
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2020 Tonix Pharmaceuticals Holding Corp. 1 June 2020 Version P0233 6 - 5 - 20 (Doc 0645) Investor Presentation NASDAQ:TNXP

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2019 , as filed with the Securities and Exchange Commission (the “SEC”) on March 24 , 2020 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

© 2020 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals © 2020 Tonix Pharmaceuticals Holding Corp. Developing novel therapies for humanity • A clinical - stage biopharmaceutical company committed to discovering and developing innovative and proprietary new therapeutics that address the needs of patients • We focus on developing small molecules and biologics: • CNS (pain, neurology, psychiatry, addiction) • Immunology (vaccines, immunosuppression, oncology, autoimmune disease) 3
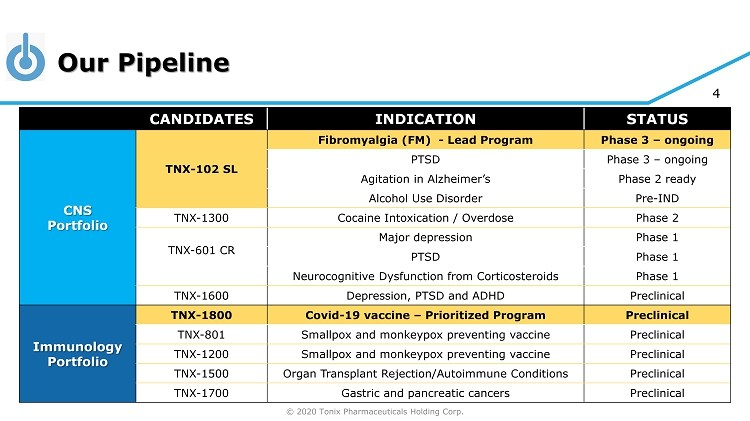
© 2020 Tonix Pharmaceuticals Holding Corp. 4 Our Pipeline CANDIDATES INDICATION STATUS CNS Portfolio TNX - 102 SL Fibromyalgia (FM) - Lead Program Phase 3 – ongoing PTSD Phase 3 – ongoing Agitation in Alzheimer’s Phase 2 ready Alcohol Use Disorder Pre - IND TNX - 1300 Cocaine Intoxication / Overdose Phase 2 TNX - 601 CR Major depression Phase 1 PTSD Phase 1 Neurocognitive Dysfunction from Corticosteroids Phase 1 TNX - 1600 Depression, PTSD and ADHD Preclinical Immunology Portfolio TNX - 1800 Covid - 19 vaccine – Prioritized Program Preclinical TNX - 801 Smallpox and monkeypox preventing vaccine Preclinical TNX - 1200 Smallpox and monkeypox preventing vaccine Preclinical TNX - 1500 Organ Transplant Rejection/Autoimmune Conditions Preclinical TNX - 1700 Gastric and pancreatic cancers Preclinical
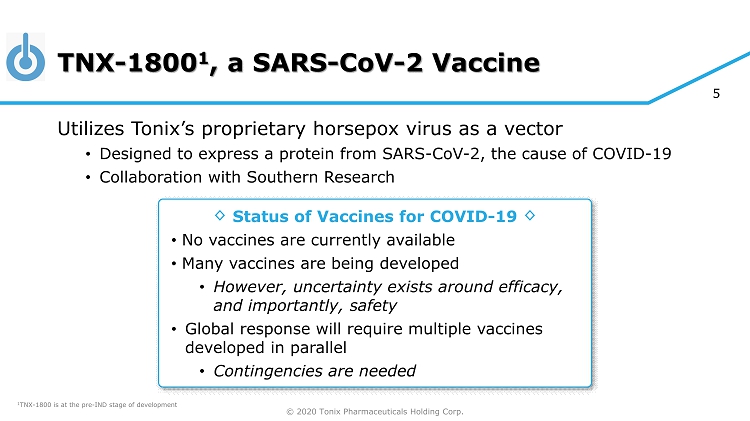
© 2020 Tonix Pharmaceuticals Holding Corp. 5 TNX - 1800 1 , a SARS - CoV - 2 Vaccine Utilizes Tonix’s proprietary horsepox virus as a vector • Designed to express a protein from SARS - CoV - 2, the cause of COVID - 19 • Collaboration with Southern Research Status of Vaccines for COVID - 19 • No vaccines are currently available • Many vaccines are being developed • However, uncertainty exists around efficacy, and importantly, safety • Global response will require multiple vaccines developed in parallel • Contingencies are needed ◊ ◊ 1 TNX - 1800 is at the pre - IND stage of development
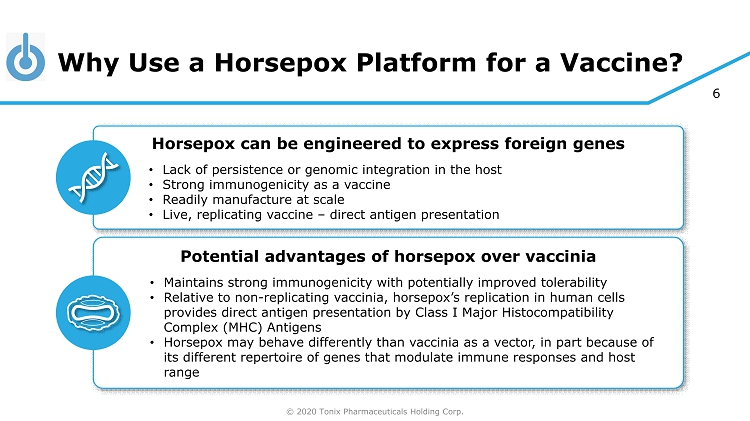
© 2020 Tonix Pharmaceuticals Holding Corp. 6 Why Use a Horsepox Platform for a Vaccine? Horsepox can be engineered to express foreign genes • Lack of persistence or genomic integration in the host • Strong immunogenicity as a vaccine • Readily manufacture at scale • Live, replicating vaccine – direct antigen presentation Potential advantages of horsepox over vaccinia • Maintains strong immunogenicity with potentially improved tolerability • Relative to non - replicating vaccinia, horsepox’s replication in human cells provides direct antigen presentation by Class I Major Histocompatibility Complex (MHC) Antigens • Horsepox may behave differently than vaccinia as a vector, in part because of its different repertoire of genes that modulate immune responses and host range
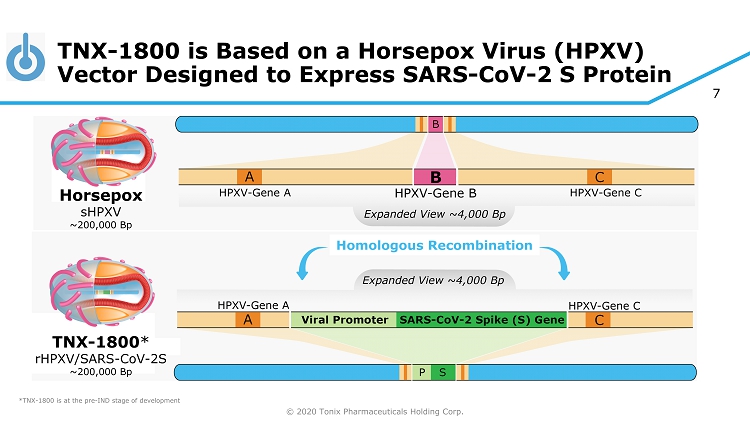
© 2020 Tonix Pharmaceuticals Holding Corp. 7 TNX - 1800 is Based on a Horsepox Virus (HPXV) Vector Designed to Express SARS - CoV - 2 S Protein *TNX - 1800 is at the pre - IND stage of development Horsepox sHPXV ~200,000 Bp TNX - 1800 * rHPXV/SARS - CoV - 2S ~200,000 Bp Homologous Recombination
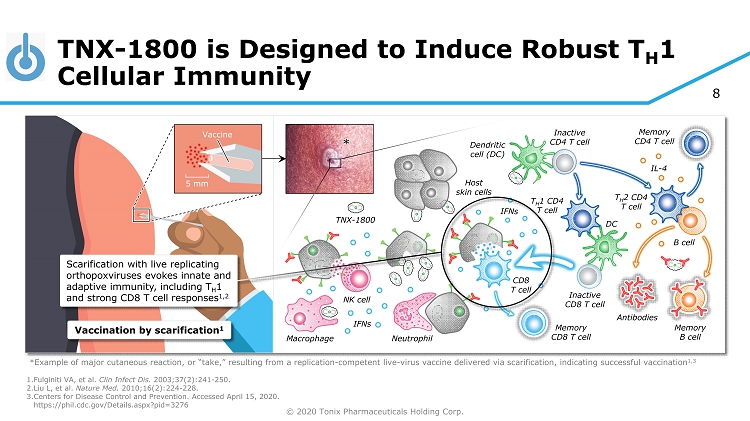
© 2020 Tonix Pharmaceuticals Holding Corp. 8 TNX - 1800 is Designed to Induce Robust T H 1 Cellular Immunity 1. Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2. Liu L, et al. Nature Med. 2010;16(2):224 - 228. 3. Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276 TNX - 1800 Host skin cells *Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine delivered via scarifi cation, indicating successful vaccination 1,3 5 mm Vaccine Vaccination by scarification 1 Inactive CD4 T cell Dendritic cell (DC) Memory B cell Memory CD8 T cell Memory CD4 T cell Neutrophil Macrophage NK cell IFNs IL - 4 T H 2 CD4 T cell B cell Antibodies * CD8 T cell Inactive CD8 T cell DC T H 1 CD4 T cell IFNs Scarification with live replicating orthopoxviruses evokes innate and adaptive immunity, including T H 1 and strong CD8 T cell responses 1,2

© 2020 Tonix Pharmaceuticals Holding Corp. 9 TNX - 1800 Development Status Southern Research will address two key questions: • Will vaccination of animals elicit an immune response to the S protein? • 4th Quarter 2020 – Small animal response expected 1 • Will immune response protect non - human primates against a challenge with SARS - CoV - 2 virus? • 4th Quarter 2020 – Primate testing results expected 1 Manufacturing development for GMP virus initiated • Clinical development will require manufacturing for clinical supplies 2 1 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
© 2020 Tonix Pharmaceuticals Holding Corp. 10 Overview of TNX - 102 SL* Protectic ® proprietary formulation of cyclobenzaprine that supports sublingual administration Scientific Rationale for Protectic ® Formulation • Engenders unique pharmacokinetic and pharmacodynamic properties that emphasize sleep properties of cyclobenzaprine while minimizing undesirable properties • Potential therapeutic value in a constellation of disorders where sleep disturbances are: • Co - morbid • Involved in the onset, progression and severity of the disease ◊ ◊ *TNX - 102 SL is in clinical stage of development and not approved for any indication
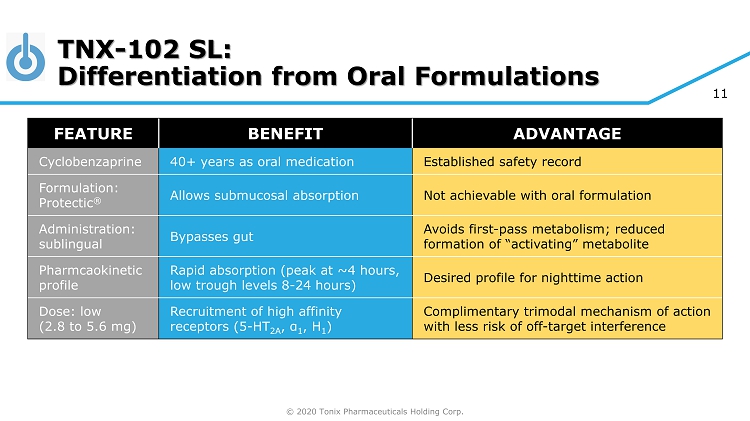
© 2020 Tonix Pharmaceuticals Holding Corp. 11 TNX - 102 SL: Differentiation from Oral Formulations FEATURE BENEFIT ADVANTAGE Cyclobenzaprine 40+ years as oral medication Established safety record Formulation: Protectic ® Allows submucosal absorption Not achievable with oral formulation Administration: sublingual Bypasses gut Avoids first - pass metabolism; reduced formation of “activating” metabolite Pharmcaokinetic profile Rapid absorption (peak at ~4 hours, low trough levels 8 - 24 hours) Desired profile for nighttime action Dose: low (2.8 to 5.6 mg) Recruitment of high affinity receptors (5 - HT 2A , α 1 , H 1 ) Complimentary trimodal mechanism of action with less risk of off - target interference
© 2020 Tonix Pharmaceuticals Holding Corp. 12 TNX - 102 SL: Results from Completed FM Trials Completed Trials in FM: • Phase 2 (F202 BESTFIT) – 205 patients randomized • Phase 3 (F301 AFFIRM) – 519 patients randomized Topline Efficacy Results: • Studies did not achieve statistical significance in the primary efficacy endpoint More In - Depth Results: • Both studies showed efficacy signals justifying continued development in FM Safety: • Well tolerated; side effects consistent with known side effects of cyclobenzaprine
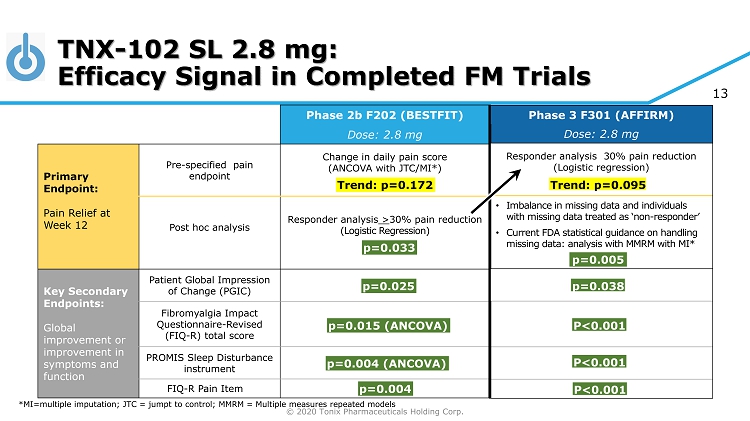
© 2020 Tonix Pharmaceuticals Holding Corp. 13 p=0.005 p=0.038 P<0.001 P<0.001 P<0.001 Phase 3 F301 (AFFIRM) Dose: 2.8 mg Responder analysis 30% pain reduction (Logistic regression) • Imbalance in missing data and individuals with missing data treated as ‘non - responder’ • Current FDA statistical guidance on handling missing data: analysis with MMRM with MI* p=0.005 p=0.038 P<0.001 P<0.001 P<0.001 Trend: p=0.095 Phase 2b F202 (BESTFIT) Dose: 2.8 mg Primary Endpoint: Pain Relief at Week 12 Pre - specified pain endpoint Change in daily pain score (ANCOVA with JTC/MI*) Post hoc analysis Responder analysis > 30% pain reduction (Logistic Regression) Key Secondary Endpoints: Global improvement or improvement in symptoms and function Patient Global Impression of Change (PGIC) Fibromyalgia Impact Questionnaire - Revised (FIQ - R) total score PROMIS Sleep Disturbance instrument FIQ - R Pain Item Trend: p=0.172 p=0.033 p=0.025 p=0.015 (ANCOVA) p=0.004 (ANCOVA) p=0.004 TNX - 102 SL 2.8 mg: Efficacy Signal in Completed FM Trials *MI=multiple imputation; JTC = jumpt to control; MMRM = Multiple measures repeated models
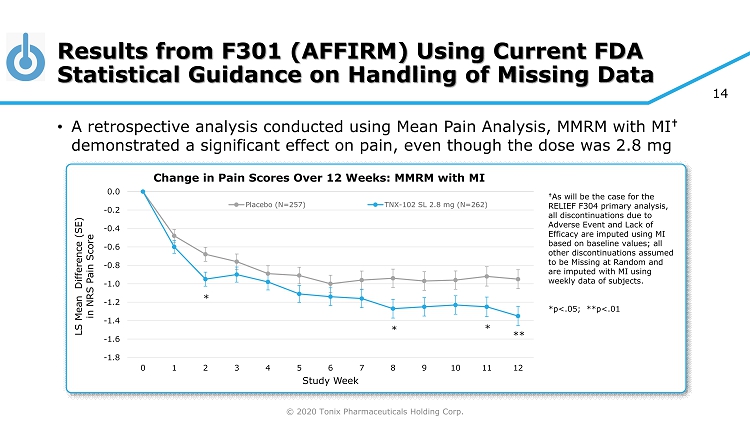
© 2020 Tonix Pharmaceuticals Holding Corp. 14 Results from F301 (AFFIRM) Using Current FDA Statistical Guidance on Handling of Missing Data • A retrospective analysis conducted using Mean Pain Analysis, MMRM with MI † demonstrated a significant effect on pain, even though the dose was 2.8 mg -1.8 -1.6 -1.4 -1.2 -1.0 -0.8 -0.6 -0.4 -0.2 0.0 0 1 2 3 4 5 6 7 8 9 10 11 12 LS Mean Difference (SE) in NRS Pain Score Study Week Change in Pain Scores Over 12 Weeks: MMRM with MI Placebo (N=257) TNX-102 SL 2.8 mg (N=262) * * * ** † As will be the case for the RELIEF F304 primary analysis, all discontinuations due to Adverse Event and Lack of Efficacy are imputed using MI based on baseline values; all other discontinuations assumed to be Missing at Random and are imputed with MI using weekly data of subjects. *p<.05; **p<.01
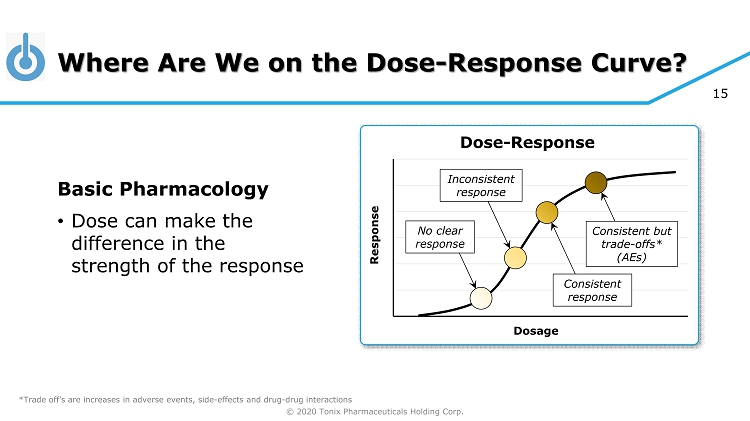
© 2020 Tonix Pharmaceuticals Holding Corp. 15 Where Are We on the Dose - Response Curve? Basic Pharmacology • Dose can make the difference in the strength of the response Response Dosage Dose - Response Inconsistent response No clear response Consistent response Consistent but trade - offs* (AEs) *Trade off’s are increases in adverse events, side - effects and drug - drug interactions
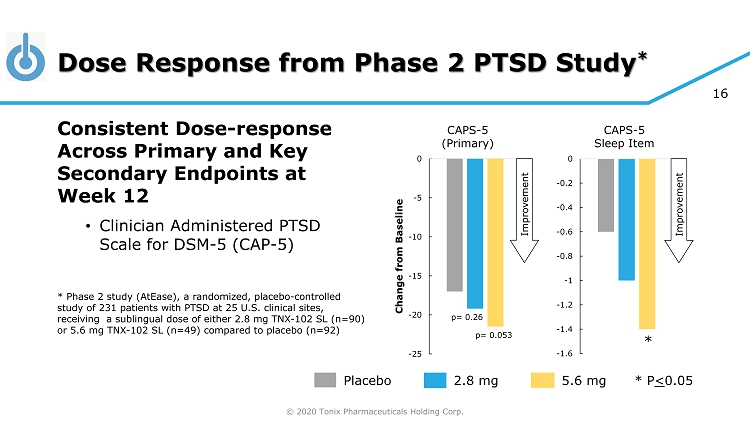
© 2020 Tonix Pharmaceuticals Holding Corp. 16 Dose Response from Phase 2 PTSD Study * Placebo 5.6 mg 2.8 mg * P < 0.05 Consistent Dose - response Across Primary and Key Secondary Endpoints at Week 12 • Clinician Administered PTSD Scale for DSM - 5 (CAP - 5) * Phase 2 study (AtEase), a randomized, placebo - controlled study of 231 patients with PTSD at 25 U.S. clinical sites, receiving a sublingual dose of either 2.8 mg TNX - 102 SL (n=90) or 5.6 mg TNX - 102 SL (n=49) compared to placebo (n=92) -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 CAPS - 5 Sleep Item -25 -20 -15 -10 -5 0 Change from Baseline CAPS - 5 (Primary) p= 0.053 * p= 0.26 Improvement Improvement
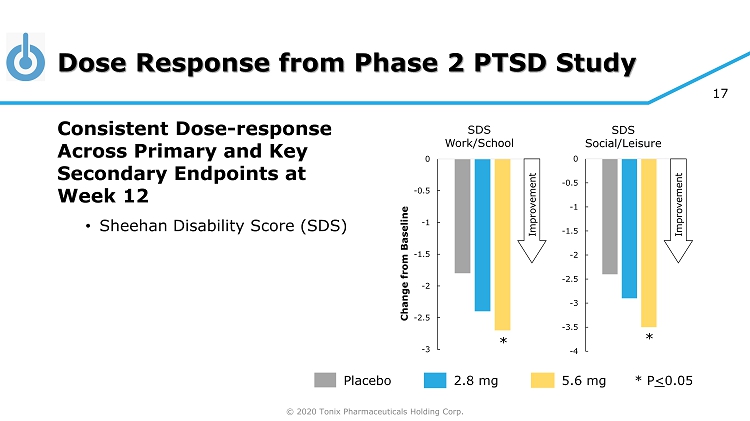
© 2020 Tonix Pharmaceuticals Holding Corp. 17 Dose Response from Phase 2 PTSD Study Placebo 5.6 mg 2.8 mg * P < 0.05 Consistent Dose - response Across Primary and Key Secondary Endpoints at Week 12 • Sheehan Disability Score (SDS) -3 -2.5 -2 -1.5 -1 -0.5 0 SDS Work/School -4 -3.5 -3 -2.5 -2 -1.5 -1 -0.5 0 SDS Social/Leisure * * Improvement Improvement Change from Baseline
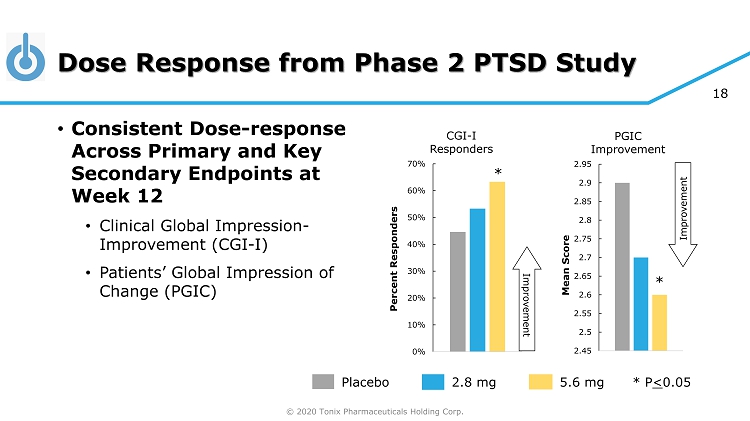
© 2020 Tonix Pharmaceuticals Holding Corp. 18 Dose Response from Phase 2 PTSD Study 0% 10% 20% 30% 40% 50% 60% 70% Percent Responders CGI - I Responders 2.45 2.5 2.55 2.6 2.65 2.7 2.75 2.8 2.85 2.9 2.95 Mean Score PGIC Improvement Placebo 5.6 mg 2.8 mg * P < 0.05 * * Improvement Improvement • Consistent Dose - response Across Primary and Key Secondary Endpoints at Week 12 • Clinical Global Impression - Improvement (CGI - I) • Patients’ Global Impression of Change (PGIC)
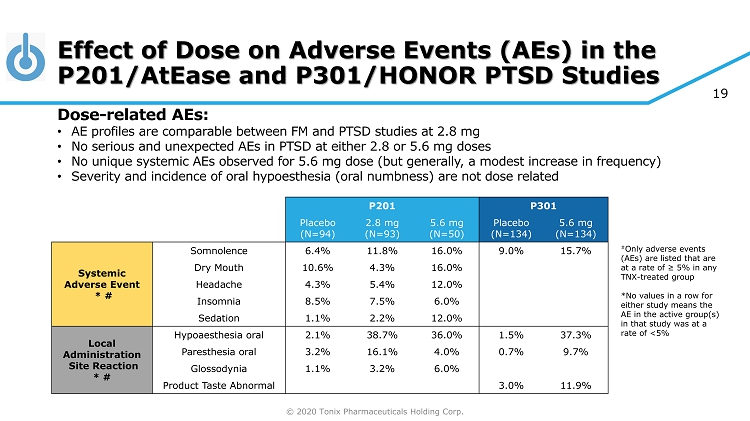
© 2020 Tonix Pharmaceuticals Holding Corp. 19 Effect of Dose on Adverse Events (AEs) in the P201/ AtEase and P301/HONOR PTSD Studies # Only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *No values in a row for either study means the AE in the active group(s) in that study was at a rate of <5% P201 P301 Placebo (N=94) 2.8 mg (N=93) 5.6 mg (N=50) Placebo (N=134) 5.6 mg (N=134) Systemic Adverse Event * # Somnolence 6.4% 11.8% 16.0% 9.0% 15.7% Dry Mouth 10.6% 4.3% 16.0% Headache 4.3% 5.4% 12.0% Insomnia 8.5% 7.5% 6.0% Sedation 1.1% 2.2% 12.0% Local Administration Site Reaction * # Hypoaesthesia oral 2.1% 38.7% 36.0% 1.5% 37.3% Paresthesia oral 3.2% 16.1% 4.0% 0.7% 9.7% Glossodynia 1.1% 3.2% 6.0% Product Taste Abnormal 3.0% 11.9% Dose - related AEs: • AE profiles are comparable between FM and PTSD studies at 2.8 mg • No serious and unexpected AEs in PTSD at either 2.8 or 5.6 mg doses • No unique systemic AEs observed for 5.6 mg dose (but generally, a modest increase in frequency) • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related

© 2020 Tonix Pharmaceuticals Holding Corp. 20 Tonix Pharmaceuticals: Lead Programs 1 © 2020 Tonix Pharmaceuticals Holding Corp. • TNX - 102 SL for fibromyalgia (FM) • Phase 3 clinical development – RELIEF study enrolling • Sublingual cyclobenzaprine tablets at higher dose of 5.6 mg • Milestones: • September 2020 – Optional interim analysis results expected 5 • 1 st Quarter 2021 - Topline data expected 5 • TNX - 1800 potential vaccine for COVID - 19 2,3 • Preclinical stage • Live virus vaccine designed on our horsepox vaccine platform 4 to express the SARS - CoV - 2 Spike (S) protein • Milestones: • 4 th Quarter 2020 – Small animal response expected 5 • 4 th Quarter 2020 – Primate testing results expected 5 1 Experimental new medicines and biologics, not approved for any indication 2 Collaboration with Southern Research 3 COVID - 19 = Coronavirus disease 2019 4 TNX - 801 is unmodified horsepox virus, which is in development as a vaccine to protect against smallpox and monkeypox 5 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones 20

© 2020 Tonix Pharmaceuticals Holding Corp. 21 TNX - 102 SL: On - going Phase 3 Study in FM (F304/RELIEF) © 2020 Tonix Pharmaceuticals Holding Corp. • Key changes to protocol from previous Phase 3 trial in FM • Exclusive use of higher dose of 5.6 mg (2 x 2.8 mg) • Primary endpoint: mean pain improvement • Analysis: MMRM with MI • Clear guidance from FDA to advance fibromyalgia program using higher dose (5.6 mg) • Long - term safety of 5.6 mg dose from PTSD studies expected to support FM NDA • Study is progressing on schedule • First patient enrolled in the new Phase 3 RELIEF study in December 2019 • Achieved 50% enrollment in April 2020 • Optional interim analysis results expected September 2020; topline results in 1Q 2021 if no delays • Potential pivotal efficacy study to support NDA approval 21
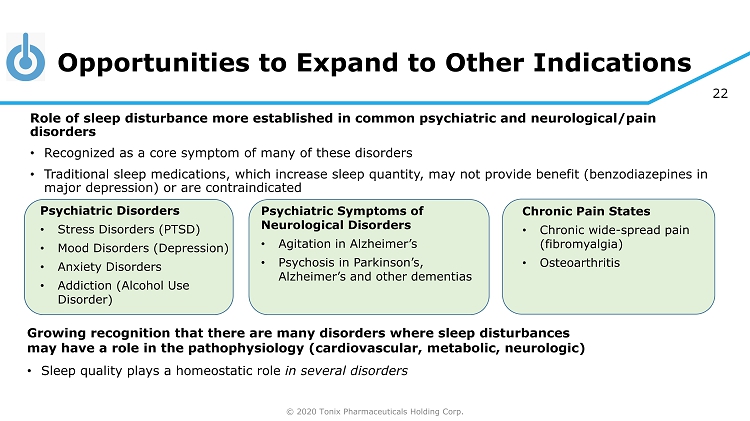
© 2020 Tonix Pharmaceuticals Holding Corp. 22 Opportunities to Expand to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Sleep quality plays a homeostatic role in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (Depression) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

© 2020 Tonix Pharmaceuticals Holding Corp. 23 TNX - 102 SL: Potential Treatment for Agitation in Alzheimer’s Disease (AAD) Agitation is one of the most distressing and debilitating of the behavioral complications of Alzheimer’s disease • Includes emotional lability, restlessness, irritability and aggression 1 Link between disturbed sleep and agitation in Alzheimer’s 1 - 3 • Agitation is commonly diurnal (e.g., “sundowning”) Prevalence • Agitation is likely to affect more than half of the 5.3 million Americans who currently suffer from moderate to severe Alzheimer’s disease; expected to nearly triple by 2050 4 Significant unmet need with no FDA approved drugs for the treatment of AAD Proposed Phase 2 study can potentially serve as a pivotal efficacy study to support NDA approval 5 1 Rose, K.et al. (2015). American Journal of Alzheimer's Disease & Other Dementias , 30 :78 2 Shih, Y. H., et al. (2017). Journal of the American Medical Directors Association , 18 , 396. 3 Canevelli, M., et al. (2016). Frontiers in medicine , 3 . 4 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/ 5 FDA comments on final protocol received October 2018

© 2020 Tonix Pharmaceuticals Holding Corp. 24 TNX - 102 SL: Potential Treatment for Alcohol Use Disorder (AUD) AUD is a chronic relapsing brain disease • Characterized by compulsive alcohol use, loss of control over alcohol intake, and a negative emotional state when not using Sleep disturbance is extremely common in alcohol recovery 1 • Significantly impacts daytime cognition, mood, and ability to participate in alcohol treatment, and is associated with increased risk of relapse Prevalence • An estimated 36 million adults in the U.S. have AUD 2 Three FDA - approved medications • Remains an unmet need due to compliance and safety issues Pre - IND meeting with the FDA completed in October 2019 • Discussed 505(b)(2) development plan for TNX - 102 SL as a treatment for AUD • FDA official meeting minutes confirmed plan to submit IND application in 2Q 2020 for a Phase 2 POC Study 3 1 Arnedt et al, J Addict Dis. 2007 ; 26(4): 41 – 54 2 Grant et al, JAMA Psychiatry 2015; 72(8): 757 - 766; www.census.gov 3 We cannot predict whether the global COVID - 19 pandemic will impact the timing of this milestone.

© 2020 Tonix Pharmaceuticals Holding Corp. 25 TNX - 1300* for the Treatment of Cocaine Intoxication Recombinant protein that degrades cocaine in the bloodstream 1 • Double - mutant cocaine esterase ( CocE ) • CocE was identified in a bacterium ( Rhodococcus ) that use cocaine as its sole source of carbon and nitrogen and that grow in soil surrounding coca plants 2 • CocE catalyzes the breakdown of cocaine into metabolites ecgonine methyl ester and benzoic acid Phase 2 study comp leted by Rickett Benckiser (TNX - 1300 was formerly RBP - 8000) 3 • Volunteer cocaine abusers received cocaine 50 mg i.v. infusion over 10 minutes • TNX - 1300 given one minute after completion of cocaine infusion • Rapidly reversed the physiologic effects of cocaine; cocaine plasma exposures dropped by 90% within two minutes • Well tolerated with the most frequently reported adverse events being gastrointestinal disorders ( including dry mouth, nausea); nervous systems disorders (including headache, dizziness) and skin and subcutaneous tissue disorders (including hyperhidrosis , dermatitis) *TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication. 1 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23. 2 Bresler MM et al, Appl Environ Microbiol . 2000. 66(3):904 - 8. 3 Nasser AF et al, J Addict Dis . 2014;33(4):289 - 302.
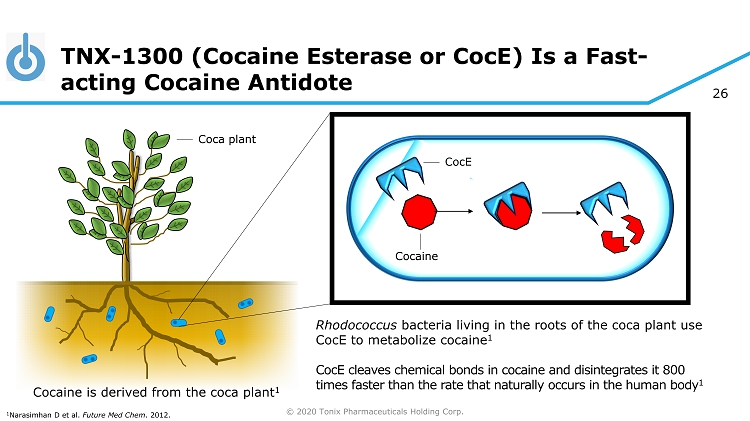
© 2020 Tonix Pharmaceuticals Holding Corp. 26 TNX - 1300 (Cocaine Esterase or CocE ) Is a Fast - acting Cocaine Antidote CocE Rhodococcus bacteria living in the roots of the coca plant use CocE to metabolize cocaine 1 CocE cleaves chemical bonds in cocaine and disintegrates it 800 times faster than the rate that naturally occurs in the human body 1 Cocaine Cocaine is derived from the coca plant 1 1 Narasimhan D et al. Future Med Chem . 2012. Coca plant
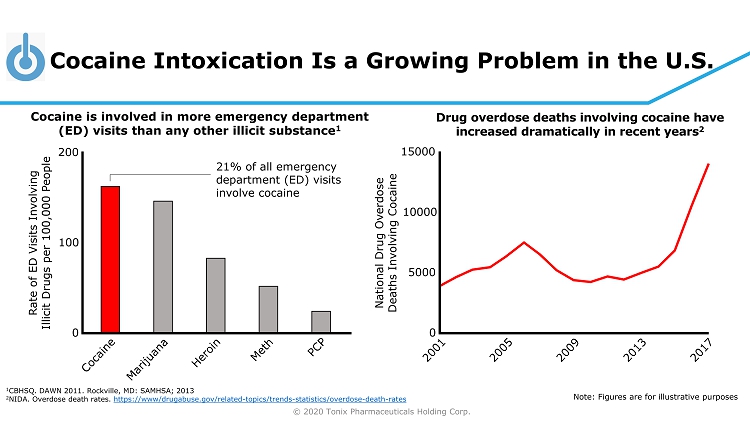
© 2020 Tonix Pharmaceuticals Holding Corp. Cocaine Intoxication Is a Growing Problem in the U.S. National Drug Overdose Deaths Involving Cocaine 0 5000 10000 15000 Cocaine is involved in more emergency department (ED) visits than any other illicit substance 1 Drug overdose deaths involving cocaine have increased dramatically in recent years 2 1 CBHSQ. DAWN 2011. Rockville, MD: SAMHSA; 2013 2 NIDA. Overdose death rates. https://www/drugabuse.gov/related - topics/trends - statistics/overdose - death - rates Note: Figures are for illustrative purposes Rate of ED Visits Involving Illicit Drugs per 100,000 People 0 100 200 21% of all emergency department (ED) visits involve cocaine
© 2020 Tonix Pharmaceuticals Holding Corp. 28 TNX - 601 CR 1 (Tianeptine Oxalate Controlled Release) Tablets Proprietary new controlled release formulation for once - daily dosing • Suitability for once - daily dosing established in Phase 1 pharmacokinetic study, completed outside of the U.S. • Well tolerated in study and side effects were consistent with the known safety profile of tianeptine sodium • Tianeptine sodium immediate release is approved and marketed outside of the U.S. for three times a day dosing for the treatment of depression • Once - daily dosing for TNX - 601 CR believed to have an adherence advantage over three times a day dosing with tianeptine sodium • Plan to request pre - IND meeting with FDA in 2020 2 • Plan for Phase 2 study in depression in 2021 2 Proprietary new oxalate salt with improved pharmaceutical properties • Tianeptine oxalate is crystalline, while tianeptine sodium is amorphous Issued patents directed to tianeptine and tianeptine oxalate • Composition of Matter: Issued US patent directed to oxalate salt, U.S. Patent No. 10,449,203 • Method of Use: Issued U.S. and European patents directed to methods of treating cognitive impairment associated with corticosteroid treatment (U.S. Patent No. 9,314,469; European Patent No. 3246031) 1 TNX - 601 CR (tianeptine oxalate controlled release tablets) is in the pre - IND stage in the U.S. and has not been approved for any indication. 2 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones.
© 2020 Tonix Pharmaceuticals Holding Corp. 29 TNX - 601 CR : A Potential Daytime Treatment for Depression and PTSD Depression: majority suffering from depression do not have an adequate response to initial antidepressant therapy • Tianeptine sodium immediate release (IR) tablets for three times a day dosing is approved as an antidepressant in the EU, Russia, Asia and Latin America; first marketed for depression in France in 1989 • Tianeptine sodium is reported to have prominent anti - anxiety effects in depression with a low incidence of sexual side effects • TNX - 601 CR leverages the established efficacy and safety of tianeptine sodium IR as a treatment for depression outside of the U.S. • Despite multiple approved products for depression in the U.S., there remains significant interest and need for new treatments, particularly for medicines that modulate the glutamatergic system PTSD: heterogeneous condition, so not all patients are expected to respond to a single medicine • Tianeptine modulates the glutamatergic system • Published studies show tianeptine is active in the treatment of PTSD 1 - 4 • Leverages Tonix expertise in PTSD (clinical and regulatory, market analysis, etc.) 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747
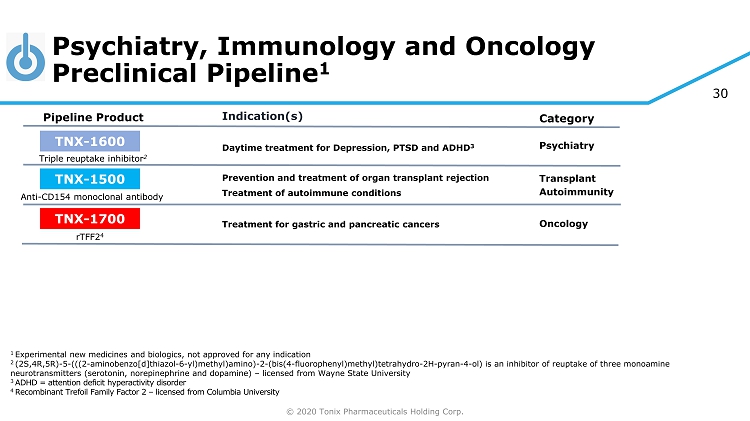
© 2020 Tonix Pharmaceuticals Holding Corp. 30 Psychiatry, Immunology and Oncology Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) – licensed from Wayne State University 3 ADHD = attention deficit hyperactivity disorder 4 R ecombinant Trefoil Family Factor 2 – licensed from Columbia University Category Pipeline Product Indication(s) Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for Depression, PTSD and ADHD 3 Psychiatry TNX - 1500 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Treatment of autoimmune conditions
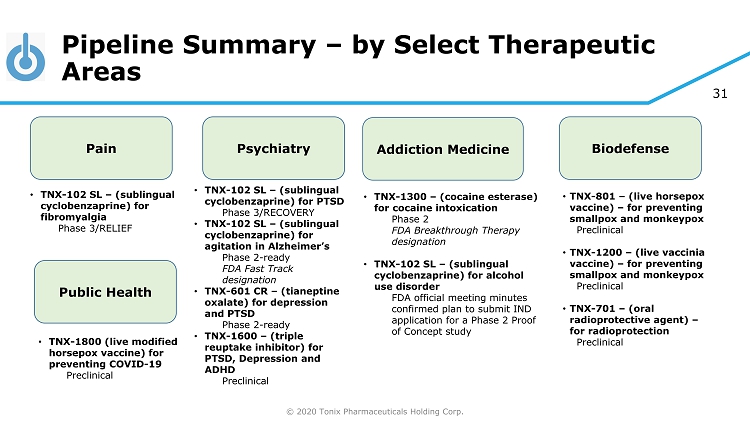
© 2020 Tonix Pharmaceuticals Holding Corp. 31 Pipeline Summary – by Select Therapeutic Areas Pain Psychiatry Addiction Medicine Biodefense • TNX - 102 SL – (sublingual cyclobenzaprine) for fibromyalgia Phase 3/RELIEF • TNX - 102 SL – (sublingual cyclobenzaprine) for PTSD Phase 3/RECOVERY • TNX - 102 SL – (sublingual cyclobenzaprine) for agitation in Alzheimer’s Phase 2 - ready FDA Fast Track designation • TNX - 601 CR – (tianeptine oxalate) for depression and PTSD Phase 2 - ready • TNX - 1600 – (triple reuptake inhibitor) for PTSD, Depression and ADHD Preclinical • TNX - 1300 – (cocaine esterase) for cocaine intoxication Phase 2 FDA Breakthrough Therapy designation • TNX - 102 SL – (sublingual cyclobenzaprine) for alcohol use disorder FDA official meeting minutes confirmed plan to submit IND application for a Phase 2 Proof of Concept study • TNX - 801 – (live horsepox vaccine) – for preventing smallpox and monkeypox Preclinical • TNX - 1200 – (live vaccinia vaccine) – for preventing smallpox and monkeypox Preclinical • TNX - 701 – (oral radioprotective agent) – for radioprotection Preclinical Public Health • TNX - 1800 (live modified horsepox vaccine) for preventing COVID - 19 Preclinical

© 2020 Tonix Pharmaceuticals Holding Corp. 32 Milestones – Recently Completed and Upcoming 1 □ 4 th Quarter 2019 Confirmed once - daily dosing for TNX - 601 CR in PK study □ 4 th Quarter 2019 Enrolled first patient in TNX - 102 SL Phase 3 F304/RELIEF study for management of fibromyalgia □ February 2020 Interim analysis results reported from TNX - 102 SL Phase 3 P302/RECOVERY study in PTSD □ 2 nd Quarter 2020 Expect to submit IND application for TNX - 102 SL to support Phase 2 POC study in AUD □ September 2020 Interim analysis results from TNX - 102 SL Phase 3 F304/RELIEF study in fibromyalgia expected □ 4 th Quarter 2020 Expect small animal data from TNX - 1800 in COVID - 19 model □ 4 th Quarter 2020 Expect primate data from TNX - 1800 in COVID - 19 model □ 1 st Quarter 2021 Topline data from TNX - 102 SL Phase 3 F304/RELIEF study in fibromyalgia expected □ 1 st Half 2021 Expect to initiate Phase 2 study of TNX - 601 CR in depression, ex - U.S. x x x 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones.

© 2020 Tonix Pharmaceuticals Holding Corp. 33 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger , CPA Chief Financial Officer

© 2020 Tonix Pharmaceuticals Holding Corp. 34 Thank You!