
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01

© 2020 Tonix Pharmaceuticals Holding Corp. 1 May 19, 2020 1 – 2 pm Version P0231 5 - 19 - 20 (Doc 0631) NYC Builds Bio+ Vanquishing the Virus

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2019 , as filed with the Securities and Exchange Commission (the “SEC”) on March 24 , 2020 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Potential COVID - 19 Vaccine 1 TNX - 1800 (modified horsepox virus) 2,3 • Pre - clinical and pre - IND stage • Live virus vaccine designed on our horsepox vaccine platform 4 to express the SARS - CoV - 2 Spike (S) protein • Milestones: • 4 th Quarter 2020 – Non - human primate testing results expected 5 1 COVID - 19 = Coronavirus disease 2019 2 Collaboration with Southern Research and University of Alberta 3 Experimental new biologic, not approved for any indication 4 TNX - 801 is unmodified horsepox virus, which is in development as a vaccine to protect against smallpox and monkeypox 5 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
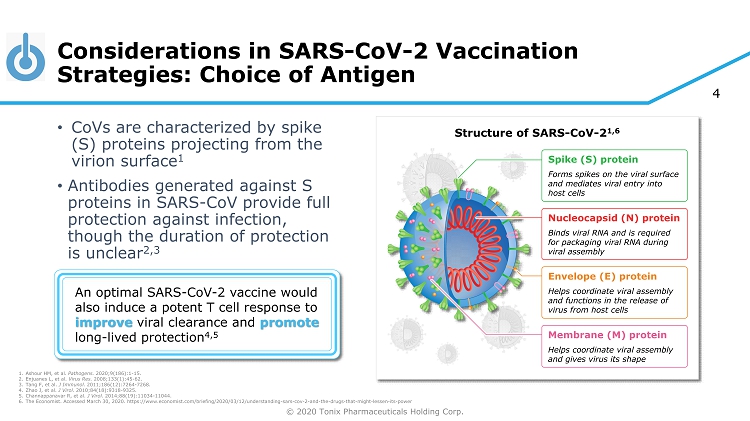
© 2020 Tonix Pharmaceuticals Holding Corp. 4 Considerations in SARS - CoV - 2 Vaccination Strategies: Choice of Antigen • CoVs are characterized by spike (S) proteins projecting from the virion surface 1 • Antibodies generated against S proteins in SARS - CoV provide full protection against infection, though the duration of protection is unclear 2,3 Structure of SARS - CoV - 2 1,6 An optimal SARS - CoV - 2 vaccine would also induce a potent T cell response to improve viral clearance and promote long - lived protection 4,5 Spike (S) protein Forms spikes on the viral surface and mediates viral entry into host cells Nucleocapsid (N) protein Binds viral RNA and is required for packaging viral RNA during viral assembly Envelope (E) protein Helps coordinate viral assembly and functions in the release of virus from host cells Membrane (M) protein Helps coordinate viral assembly and gives virus its shape 1. Ashour HM, et al. Pathogens. 2020;9(186):1 - 15. 2. Enjuanes L, et al. Virus Res. 2008;133(1):45 - 62. 3. Tang F, et al. J Immunol. 2011;186(12):7264 - 7268. 4. Zhao J, et al. J Virol. 2010;84(18):9318 - 9325. 5. Channappanavar R, et al. J Virol. 2014;88(19):11034 - 11044. 6. The Economist. Accessed March 30, 2020. https://www.economist.com/briefing/2020/03/12/understanding - sars - cov - 2 - and - the - drugs - tha t - might - lessen - its - power
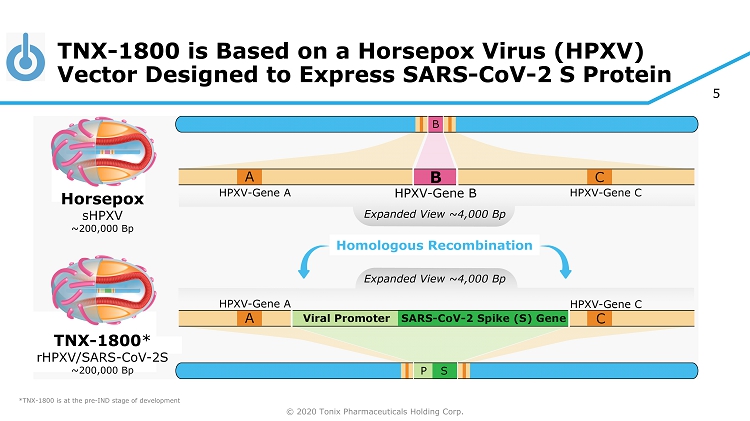
© 2020 Tonix Pharmaceuticals Holding Corp. 5 TNX - 1800 is Based on a Horsepox Virus (HPXV) Vector Designed to Express SARS - CoV - 2 S Protein *TNX - 1800 is at the pre - IND stage of development Horsepox sHPXV ~200,000 Bp Homologous Recombination TNX - 1800 * rHPXV/SARS - CoV - 2S ~200,000 Bp
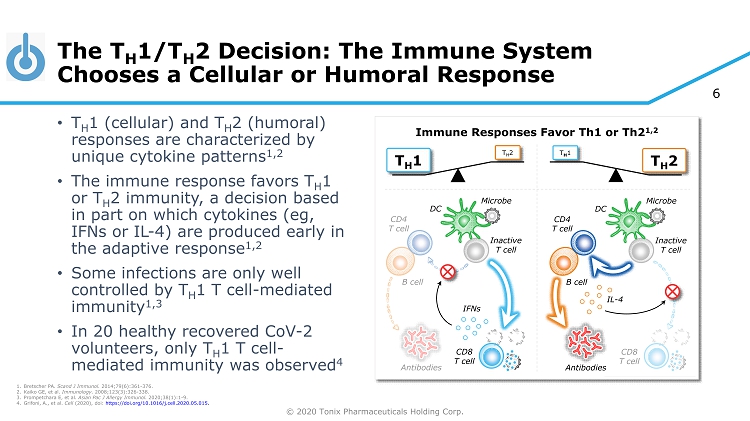
© 2020 Tonix Pharmaceuticals Holding Corp. 6 CD8 T cell Antibodies CD4 T cell B cell The T H 1/T H 2 Decision: The Immune System Chooses a Cellular or Humoral Response • T H 1 (cellular) and T H 2 (humoral) responses are characterized by unique cytokine patterns 1,2 • The immune response favors T H 1 or T H 2 immunity, a decision based in part on which cytokines (eg, IFNs or IL - 4) are produced early in the adaptive response 1,2 • Some infections are only well controlled by T H 1 T cell - mediated immunity 1,3 • In 20 healthy recovered CoV - 2 volunteers, only T H 1 T cell - mediated immunity was observed 4 Immune Responses Favor Th1 or Th2 1,2 CD8 T cell Inactive T cell DC Microbe Inactive T cell DC Microbe Antibodies CD4 T cell B cell IFNs IL - 4 1. Bretscher PA. Scand J Immunol. 2014;79(6):361 - 376. 2. Kaiko GE, et al. Immunology. 2008;123(3):326 - 338. 3. Prompetchara E, et al. Asian Pac J Allergy Immunol. 2020;38(1):1 - 9. 4. Grifoni , A., et al. Cell (2020), doi : https://doi.org/10.1016/j.cell.2020.05.015 . T H 1 T H 2 T H 2 T H 1
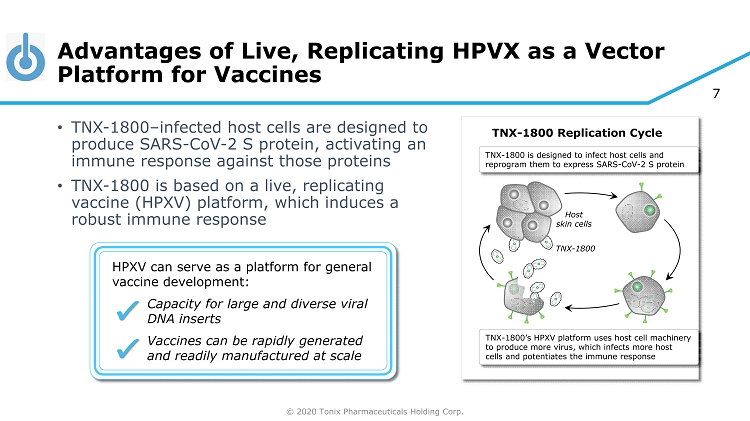
© 2020 Tonix Pharmaceuticals Holding Corp. 7 Advantages of Live, Replicating HPVX as a Vector Platform for Vaccines • TNX - 1800 – infected host cells are designed to produce SARS - CoV - 2 S protein, activating an immune response against those proteins • TNX - 1800 is based on a live, replicating vaccine (HPXV) platform, which induces a robust immune response HPXV can serve as a platform for general vaccine development: Capacity for large and diverse viral DNA inserts Vaccines can be rapidly generated and readily manufactured at scale ✔ ✔ TNX - 1800 Replication Cycle TNX - 1800 is designed to infect host cells and reprogram them to express SARS - CoV - 2 S protein TNX - 1800’s HPXV platform uses host cell machinery to produce more virus, which infects more host cells and potentiates the immune response Host skin cells TNX - 1800
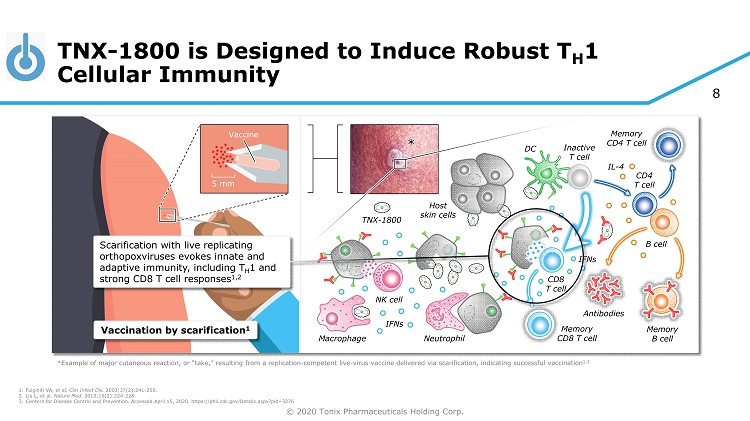
© 2020 Tonix Pharmaceuticals Holding Corp. 8 TNX - 1800 is Designed to Induce Robust T H 1 Cellular Immunity 1. Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2. Liu L, et al. Nature Med. 2010;16(2):224 - 228. 3. Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276 TNX - 1800 Host skin cells *Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine delivered via scarifi cation, indicating successful vaccination 1,3 5 mm Vaccine Vaccination by scarification 1 Inactive T cell DC Neutrophil Macrophage NK cell CD4 T cell B cell Antibodies CD8 T cell * Memory B cell Memory CD8 T cell Memory CD4 T cell IFNs IL - 4 IFNs Scarification with live replicating orthopoxviruses evokes innate and adaptive immunity, including T H 1 and strong CD8 T cell responses 1,2
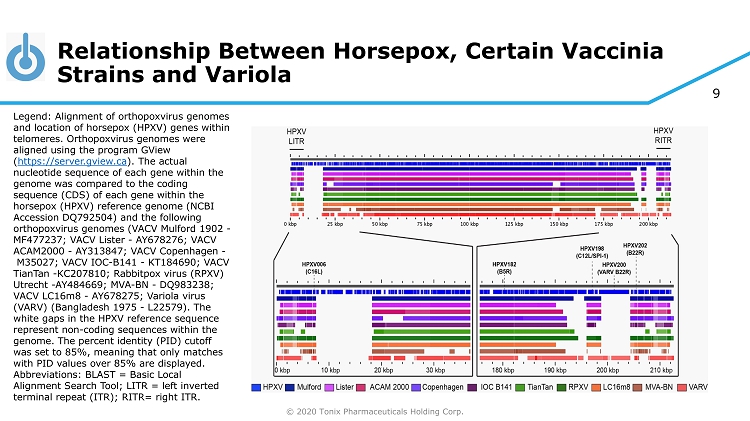
© 2020 Tonix Pharmaceuticals Holding Corp. 9 Relationship Between Horsepox, Certain Vaccinia Strains and Variola Legend: Alignment of orthopoxvirus genomes and location of horsepox (HPXV) genes within telomeres. Orthopoxvirus genomes were aligned using the program GView ( https://server.gview.ca ). The actual nucleotide sequence of each gene within the genome was compared to the coding sequence (CDS) of each gene within the horsepox (HPXV) reference genome (NCBI Accession DQ792504) and the following orthopoxvirus genomes (VACV Mulford 1902 - MF477237; VACV Lister - AY678276; VACV ACAM2000 - AY313847; VACV Copenhagen - M35027; VACV IOC - B141 - KT184690; VACV TianTan - KC207810; Rabbitpox virus (RPXV) Utrecht - AY484669; MVA - BN - DQ983238; VACV LC16m8 - AY678275 ; Variola virus (VARV) (Bangladesh 1975 - L22579). The white gaps in the HPXV reference sequence represent non - coding sequences within the genome. The percent identity (PID) cutoff was set to 85%, meaning that only matches with PID values over 85% are displayed. Abbreviations: BLAST = Basic Local Alignment Search Tool; LITR = left inverted terminal repeat (ITR); RITR= right ITR.

© 2020 Tonix Pharmaceuticals Holding Corp. 10 Development of TNX - 1800 as a COVID - 19 Vaccine Collaboration with Southern Research • Southern Research will develop and test TNX - 1800, which is designed to express Spike (S) protein from the virus that causes COVID - 19, which is called SARS - CoV - 2. • We plan to test whether vaccination of animals with TNX - 1800 will elicit an immune response to the S protein from SARS - CoV - 2 and if so, whether such an immune response will protect mice and non - human primates against a challenge with SARS - CoV - 2 virus • We expect to receive data from small animal experiments and from primates in the fourth quarter of 2020 1 Further Development • The further development of TNX - 1800 for human clinical trials will require manufacturing according to Good Manufacturing Practice, or GMP • TNX - 1810, TNX - 1820 and TNX - 1830 2 are in early development as vaccines to elicit almost pure T cell responses vaccines 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones 2 TNX - 1810, - 1820 and - 1830 are experimental new biologics, at the pre - IND and pre - clinical stage of development and are not approved for any indication

© 2020 Tonix Pharmaceuticals Holding Corp. 11 Thank you ! NASDAQ: TNXP