
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.1

1 March 20, 2020 Version P0224 3 - 20 - 20 (Doc 0607) WHO Presentation – COVID Vaccine Manufacturers © 2020 Tonix Pharmaceuticals Holding Corp.

Cautionary Note on Forward - Looking Statements © 2020 Tonix Pharmaceuticals Holding Corp. 2 Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2018 , as filed with the Securities and Exchange Commission (the “SEC”) on March 18 , 2019 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
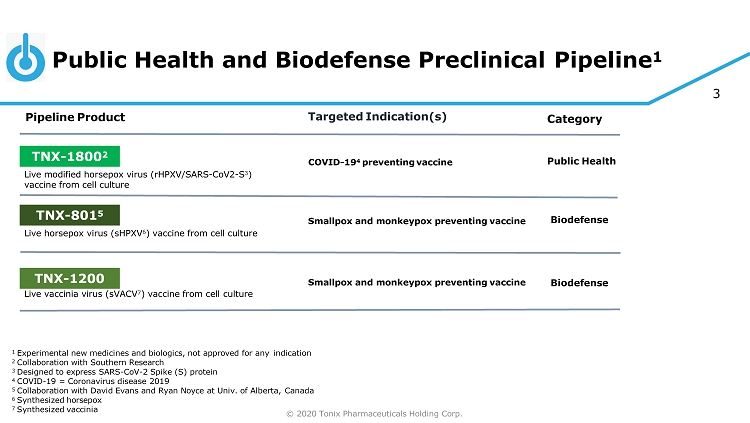
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Public Health and Biodefense Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 Collaboration with Southern Research 3 Designed to express SARS - CoV - 2 Spike (S) protein 4 COVID - 19 = Coronavirus disease 2019 5 Collaboration with David Evans and Ryan Noyce at Univ. of Alberta, Canada 6 Synthesized horsepox 7 Synthesized vaccinia C at eg or y Pipeline Product Targeted Indication(s) TNX - 801 5 Smallpox and monkeypox preventing vaccine Live horsepox virus (sHPXV 6 ) vaccine from cell culture Bi od ef e ns e TNX - 1800 2 COVID - 19 4 preventing vaccine Live modified horsepox virus (rHPXV/SARS - CoV2 - S 3 ) vaccine from cell culture Public Health TNX - 1200 Smallpox and monkeypox preventing vaccine Live vaccinia virus (sVACV 7 ) vaccine from cell culture Bi od ef e ns e
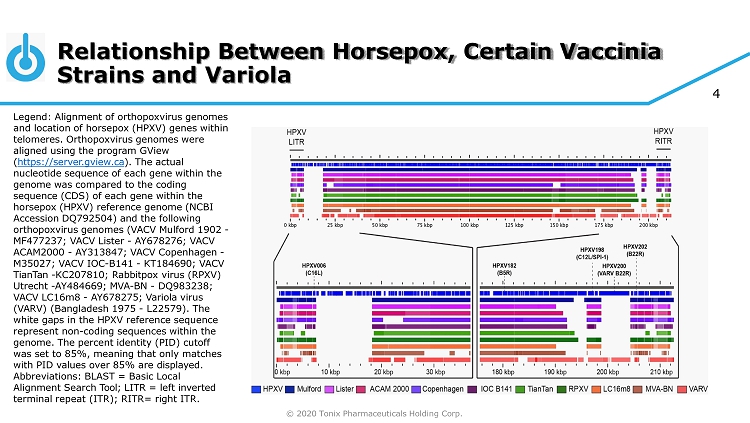
4 Relationship Between Horsepox, Certain Vaccinia Strains and Variola Legend: Alignment of orthopoxvirus genomes and location of horsepox (HPXV) genes within telomeres. Orthopoxvirus genomes were aligned using the program GView ( https://server.gview.ca ). The actual nucleotide sequence of each gene within the genome was compared to the coding sequence (CDS) of each gene within the horsepox (HPXV) reference genome (NCBI Accession DQ792504) and the following orthopoxvirus genomes (VACV Mulford 1902 - MF477237; VACV Lister - AY678276; VACV ACAM2000 - AY313847; VACV Copenhagen - M35027; VACV IOC - B141 - KT184690; VACV TianTan - KC207810; Rabbitpox virus (RPXV) Utrecht - AY484669; MVA - BN - DQ983238; VACV LC16m8 - AY678275; Variola virus (VARV) (Bangladesh 1975 - L22579). The white gaps in the HPXV reference sequence represent non - coding sequences within the genome. The percent identity (PID) cutoff was set to 85%, meaning that only matches with PID values over 85% are displayed. Abbreviations: BLAST = Basic Local Alignment Search Tool; LITR = left inverted terminal repeat (ITR); RITR= right ITR. © 2020 Tonix Pharmaceuticals Holding Corp.

TNX - 801 (live horsepox virus vaccine for percutaneous (scarification) administration) © 2020 Tonix Pharmaceuticals Holding Corp. 5 Vaccine based on sequence of isolated horsepox clone 1,2 • No new gene elements and coding sequence is identical to environmental horsepox isolate • May be considered “primordial” since Left and Right ITRs are “complete” • In contrast, modern vaccinia strains contain deletions and mutations Small plaque size in culture • Appears similar to CDC publication of 1976 horsepox isolate 3 Substantially decreased virulence in mice 2 • Relative to a vaccinia vaccine strain Protects macaques from monkeypox 4 • No overt sign of clinical symptoms and no lesions in 8/8 animals at two doses of TNX - 801 Historical evidence for horsepox - like vaccines • Jenner and others demonstrated their horse originated vaccine was protective against variola in challenge studies with variola (what was then called “variolation”) • Used when smallpox was endemic Horsepox has not been reported in >40 years • Improved hygiene in animal husbandry led to its elimination • Probable natural hosts are rodents • Horse - to - cow transmission by human vector reported by Jenner 1 Tulman ER, et al. (2006) J Virol. 80(18):9244 - 58.PMID:16940536 2 Noyce RS, et al. (2018) PLoS One. 13(1):e0188453. 3 Trindale GS et al. Viruses (2016) (12). pii: E328. PMID:27973399 4 Noyce, RS, et al. Synthetic Chimeric Horsepox Virus (scHPXV) Vaccination Protects Macaques from Monkeypox* Presented as a poster at the American Society of Microbiology BioThreats Conference - January 29, 2020, Arlington, VA. ( https://content.equisolve.net/tonixpharma/media/10929ac27f4fb5f5204f5cf41d59a121.pdf )
© 2020 Tonix Pharmaceuticals Holding Corp. Potential for Use of Horsepox as a Vector Platform for a SARS - CoV - 2 Vaccine 6 Horsepox can be engineered to express foreign genes and serve as a platform for vaccine development • Large packaging capacity for exogenous DNA inserts (i.e. encoding antigens) • Precise virus - specific control of exogenous gene insert expression • Lack of persistence or genomic integration in the host • Strong immunogenicity as a vaccine • Ability to rapidly generate vector/insert constructs • Readily manufacture at scale • Live, replicating vaccine – direct antigen presentation Potential advantages of horsepox over vaccinia • Maintains strong immunogenicity with potentially improved tolerability • Relative to non - replicating vaccinia, horsepox’s replication in human cells provides direct antigen presentation by Class I MHC • Horsepox may behave differently as a vector, in part because of its different repertoire of genes that modulate immune responses and host range Strong immunogenicity for adaptive and innate immunity – believed important in SARS • Humoral immunity against Spike protein is sufficient to protect against SARS - CoV in mice 1,2 • T cells are sufficient to clear SARS - CoV in mice 3 • T cells can protect mice from SARS - CoV after vaccination with vaccinia - virus encoding a SARS Spike protein peptide 3,4 • T cell response to Spike protein is durable (>1 year) in humans post - SARS 5 • Innate immunity can clear SARS - CoV from mice 6 • Interferon responses are important for mice to limit SARS - CoV in mice 7 1 Yang ZY, et al. (2004) Nature. ;428:561 – 564. 2 Enjuanes L, et al. (Review) (2008) Virus Res. 133:45 – 62. 3 Zhao J et al. (2010) J Virol 84(18):9318 - 9325. 4 Channappanavar R, et al. (2014) J Virol 88(19):11034 - 11044. 5 Yang L - T et al. (2006) Clinical Immunology 120, 171 — 178. 6 Glass WG, et al. (2004) J Immunol. 173:4030 – 4039. 7 Hogan RJ, et al. (2004) J Virol. 78:11416 – 11421.
7 TNX - 1800 is Designed to Express SARS - CoV - 2 Spike Protein © 2020 Tonix Pharmaceuticals Holding Corp.

8 Thank you ! NASDAQ: TNXP © 2020 Tonix Pharmaceuticals Holding Corp.