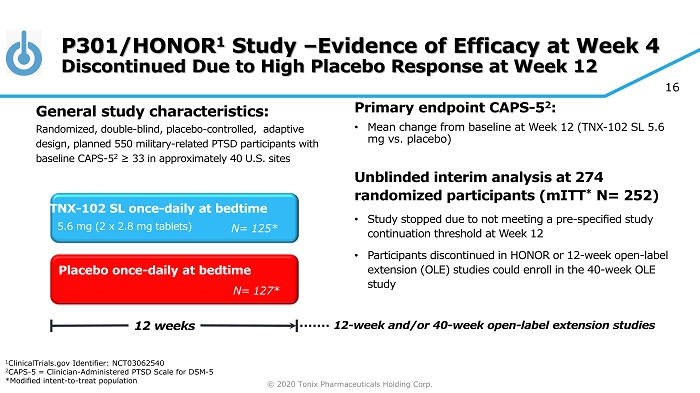
© 2020 Tonix Pharmaceuticals Holding Corp. 16 P301/HONOR 1 Study – Evidence of Efficacy at Week 4 Discontinued Due to High Placebo Response at Week 12 Primary e ndpoint CAPS - 5 2 : • Mean change from baseline at W eek 12 (TNX - 102 SL 5.6 mg vs. placebo ) Unblinded interim analysis at 274 randomized participants ( mITT * N= 252) • Study stopped due to not meeting a pre - specified study continuation threshold at Week 12 • Participants discontinued in HONOR or 12 - week open - label extension (OLE) studies could enroll in the 40 - week OLE study Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime N= 127* N= 125* 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: Randomized, double - blind, placebo - controlled , adaptive design, planned 550 military - related PTSD participants with baseline CAPS - 5 2 ≥ 33 in approximately 40 U.S. sites 12 - week and/or 40 - week open - label extension studies 1 ClinicalTrials.gov Identifier: NCT03062540 2 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 *Modified intent - to - treat population
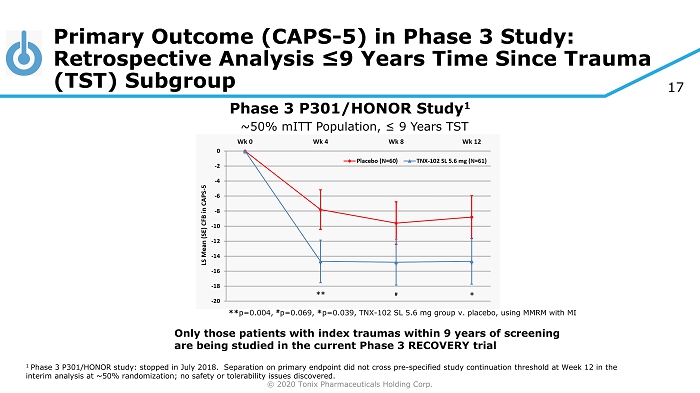
© 2020 Tonix Pharmaceuticals Holding Corp. 17 Primary Outcome (CAPS - 5) in Phase 3 Study: Retrospective Analysis ≤9 Years Time Since Trauma (TST) Subgroup Phase 3 P301/HONOR Study 1 ** p=0.004, # p=0.069, * p=0.039, TNX - 102 SL 5.6 mg group v. placebo, using MMRM with MI ~50% mITT Population, ≤ 9 Years TST 1 Phase 3 P301/HONOR study: stopped in July 2018. Separation on primary endpoint did not cross pre - specified study continuation t hreshold at Week 12 in the interim analysis at ~50% randomization; no safety or tolerability issues discovered. Only those patients with index traumas within 9 years of screening are being studied in the current Phase 3 RECOVERY trial
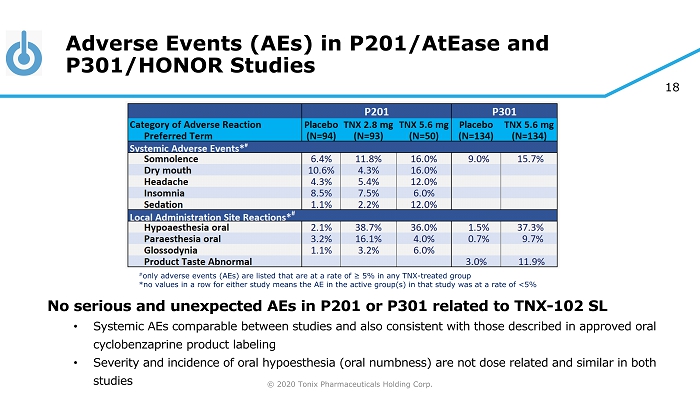
© 2020 Tonix Pharmaceuticals Holding Corp. 18 Adverse Events (AEs) in P201/AtEase and P301/HONOR Studies No serious and unexpected AEs in P201 or P301 related to TNX - 102 SL • Systemic AEs comparable between studies and also consistent with those described in approved oral cyclobenzaprine product labeling • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related and similar in both studies # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%
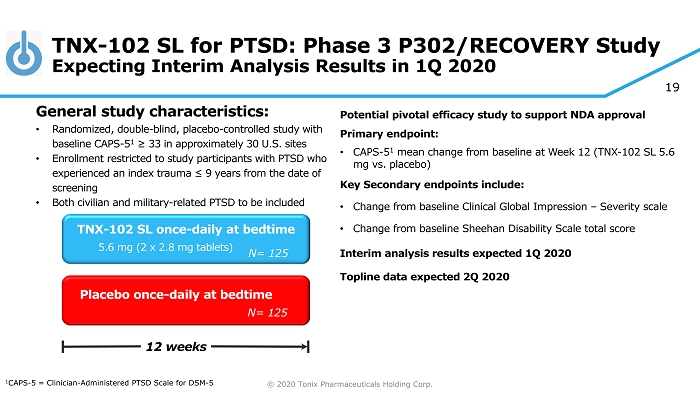
© 2020 Tonix Pharmaceuticals Holding Corp. 19 TNX - 102 SL for PTSD: Phase 3 P302/RECOVERY Study Expecting Interim Analysis Results in 1Q 2020 Potential pivotal efficacy study to support NDA approval Primary e ndpoint: • CAPS - 5 1 mean change from baseline at Week 12 (TNX - 102 SL 5.6 mg vs. placebo) Key Secondary e ndpoint s include: • Change from baseline Clinical Global Impression – Severity scale • Change from baseline Sheehan Disability Scale total score Interim analysis results expected 1Q 2020 Topline data expected 2Q 2020 Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study with baseline CAPS - 5 1 ≥ 33 in approximately 30 U.S. sites • Enrollment restricted to study participants with PTSD who experienced an index trauma ≤ 9 years from the date of screening • Both civilian and military - related PTSD to be included 1 CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5 N= 125 N= 125
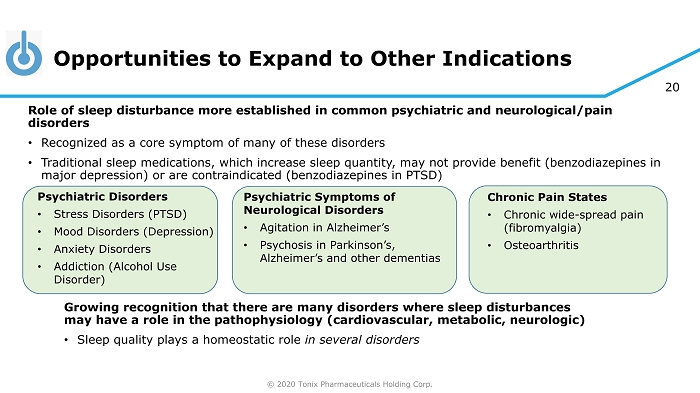
© 2020 Tonix Pharmaceuticals Holding Corp. 20 Opportunities to Expand to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Sleep quality plays a homeostatic role in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (Depression) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated (benzodiazepines in PTSD) Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

© 2020 Tonix Pharmaceuticals Holding Corp. 21 Volkswagen Check Engine [Photograph]. (2011, October 14). Wikipedia When the check engine light malfunctions, the light is on even though the car is not malfunctioning • Fibromyalgia is considered a neurobiological disorder characterized by 1 : chronic widespread pain, non - restorative sleep, fatigue, diminished cognition • Believed to result from inappropriate pain signaling in central nervous system in the absence of peripheral injury 1 • An estimated 6 - 12 million adults in the U.S. have fibromyalgia 2 • Causes significant impairment in all areas of life 3 • Lower levels of health - related quality of life – reduced daily functioning • Interference with work (loss of productivity, disability) • Fewer than half of those treated for fibromyalgia receive complete relief from the three FDA - approved drugs 4 • Inflicts substantial strain on the healthcare system • Average patient has 20 physician office visits per year 5 • Annual direct medical costs are twice those of non - fibromyalgia individuals 6 TNX - 102 SL: Potential Treatment for Fibromyalgia 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141. 2 American Chronic Pain Association (www.theacpa.org, 2019) 3 Schaefer et al., Pain Pract , 2015. 4 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 5 Robinson et al, Pain Medicine 2013;14:1400. 6 White et al, J Occupational Environ Med 2008;50:13.
© 2020 Tonix Pharmaceuticals Holding Corp. 22 Large Need for New Fibromyalgia Therapies that Provide Broad Symptom Improvement with Better Tolerability • Currently - approved medications may have side effects that limit long - term use 1 • High rates of discontinuation, switching and augmentation • A ttempts to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications used simultaneously 2 • Typical patient has tried six different medications 3 • Medication - related side effects may be similar to fibromyalgia symptoms • Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 • TNX - 102 SL is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources, 2011. 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J Clin Pract , 2007; 61(9):1498 – 1508.
© 2020 Tonix Pharmaceuticals Holding Corp. 23 TNX - 102 SL 2.8 mg for Fibromyalgia Summary of Completed Phase 3 AFFIRM Study (F301) MMRM = mixed model repeated measures • 519 patients enrolled in 12 - week, double - blind trial • Randomized 1:1, placebo or TNX - 102 SL 2.8 mg (lowest possible dose), taken once daily at bedtime • Narrowly missed primary endpoint (30% responder analysis), p = 0.095 • Pre - specified key secondary endpoint (mean pain improvement after 12 weeks of treatment) showed statistically significant benefit (MMRM statistical method) with p < 0.001 • Significant improvements in other secondary endpoints measuring sleep quality and sleep disturbances, fatigue, patient global impression of change, global physical health, and fibromyalgia symptom and function domains • Good tolerability with most common adverse events generally mild and transient events related to the sublingual administration of the drug
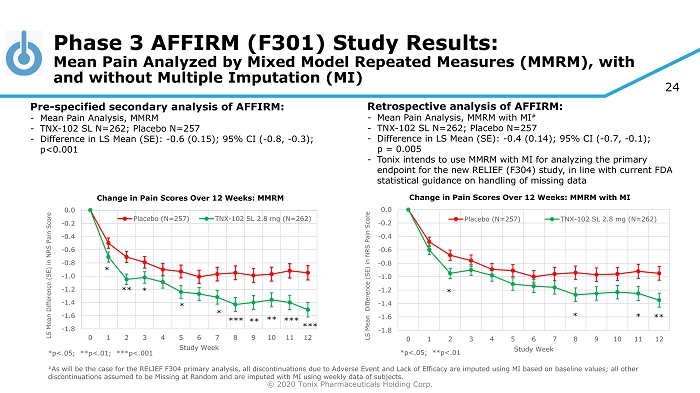
© 2020 Tonix Pharmaceuticals Holding Corp. 24 *p<.05; **p<.01; ***p<.001 # As will be the case for the RELIEF F304 primary analysis, all discontinuations due to Adverse Event and Lack of Efficacy are imp uted using MI based on baseline values; all other discontinuations assumed to be Missing at Random and are imputed with MI using weekly data of subjects. Phase 3 AFFIRM (F301) Study Results: Mean Pain Analyzed by Mixed Model Repeated Measures (MMRM), with and without Multiple Imputation (MI) Pre - specified secondary analysis of AFFIRM: - Mean Pain Analysis, MMRM - TNX - 102 SL N=262; Placebo N=257 - Difference in LS Mean (SE): - 0.6 (0.15); 95% CI ( - 0.8, - 0.3); p<0.001 Retrospective analysis of AFFIRM: - Mean Pain Analysis, MMRM with MI # - TNX - 102 SL N=262; Placebo N=257 - Difference in LS Mean (SE): - 0.4 (0.14); 95% CI ( - 0.7, - 0.1); p = 0.005 - Tonix intends to use MMRM with MI for analyzing the primary endpoint for the new RELIEF (F304) study, in line with current FDA statistical guidance on handling of missing data -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM with MI Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * * * ** *p<.05; **p<.01 -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * ** *** * * * *** *** ** **
© 2020 Tonix Pharmaceuticals Holding Corp. 25 TNX - 102 SL for Fibromyalgia New Phase 3 Study: Higher (2x) Dose, New Primary Endpoint • Clear guidance from FDA to advance fibromyalgia program using higher dose (5.6 mg) • Long - term safety of 5.6 mg dose used in PTSD expected to support fibromyalgia NDA • Retrospective analysis of mean pain improvement after 12 weeks of treatment showed statistically significant improvement using both statistical methods: MMRM (p < 0.001) and MMRM with MI (p < 0.01) • MMRM with MI to be used going forward • First patient enrolled in December 2019
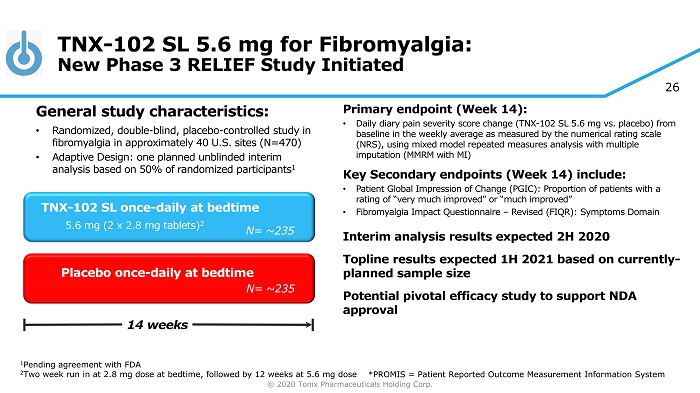
© 2020 Tonix Pharmaceuticals Holding Corp. 26 TNX - 102 SL 5.6 mg for Fibromyalgia: New Phase 3 RELIEF Study Initiated Primary e ndpoint (Week 14) : • Daily diary pain severity score change (TNX - 102 SL 5.6 mg vs. placebo) from baseline in the weekly average as measured by the numerical rating scale (NRS ), using mixed model repeated measures analysis with multiple imputation (MMRM with MI) Key Secondary e ndpoint s (Week 14) include: • Patient Global Impression of Change (PGIC): Proportion of patients with a rating of “very much improved” or “much improved” • Fibromyalgia Impact Questionnaire – Revised (FIQR): Symptoms Domain Interim analysis results expected 2H 2020 Topline results expected 1H 2021 based on currently - planned sample size Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) 2 General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia in approximately 40 U.S. sites (N=470) • Adaptive Design: one planned unblinded interim analysis based on 50% of randomized participants 1 1 Pending agreement with FDA 2 Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose N= ~235 N= 250 N= ~235 *PROMIS = Patient Reported Outcome Measurement Information System
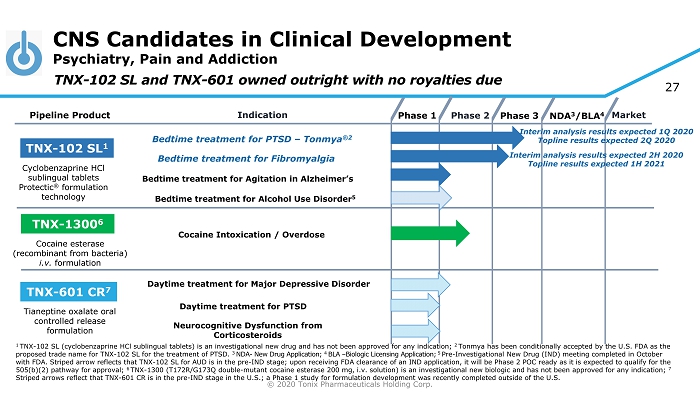
© 2020 Tonix Pharmaceuticals Holding Corp. 27 CNS Candidates in Clinical Development Psychiatry, Pain and Addiction Phase 2 NDA 3 /BLA 4 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Bedtime treatment for PTSD – Tonmya ®2 Daytime treatment for PTSD TNX - 601 CR 7 TNX - 1300 6 Cocaine Intoxication / Overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral controlled release formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 Bedtime t reatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due Bedtime t reatment for Agitation in Alzheimer’s Neurocognitive Dysfunction from Corticosteroids Bedtime treatment for Alcohol Use Disorder 5 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 Tonmya has been conditionally accepted by the U.S. FDA as the proposed trade name for TNX - 102 SL for the treatment of PTSD. 3 NDA - New Drug Application; 4 BLA – Biologic Licensing Application; 5 Pre - Investigational New Drug (IND) meeting completed in October with FDA. Striped arrow reflects that TNX - 102 SL for AUD is in the pre - IND stage; upon receiving FDA clearance of an IND applica tion, it will be Phase 2 POC ready as it is expected to qualify for the 505(b)(2) pathway for approval; 6 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 7 Striped arrows reflect that TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 study for formulation development was recently completed outside of th e U.S. Interim analysis results expected 1Q 2020 Topline results expected 2Q 2020 Interim analysis results expected 2H 2020 Topline results expected 1H 2021 Daytime treatment for Major Depressive Disorder
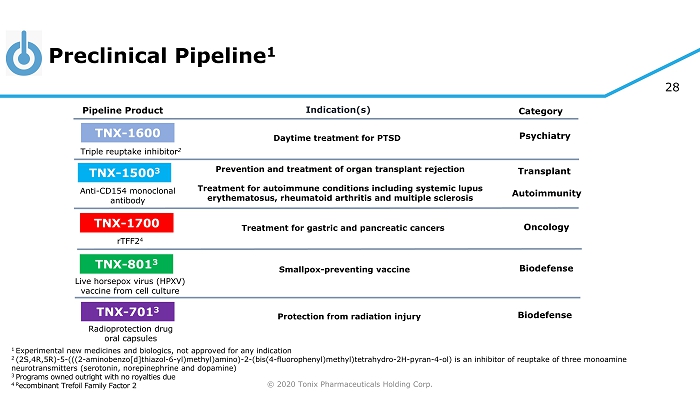
© 2020 Tonix Pharmaceuticals Holding Corp. 28 Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) 3 Programs owned outright with no royalties due 4 R ecombinant Trefoil Family Factor 2 Category Pipeline Product Indication(s) TNX - 801 3 Smallpox - preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture TNX - 701 3 Protection from radiation injury Radioprotection drug oral capsules Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for PTSD Psychiatry TNX - 1500 3 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Treatment for autoimmune conditions including systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Biodefense Biodefense
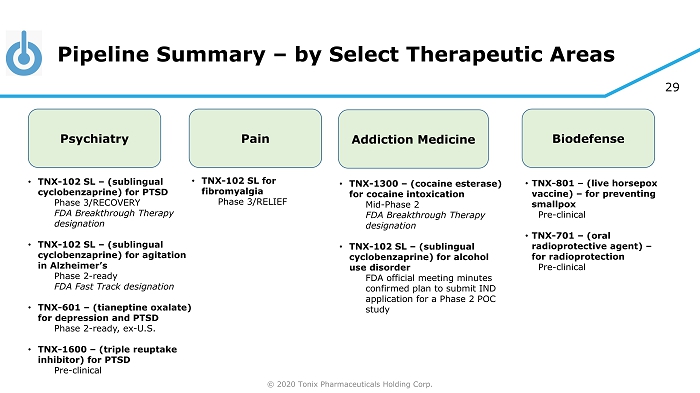
© 2020 Tonix Pharmaceuticals Holding Corp. 29 Pipeline Summary – by Select Therapeutic Areas Psychiatry Pain Addiction Medicine Biodefense • TNX - 102 SL – (sublingual cyclobenzaprine) for PTSD Phase 3/RECOVERY FDA Breakthrough Therapy designation • TNX - 102 SL – (sublingual cyclobenzaprine) for agitation in Alzheimer’s Phase 2 - ready FDA Fast Track designation • TNX - 601 – (tianeptine oxalate) for depression and PTSD Phase 2 - ready, ex - U.S. • TNX - 1600 – (triple reuptake inhibitor) for PTSD Pre - clinical • TNX - 102 SL for fibromyalgia Phase 3/RELIEF • TNX - 1300 – (cocaine esterase) for cocaine intoxication Mid - Phase 2 FDA Breakthrough Therapy designation • TNX - 102 SL – (sublingual cyclobenzaprine) for alcohol use disorder FDA official meeting minutes confirmed plan to submit IND application for a Phase 2 POC study • TNX - 801 – (live horsepox vaccine) – for preventing smallpox Pre - clinical • TNX - 701 – (oral radioprotective agent) – for radioprotection Pre - clinical

© 2020 Tonix Pharmaceuticals Holding Corp. 30 Milestones – Recently Completed and Upcoming □ May 2019 In - licensed TNX - 1300, in Phase 2 development for cocaine intoxication □ October 2019 Completed long - term exposure studies in PTSD to evaluate tolerability of TNX - 102 SL 5.6 mg □ October 2019 Met with FDA to discuss Phase 2 study for TNX - 102 SL to treat AUD □ 4 th Quarter 2019 Confirmed once - daily dosing for TNX - 601 CR in PK study □ 4 th Quarter 2019 Enrolled first patient in Phase 3 F304/RELIEF study for management of fibromyalgia □ 1 st Quarter 2020 Interim analysis results from Phase 3 P302/RECOVERY study in PTSD expected □ 1 st Quarter 2020 Expect to submit IND application to support Phase 2 POC study in AUD □ 2 nd Quarter 2020 Topline data from Phase 3 P302/RECOVERY study in PTSD expected □ 2 nd Half 2020 Interim analysis results from Phase 3 F304/RELIEF study in fibromyalgia expected □ 2 nd Half 2020 Expect to initiate Phase 2 study of TNX - 601 CR in depression, ex - U.S. □ 1 st Half 2021 Topline data from Phase 3 F304/RELIEF study in fibromyalgia expected x x x x x

© 2020 Tonix Pharmaceuticals Holding Corp. 31 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
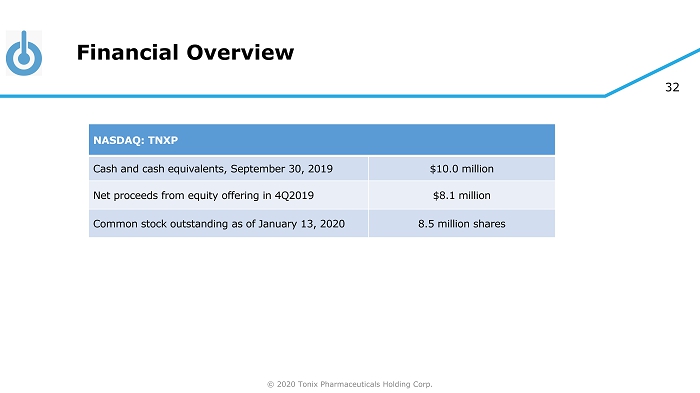
© 2020 Tonix Pharmaceuticals Holding Corp. 32 Financial Overview NASDAQ: TNXP Cash and cash equivalents, September 30, 2019 $10.0 million Net proceeds from equity offering in 4Q2019 $8.1 million Common stock outstanding as of January 13, 2020 8.5 million shares

© 2020 Tonix Pharmaceuticals Holding Corp. 33 Thank you ! NASDAQ: TNXP