
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.01

© 2020 Tonix Pharmaceuticals Holding Corp. 1 February 2020 Version P0220 2 - 5 - 20 (Doc 0593) Investor Presentation

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2018 , as filed with the Securities and Exchange Commission (the “SEC”) on March 18 , 2019 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

© 2020 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals: Lead Program In Phase 3 clinical development of TNX - 102 SL 1 for Fibromyalgia • Chronic pain condition Fibromyalgia milestones ( Phase 3 RELIEF study ): • 2 nd Half 2020 - Interim analysis results expected • 1 st Half 2021 - Topline data expected 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication
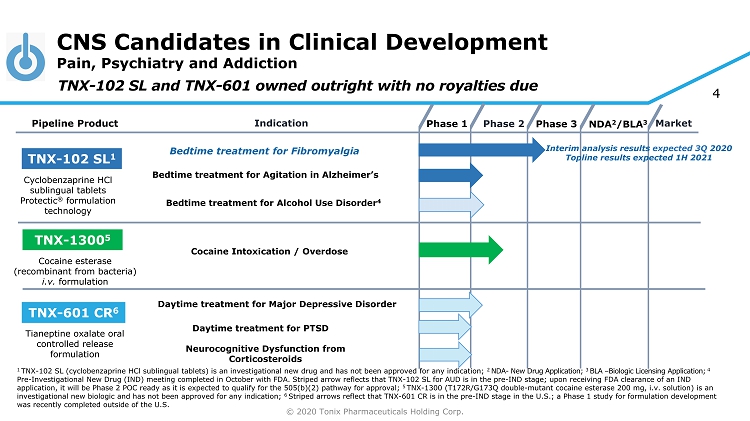
© 2020 Tonix Pharmaceuticals Holding Corp. 4 CNS Candidates in Clinical Development Pain, Psychiatry and Addiction Phase 2 NDA 2 /BLA 3 Market Pipeline Product Indication Phase 3 TNX - 102 SL 1 Daytime treatment for PTSD TNX - 601 CR 6 TNX - 1300 5 Cocaine Intoxication / Overdose Cyclobenzaprine HCl sublingual tablets Protectic ® formulation technology Tianeptine oxalate oral controlled release formulation Cocaine esterase (recombinant from bacteria) i.v. formulation Phase 1 Bedtime t reatment for Fibromyalgia TNX - 102 SL and TNX - 601 owned outright with no royalties due Bedtime t reatment for Agitation in Alzheimer’s Neurocognitive Dysfunction from Corticosteroids Bedtime treatment for Alcohol Use Disorder 4 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication; 2 NDA - New Drug Application; 3 BLA – Biologic Licensing Application; 4 Pre - Investigational New Drug (IND) meeting completed in October with FDA. Striped arrow reflects that TNX - 102 SL for AUD is in t he pre - IND stage; upon receiving FDA clearance of an IND application, it will be Phase 2 POC ready as it is expected to qualify for the 505(b)(2) pathway for approval; 5 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; 6 Striped arrows reflect that TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 study for formulation development was recently completed outside of the U.S. Interim analysis results expected 3Q 2020 Topline results expected 1H 2021 Daytime treatment for Major Depressive Disorder
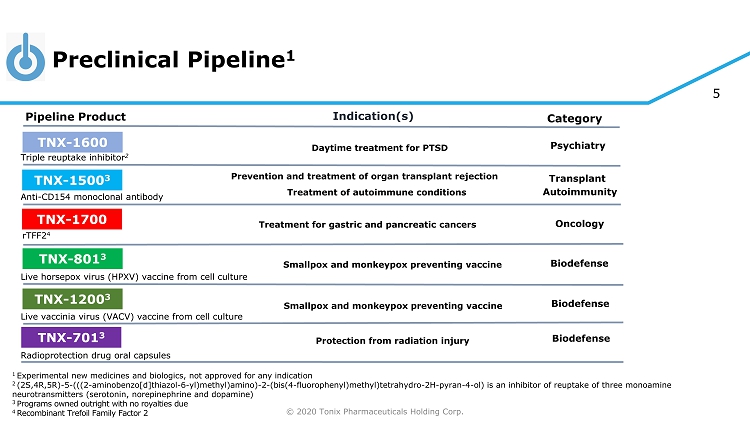
© 2020 Tonix Pharmaceuticals Holding Corp. 5 Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) 3 Programs owned outright with no royalties due 4 R ecombinant Trefoil Family Factor 2 Category Pipeline Product Indication(s) TNX - 801 3 Smallpox and monkeypox preventing vaccine Live horsepox virus (HPXV) vaccine from cell culture TNX - 701 3 Protection from radiation injury Radioprotection drug oral capsules Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for PTSD Psychiatry TNX - 1500 3 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Biodefense Biodefense TNX - 1200 3 Smallpox and monkeypox preventing vaccine Live vaccinia virus (VACV) vaccine from cell culture Biodefense Treatment of autoimmune conditions
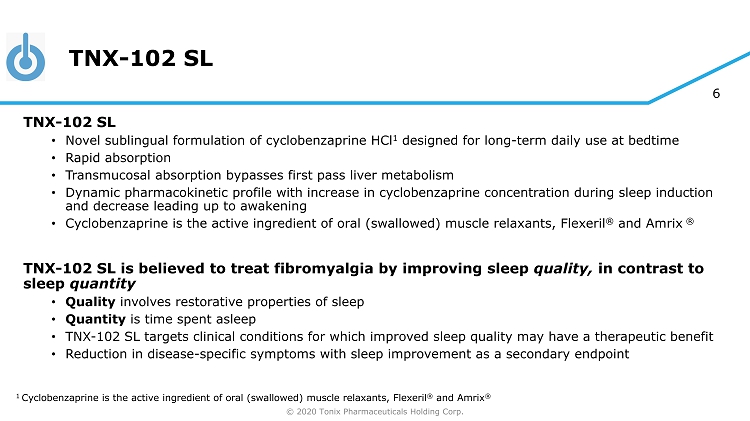
© 2020 Tonix Pharmaceuticals Holding Corp. 6 TNX - 102 SL TNX - 102 SL • Novel sublingual formulation of cyclobenzaprine HCl 1 designed for long - term daily use at bedtime • Rapid absorption • Transmucosal absorption bypasses first pass liver metabolism • Dynamic pharmacokinetic profile with increase in cyclobenzaprine concentration during sleep induction and decrease leading up to awakening • Cyclobenzaprine is the active ingredient of oral (swallowed) muscle relaxants, Flexeril ® and Amrix ® TNX - 102 SL is believed to treat fibromyalgia by improving sleep quality, in contrast to sleep quantity • Quality involves restorative properties of sleep • Quantity is time spent asleep • TNX - 102 SL targets clinical conditions for which improved sleep quality may have a therapeutic benefit • Reduction in disease - specific symptoms with sleep improvement as a secondary endpoint 1 Cyclobenzaprine is the active ingredient of oral (swallowed) muscle relaxants, Flexeril ® and Amrix ®
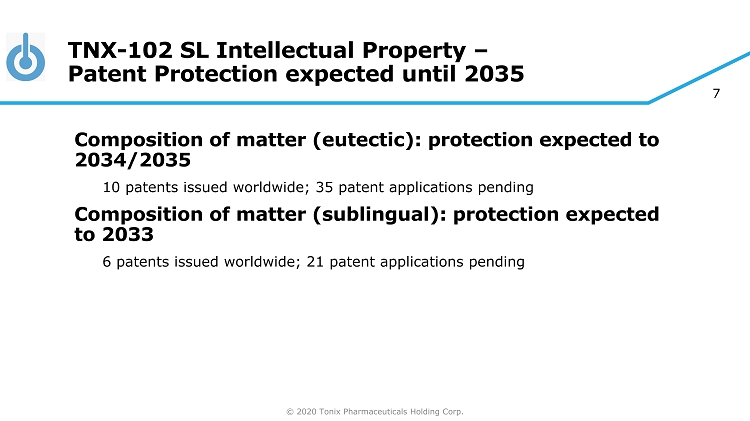
© 2020 Tonix Pharmaceuticals Holding Corp. 7 TNX - 102 SL Intellectual Property – Patent Protection expected until 2035 Composition of matter (eutectic): protection expected to 2034/2035 10 patents issued worldwide; 35 patent applications pending Composition of matter (sublingual): protection expected to 2033 6 patents issued worldwide; 21 patent applications pending

© 2020 Tonix Pharmaceuticals Holding Corp. 8 When the check engine light malfunctions, the light is on even though the car is not malfunctioning Fibromyalgia is considered a neurobiological disorder characterized by 1 : chronic widespread pain, non - restorative sleep, fatigue, diminished cognition Believed to result from inappropriate pain signaling in central nervous system in the absence of peripheral injury 1 An estimated 6 - 12 million adults in the U.S. have fibromyalgia 2 Causes significant impairment in all areas of life 3 • Lower levels of health - related quality of life – reduced daily functioning • Interference with work (loss of productivity, disability) Fewer than half of those treated for fibromyalgia receive complete relief from the three FDA - approved drugs 4 Inflicts substantial strain on the healthcare system • Average patient has 20 physician office visits per year 5 • Annual direct medical costs are twice those of non - fibromyalgia individuals 6 Fibromyalgia 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141. 2 American Chronic Pain Association (www.theacpa.org, 2019) 3 Schaefer et al., Pain Pract , 2015. 4 The three drugs with FDA approval for the treatment of fibromyalgia: Pregabalin (Lyrica); Duloxetine (Cymbalta); Milnacipran ( Savella ) 5 Robinson et al, Pain Medicine 2013;14:1400. 6 White et al, J Occupational Environ Med 2008;50:13.
© 2020 Tonix Pharmaceuticals Holding Corp. 9 Large Need for New Fibromyalgia Therapies that Provide Broad Symptom Improvement with Better Tolerability Currently - approved medications may have side effects that limit long - term use 1 High rates of discontinuation, switching and augmentation • A ttempts to treat multiple symptoms and/or avoid intolerable side effects • Average of 2 - 3 medications used simultaneously 2 • Typical patient has tried six different medications 3 • Medication - related side effects may be similar to fibromyalgia symptoms Substantial off - label use of narcotic painkillers and prescription sleep aids 3 • Among those diagnosed, more than one - third have used prescription opioids as a means of treatment 4 TNX - 102 SL is a non - opioid, centrally - acting analgesic that could provide a new therapeutic option for fibromyalgia patients 1 Nuesch et al, Ann Rheum Dis 2013;72:955 - 62. 2 Robinson RL et al, Pain Medicine 2012;13:1366. 3 Patient Trends: Fibromyalgia”, Decision Resources, 2011. 4 Berger A, Dukes E, Martin S, Edelsberg J, Oster G, Int J Clin Pract , 2007; 61(9):1498 – 1508.

© 2020 Tonix Pharmaceuticals Holding Corp. 10 Volkswagen Check Engine [Photograph]. (2011, October 14). Wikipedia When the check engine light malfunctions, the light is on even though the car is not malfunctioning Believed to result from inappropriate pain signaling in central nervous system • Absence of peripheral injury 1 Pain is a sensor system in the brain • When the system malfunctions, the pain alarm is turned on even through there has been no peripheral nerve tissue injury Improving sleep quality is believed to reduce pain and fatigue in FM • Suggesting sleep dysfunction is pathogenic in FM TNX - 102 SL acts as a non - opioid, centrally - acting analgesic to aid in the management of fibromyalgia Potential Role of Sleep Quality in Fibromyalgia 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141.
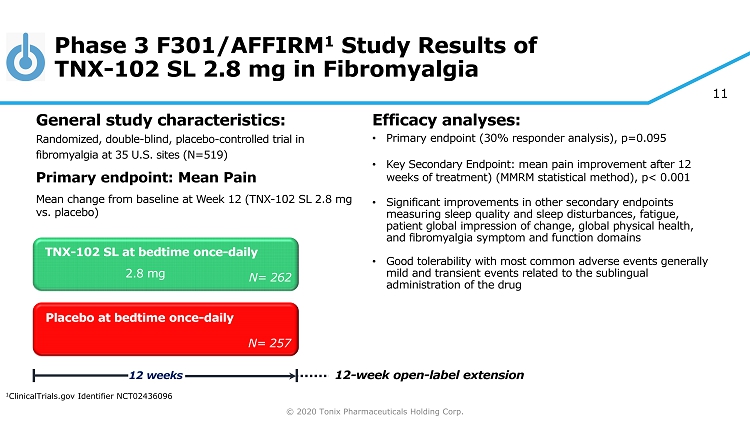
© 2020 Tonix Pharmaceuticals Holding Corp. 11 Phase 3 F301/AFFIRM 1 Study Results of TNX - 102 SL 2.8 mg in Fibromyalgia Efficacy analyses: • Primary endpoint (30% responder analysis), p=0.095 • Key Secondary Endpoint: mean pain improvement after 12 weeks of treatment) (MMRM statistical method), p< 0.001 • Significant improvements in other secondary endpoints measuring sleep quality and sleep disturbances, fatigue, patient global impression of change, global physical health, and fibromyalgia symptom and function domains • Good tolerability with most common adverse events generally mild and transient events related to the sublingual administration of the drug TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 262 N= 257 2.8 mg 12 - week open - label extension 1 ClinicalTrials.gov Identifier NCT02436096 General s tudy c haracteristics: Randomized, double - blind, placebo - controlled trial in fibromyalgia at 35 U.S. sites (N=519) Primary e ndpoint: Mean Pain Mean change from baseline at W eek 12 (TNX - 102 SL 2.8 mg vs. placebo )
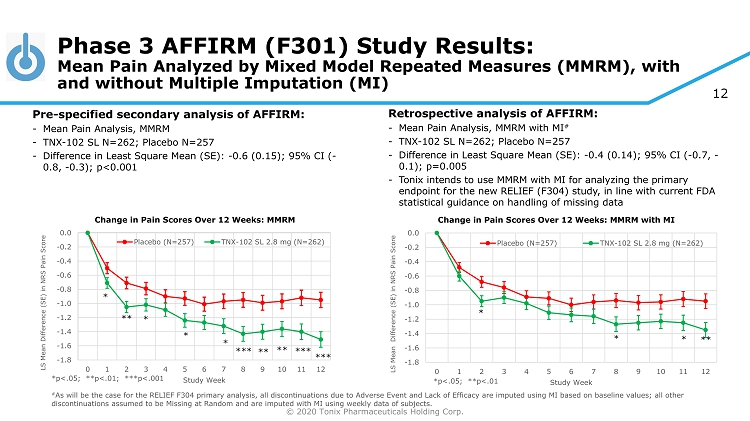
© 2020 Tonix Pharmaceuticals Holding Corp. 12 *p<.05; **p<.01; ***p<.001 # As will be the case for the RELIEF F304 primary analysis, all discontinuations due to Adverse Event and Lack of Efficacy are imp uted using MI based on baseline values; all other discontinuations assumed to be Missing at Random and are imputed with MI using weekly data of subjects. Phase 3 AFFIRM (F301) Study Results: Mean Pain Analyzed by Mixed Model Repeated Measures (MMRM), with and without Multiple Imputation (MI) Pre - specified secondary analysis of AFFIRM: - Mean Pain Analysis, MMRM - TNX - 102 SL N=262; Placebo N=257 - Difference in Least Square Mean (SE): - 0.6 (0.15); 95% CI ( - 0.8, - 0.3); p<0.001 Retrospective analysis of AFFIRM: - Mean Pain Analysis, MMRM with MI # - TNX - 102 SL N=262; Placebo N=257 - Difference in Least Square Mean (SE): - 0.4 (0.14); 95% CI ( - 0.7, - 0.1); p=0.005 - Tonix intends to use MMRM with MI for analyzing the primary endpoint for the new RELIEF (F304) study, in line with current FDA statistical guidance on handling of missing data -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM with MI Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * * * ** *p<.05; **p<.01 -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * ** *** * * * *** *** ** **

© 2020 Tonix Pharmaceuticals Holding Corp. 13 TNX - 102 SL for Fibromyalgia New Phase 3 Study: Higher (2x) Dose, New Primary Endpoint Clear guidance from FDA to advance fibromyalgia program using higher dose (5.6 mg) Long - term safety of 5.6 mg dose collected in PTSD studies expected to support fibromyalgia NDA Retrospective analysis of mean pain improvement after 12 weeks of treatment showed statistically significant improvement using both statistical methods: MMRM (p < 0.001) and MMRM with MI (p < 0.01) MMRM with MI to be used going forward First patient enrolled in the new Phase 3 RELIEF study in December 2019
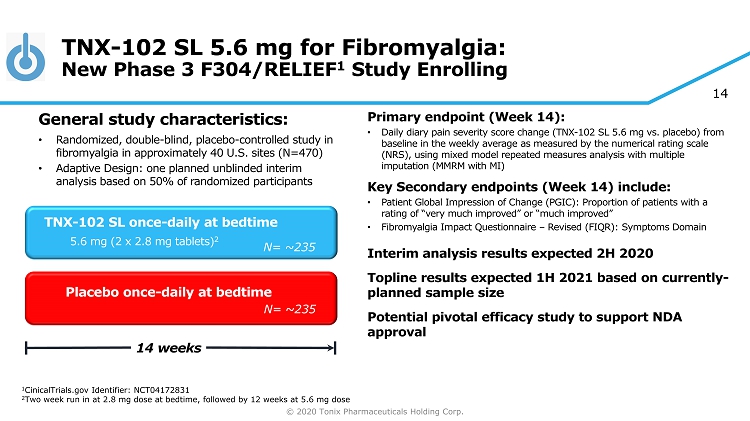
© 2020 Tonix Pharmaceuticals Holding Corp. 14 TNX - 102 SL 5.6 mg for Fibromyalgia: New Phase 3 F304/RELIEF 1 Study Enrolling Primary e ndpoint (Week 14) : • Daily diary pain severity score change (TNX - 102 SL 5.6 mg vs. placebo) from baseline in the weekly average as measured by the numerical rating scale (NRS), using mixed model repeated measures analysis with multiple imputation (MMRM with MI) Key Secondary e ndpoint s (Week 14) include: • Patient Global Impression of Change (PGIC): Proportion of patients with a rating of “very much improved” or “much improved” • Fibromyalgia Impact Questionnaire – Revised (FIQR): Symptoms Domain Interim analysis results expected 2H 2020 Topline results expected 1H 2021 based on currently - planned sample size Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) 2 General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia in approximately 40 U.S. sites (N=470) • Adaptive Design: one planned unblinded interim analysis based on 50% of randomized participants 1 C inicalTrials.gov Identifier: NCT04172831 2 Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose N= ~235 N= 250 N= ~235
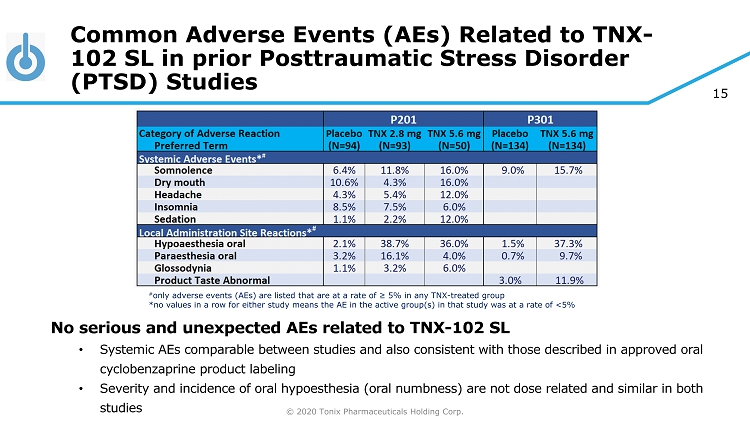
© 2020 Tonix Pharmaceuticals Holding Corp. 15 Common Adverse Events (AEs) Related to TNX - 102 SL in prior Posttraumatic Stress Disorder (PTSD) Studies No serious and unexpected AEs related to TNX - 102 SL • Systemic AEs comparable between studies and also consistent with those described in approved oral cyclobenzaprine product labeling • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related and similar in both studies # only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *no values in a row for either study means the AE in the active group(s) in that study was at a rate of <5%
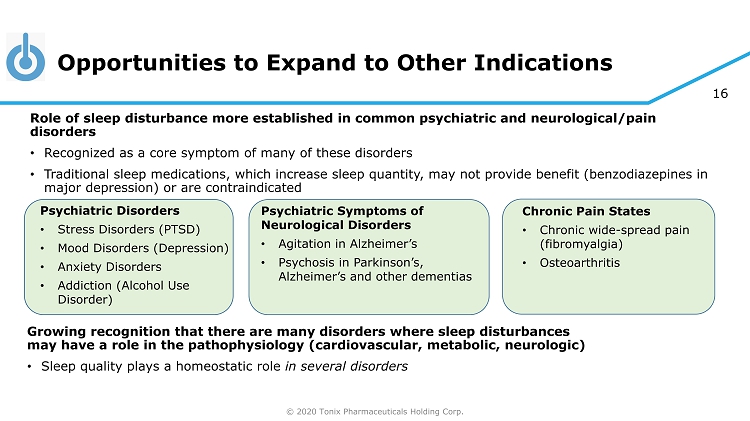
© 2020 Tonix Pharmaceuticals Holding Corp. 16 Opportunities to Expand to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Sleep quality plays a homeostatic role in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (Depression) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

© 2020 Tonix Pharmaceuticals Holding Corp. 17 TNX - 102 SL: Potential Treatment for Agitation in Alzheimer’s Disease (AAD) Agitation is one of the most distressing and debilitating of the behavioral complications of Alzheimer’s disease • Includes emotional lability, restlessness, irritability and aggression 1 Link between disturbed sleep and agitation in Alzheimer’s 1 - 3 • Agitation is commonly diurnal (e.g., “sundowning”) Prevalence • Agitation is likely to affect more than half of the 5.3 million Americans who currently suffer from moderate to severe Alzheimer’s disease; expected to nearly triple by 2050 4 Significant unmet need with no FDA approved drugs for the treatment of AAD Proposed Phase 2 study can potentially serve as a pivotal efficacy study to support NDA approval 5 1 Rose, K.et al. (2015). American Journal of Alzheimer's Disease & Other Dementias , 30 :78 2 Shih, Y. H., et al. (2017). Journal of the American Medical Directors Association , 18 , 396. 3 Canevelli, M., et al. (2016). Frontiers in medicine , 3 . 4 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/ 5 FDA comments on final protocol received October 2018

© 2020 Tonix Pharmaceuticals Holding Corp. 18 TNX - 102 SL: Potential Treatment for Alcohol Use Disorder (AUD) AUD is a chronic relapsing brain disease • Characterized by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not using Sleep disturbance is extremely common in alcohol recovery 1 • Significantly impacts daytime cognition, mood, and ability to participate in alcohol treatment, and is associated with increased risk of relapse Prevalence • An estimated 36 million adults in the U.S. have AUD 2 Three FDA - approved medications • Remains an unmet need due to compliance and safety issues Pre - IND meeting with the FDA completed in October 2019 • Discussed 505(b)(2) development plan for TNX - 102 SL as a treatment for AUD • FDA official meeting minutes confirmed plan to submit IND application in 1Q 2020 for a Phase 2 Proof of Concept Study 1 Arnedt et al, J Addict Dis. 2007 ; 26(4): 41 – 54 2 Grant et al, JAMA Psychiatry 2015; 72(8): 757 - 766; www.census.gov

© 2020 Tonix Pharmaceuticals Holding Corp. 19 TNX - 1300* for the Treatment of Cocaine Intoxication Recombinant protein that degrades cocaine in the bloodstream 1 • Double - mutant cocaine esterase ( CocE ) • CocE was identified in bacteria ( Rhodococcus ) that use cocaine as its sole source of carbon and nitrogen and that grow in soil surrounding coca plants 2 • CocE catalyzes the breakdown of cocaine into metabolites ecgonine methyl ester and benzoic acid Phase 2 study comp leted by Rickett Benckiser (TNX - 1300 was formerly RBP - 8000) 3 • Volunteer cocaine abusers received cocaine 50 mg i.v. infusion over 10 minutes • TNX - 1300 given one minute after completion of cocaine infusion • Rapidly reversed the physiologic effects of cocaine; cocaine plasma exposures dropped by 90% within two minutes • Well tolerated with the most frequently reported adverse events being gastrointestinal disorders ( including dry mouth, nausea); nervous systems disorders (including headache, dizziness) and skin and subcutaneous tissue disorders (including hyperhidrosis , dermatitis) *TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication. 1 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23. 2 Bresler MM et al, Appl Environ Microbiol . 2000. 66(3):904 - 8. 3 Nasser AF et al, J Addict Dis . 2014;33(4):289 - 302.
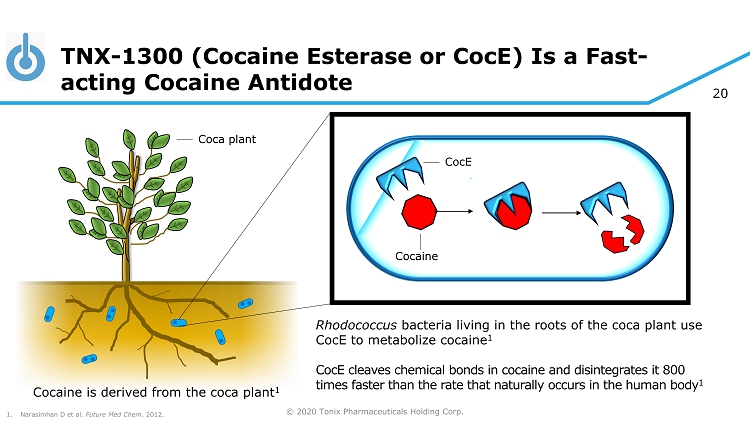
© 2020 Tonix Pharmaceuticals Holding Corp. 20 TNX - 1300 (Cocaine Esterase or CocE ) Is a Fast - acting Cocaine Antidote CocE Rhodococcus bacteria living in the roots of the coca plant use CocE to metabolize cocaine 1 CocE cleaves chemical bonds in cocaine and disintegrates it 800 times faster than the rate that naturally occurs in the human body 1 Cocaine Cocaine is derived from the coca plant 1 1. Narasimhan D et al. Future Med Chem . 2012. Coca plant
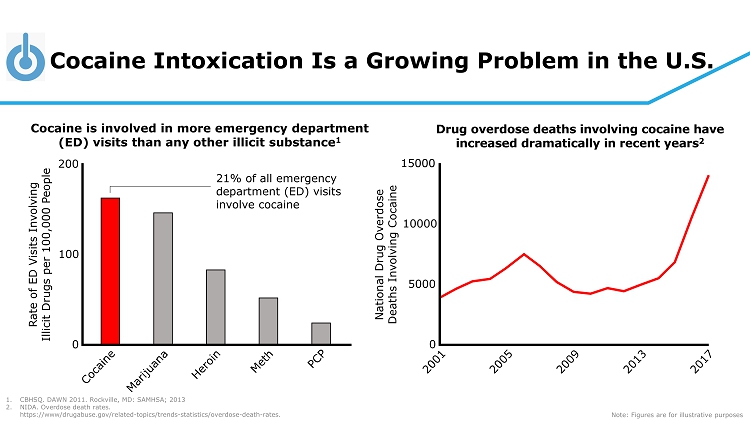
Cocaine Intoxication Is a Growing Problem in the U.S. National Drug Overdose Deaths Involving Cocaine 0 5000 10000 15000 Cocaine is involved in more emergency department (ED) visits than any other illicit substance 1 Drug overdose deaths involving cocaine have increased dramatically in recent years 2 1. CBHSQ. DAWN 2011. Rockville, MD: SAMHSA; 2013 2. NIDA. Overdose death rates. https://www/drugabuse.gov/related - topics/trends - statistics/overdose - death - rates. Note: Figures are for illustrative purposes Rate of ED Visits Involving Illicit Drugs per 100,000 People 0 100 200 21% of all emergency department (ED) visits involve cocaine
© 2020 Tonix Pharmaceuticals Holding Corp. 22 TNX - 601 CR* (Tianeptine Oxalate Controlled Release) Tablets Proprietary new controlled release formulation for once - daily dosing • Suitability for once - daily dosing established in Phase 1 pharmacokinetic study, completed outside of the U.S. • Well tolerated in study and side effects were consistent with the known safety profile of tianeptine sodium • Tianeptine sodium immediate release is approved and marketed outside of the U.S. for three times a day dosing for the treatment of depression • Once - daily dosing for TNX - 601 CR believed to have an adherence advantage over three times a day dosing with tianeptine sodium • Plan to request pre - IND meeting with FDA in first half 2020 • Plan for Phase 2 study in depression, ex - U.S., in second half 2020 Proprietary new oxalate salt with improved pharmaceutical properties • Tianeptine oxalate is crystalline, while tianeptine sodium is amorphous Issued patents directed to tianeptine and tianeptine oxalate • Composition of Matter: Issued US patent directed to oxalate salt, U.S. Patent No. 10,449,203 • Method of Use: Issued U.S. and European patents directed to methods of treating cognitive impairment associated with corticosteroid treatment (U.S. Patent No. 9,314,469; European Patent No. 3246031) *TNX - 601 (tianeptine oxalate CR tablets) is in the pre - IND stage in the U.S. and has not been approved for any indication.
© 2020 Tonix Pharmaceuticals Holding Corp. 23 TNX - 601 CR : A Potential Daytime Treatment for Depression and PTSD Depression: majority suffering from depression do not have an adequate response to initial antidepressant therapy • Tianeptine sodium immediate release (IR) tablets for three times a day dosing is approved as an antidepressant in the EU, Russia, Asia and Latin America; first marketed for depression in France in 1989 • Tianeptine sodium is reported to have prominent anti - anxiety effects in depression with a low incidence of sexual side effects • TNX - 601 CR leverages the established efficacy and safety of tianeptine sodium IR as a treatment for depression outside of the U.S. • Despite multiple approved products for depression in the U.S., there remains significant interest and need for new treatments, particularly for medicines that modulate the glutamatergic system PTSD: heterogeneous condition, so not all patients are expected to respond to a single medicine • TNX - 601 CR modulates the glutamatergic system • Published studies show tianeptine is active in the treatment of PTSD 1 - 4 • Leverages Tonix expertise in PTSD (clinical and regulatory, market analysis, etc.) 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747

© 2020 Tonix Pharmaceuticals Holding Corp. 24 TNX - 801 (Synthesized Live Horsepox Virus): A Potential Smallpox and Monkeypox Preventing Vaccine Pre - IND Stage Potential improvement over current biodefense tools against smallpox ✓ Demonstrated protective vaccine activity in mice and macaques ✓ Collaboration with Professor David Evans and Dr. Ryan Noyce at University of Alberta Currently approved smallpox and monkeypox vaccines ✓ Two vaccines are FDA approved for smallpox: ACAM2000 ® (vaccinia) and Jynneos ® (MVA - BN) and only Jynneos is approved for monkeypox 1 Regulatory strategy • We intend to meet with FDA to discuss the most efficient and appropriate investigational plan to support the licensure ✓ Planning non - inferiority, active comparator study using an FDA approved product Targeting a Potential Public Health Issue Material threat medical countermeasure under 21 st Century Cures Act • Qualifies for Priority Review Voucher (PRV) upon licensure 2 ✓ PRVs have no expiration date, are transferrable and have sold for ~$125 M 1 ACAM2000 is a registered trademark of Emergent BioSolutions and Jynneos is a registered trademark of Bavarian Nordic 2 BLA/NDA priority 6 - month review is expected.
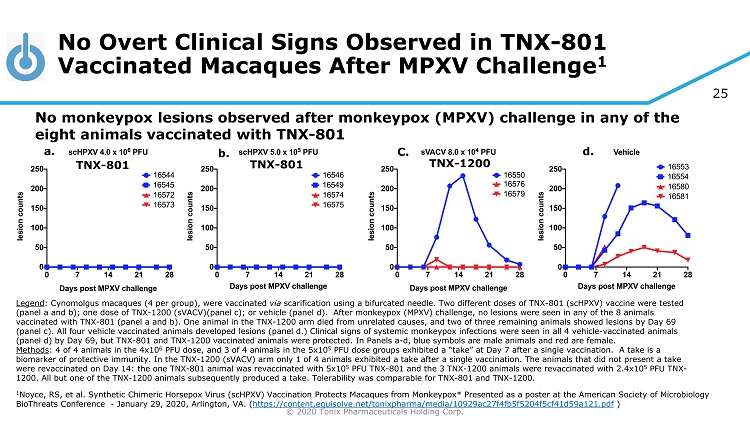
25 © 2020 Tonix Pharmaceuticals Holding Corp. No Overt Clinical Signs Observed in TNX - 801 Vaccinated Macaques After MPXV Challenge 1 1 Noyce, RS, et al. Synthetic Chimeric Horsepox Virus ( scHPXV ) Vaccination Protects Macaques from Monkeypox* Presented as a poster at the American Society of Microbiology BioThreats Conference - January 29, 2020, Arlington, VA . ( https://content.equisolve.net/tonixpharma/media/10929ac27f4fb5f5204f5cf41d59a121.pdf ) No monkeypox lesions observed after monkeypox (MPXV) challenge in any of the eight animals vaccinated with TNX - 801 Legend : Cynomolgus macaques (4 per group), were vaccinated via scarification using a bifurcated needle. Two different doses of TNX - 801 ( scHPXV ) vaccine were tested (panel a and b); one dose of TNX - 1200 ( sVACV )(panel c); or vehicle (panel d). After monkeypox (MPXV) challenge, no lesions were seen in any of the 8 animals vaccinated with TNX - 801 (panel a and b). One animal in the TNX - 1200 arm died from unrelated causes, and two of three remaining a nimals showed lesions by Day 69 (panel c). All four vehicle vaccinated animals developed lesions (panel d.) Clinical signs of systemic monkeypox infections w ere seen in all 4 vehicle - vaccinated animals (panel d) by Day 69, but TNX - 801 and TNX - 1200 vaccinated animals were protected. In Panels a - d, blue symbols are male animals an d red are female. Methods : 4 of 4 animals in the 4x10 6 PFU dose, and 3 of 4 animals in the 5x10 5 PFU dose groups exhibited a “take” at Day 7 after a single vaccination. A take is a biomarker of protective immunity. In the TNX - 1200 ( sVACV ) arm only 1 of 4 animals exhibited a take after a single vaccination. The animals that did not present a take were revaccinated on Day 14: the one TNX - 801 animal was revaccinated with 5x10 5 PFU TNX - 801 and the 3 TNX - 1200 animals were revaccinated with 2.4x10 5 PFU TNX - 1200. All but one of the TNX - 1200 animals subsequently produced a take. Tolerability was comparable for TNX - 801 and TNX - 1200. a. b. C. d. TNX - 801 TNX - 801 TNX - 1200
© 2020 Tonix Pharmaceuticals Holding Corp. 26 Pipeline Summary – by Select Therapeutic Areas Pain Psychiatry Addiction Medicine Biodefense • TNX - 102 SL – (sublingual cyclobenzaprine) for fibromyalgia Phase 3/RELIEF • TNX - 102 SL – (sublingual cyclobenzaprine) for agitation in Alzheimer’s Phase 2 - ready FDA Fast Track designation • TNX - 601 – (tianeptine oxalate) for depression and PTSD Phase 2 - ready, ex - U.S. • TNX - 1600 – (triple reuptake inhibitor) for PTSD Pre - clinical • TNX - 1300 – (cocaine esterase) for cocaine intoxication Phase 2 FDA Breakthrough Therapy designation • TNX - 102 SL – (sublingual cyclobenzaprine) for alcohol use disorder FDA official meeting minutes confirmed plan to submit IND application for a Phase 2 PoC study • TNX - 801 – (live horsepox vaccine) – for preventing smallpox Pre - clin ical • TNX - 1200 – (live vaccinia vaccine) – for preventing smallpox Pre - clinical • TNX - 701 – (oral radioprotective agent) – for radioprotection Pre - clinical

© 2020 Tonix Pharmaceuticals Holding Corp. 27 Milestones – Recently Completed and Upcoming □ May 2019 In - licensed TNX - 1300, in Phase 2 development for cocaine intoxication □ October 2019 Completed long - term exposure studies in PTSD to evaluate tolerability of TNX - 102 SL 5.6 mg □ October 2019 Met with FDA to discuss Phase 2 study for TNX - 102 SL to treat AUD □ 4 th Quarter 2019 Confirmed once - daily dosing for TNX - 601 CR in PK study □ 4 th Quarter 2019 Enrolled first patient in Phase 3 F304/RELIEF study for management of fibromyalgia □ February, 2020 Interim analysis results reported from Phase 3 P302/RECOVERY study in PTSD □ 1 st Quarter 2020 Expect to submit IND application to support Phase 2 POC study in AUD □ 3 rd Quarter 2020 Interim analysis results from Phase 3 F304/RELIEF study in fibromyalgia expected □ 2 nd Half 2020 Expect to initiate Phase 2 study of TNX - 601 CR in depression, ex - U.S. □ 1 st Half 2021 Topline data from Phase 3 F304/RELIEF study in fibromyalgia expected x x x x x x

© 2020 Tonix Pharmaceuticals Holding Corp. 28 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
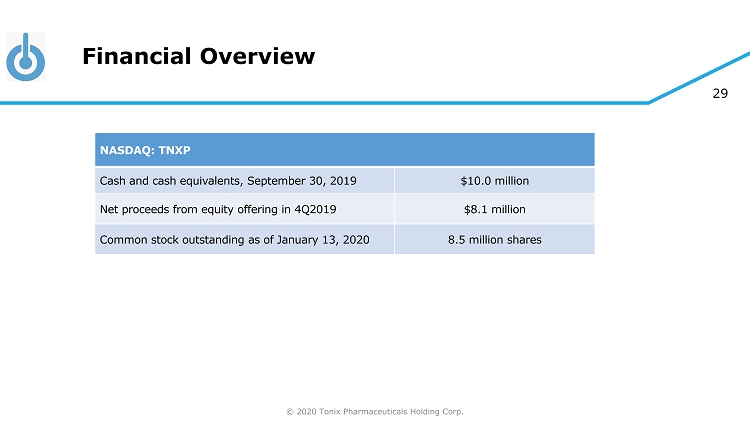
© 2020 Tonix Pharmaceuticals Holding Corp. 29 Financial Overview NASDAQ: TNXP Cash and cash equivalents, September 30, 2019 $10.0 million Net proceeds from equity offering in 4Q2019 $8.1 million Common stock outstanding as of January 13, 2020 8.5 million shares

© 2020 Tonix Pharmaceuticals Holding Corp. 30 Thank you ! NASDAQ: TNXP