
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.2

© 2020 Tonix Pharmaceuticals Holding Corp. 1 August 2020 Version P0241 (Doc 0692) Investor Presentation NASDAQ:TNXP 1

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2019 , as filed with the Securities and Exchange Commission (the “SEC”) on March 24 , 2020 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
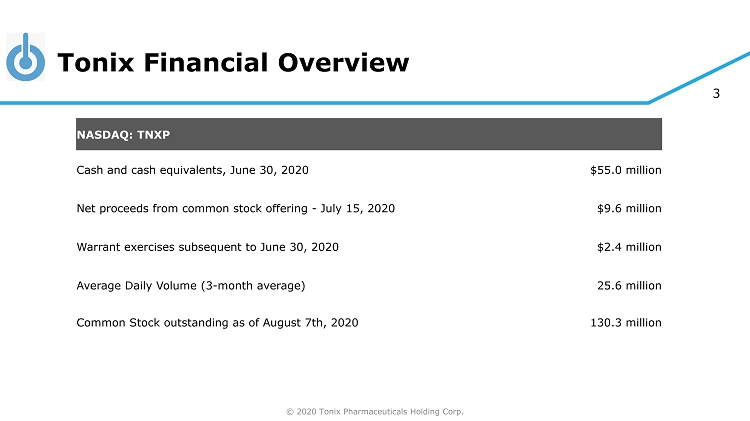
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Tonix Financial Overview NASDAQ: TNXP Cash and cash equivalents, June 30, 2020 $55.0 million Net proceeds from common stock offering - July 15, 2020 $9.6 million Warrant exercises subsequent to June 30, 2020 $2.4 million Average Daily Volume (3 - month average) 25.6 million Common Stock outstanding as of August 7th, 2020 130.3 million

© 2020 Tonix Pharmaceuticals Holding Corp. 4 Tonix Pharmaceuticals: Lead Programs 1 © 2020 Tonix Pharmaceuticals Holding Corp. • TNX - 102 SL for fibromyalgia (FM) • Phase 3 clinical development – RELIEF study enrolling • Sublingual cyclobenzaprine tablets at higher dose of 5.6 mg • Milestones: • Sept 2020 – Optional interim analysis results expected 5 • 4Q2020 – Topline data expected 5 • TNX - 1800 potential vaccine for COVID - 19 2,3 • Preclinical stage • Live virus vaccine designed elicit predominately T cell response for durable immunity - horsepox platform 4 to express the SARS - CoV - 2 Spike (S) protein • Milestones: • 4Q2020 – Small animal response expected 5 • 4Q2020 – Primate testing results expected 5 1 Experimental new medicines and biologics, not approved for any indication 2 Collaboration with Southern Research 3 COVID - 19 = Coronavirus disease 2019 4 TNX - 801 is unmodified horsepox virus, which is in development as a vaccine to protect against smallpox and monkeypox 5 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
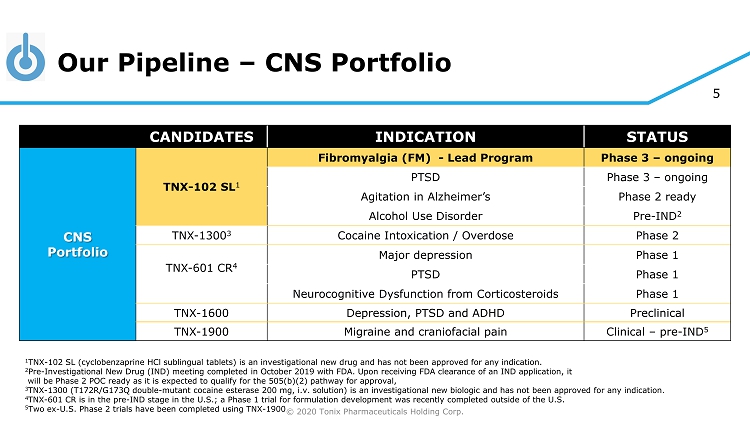
© 2020 Tonix Pharmaceuticals Holding Corp. 5 Our Pipeline – CNS Portfolio 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication. 2 Pre - Investigational New Drug (IND) meeting completed in October 2019 with FDA. Upon receiving FDA clearance of an IND applicatio n, it will be Phase 2 POC ready as it is expected to qualify for the 505(b)(2) pathway for approval, 3 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication. 4 TN X - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was recently completed outside of the U.S. 5 Two ex - U.S. Phase 2 trials have been completed using TNX - 1900 CANDIDATES INDICATION STATUS CNS Portfolio TNX - 102 SL 1 Fibromyalgia (FM) - Lead Program Phase 3 – ongoing PTSD Phase 3 – ongoing Agitation in Alzheimer’s Phase 2 ready Alcohol Use Disorder Pre - IND 2 TNX - 1300 3 Cocaine Intoxication / Overdose Phase 2 TNX - 601 CR 4 Major depression Phase 1 PTSD Phase 1 Neurocognitive Dysfunction from Corticosteroids Phase 1 TNX - 1600 Depression, PTSD and ADHD Preclinical TNX - 1900 Migraine and craniofacial pain Clinical – pre - IND 5
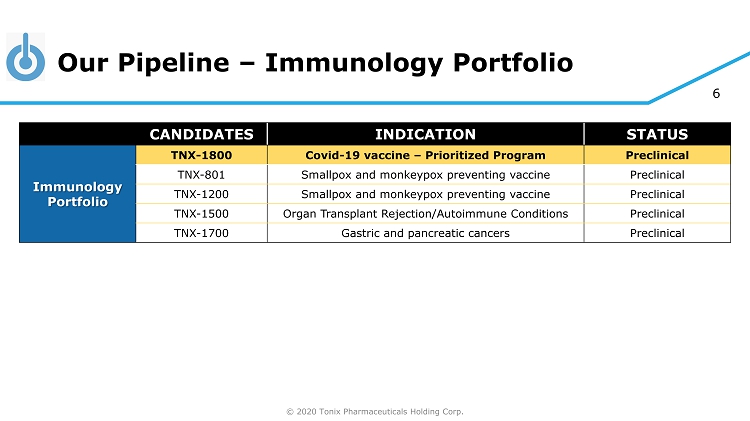
© 2020 Tonix Pharmaceuticals Holding Corp. 6 Our Pipeline – Immunology Portfolio CANDIDATES INDICATION STATUS Immunology Portfolio TNX - 1800 Covid - 19 vaccine – Prioritized Program Preclinical TNX - 801 Smallpox and monkeypox preventing vaccine Preclinical TNX - 1200 Smallpox and monkeypox preventing vaccine Preclinical TNX - 1500 Organ Transplant Rejection/Autoimmune Conditions Preclinical TNX - 1700 Gastric and pancreatic cancers Preclinical
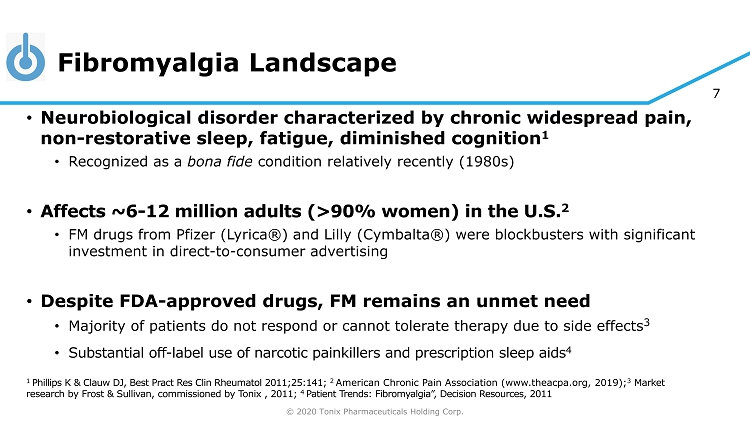
© 2020 Tonix Pharmaceuticals Holding Corp. 7 Fibromyalgia Landscape • Neurobiological disorder characterized by chronic widespread pain, non - restorative sleep, fatigue, diminished cognition 1 • Recognized as a bona fide condition relatively recently (1980s) • Affects ~6 - 12 million adults (>90% women) in the U.S. 2 • FM drugs from Pfizer (Lyrica®) and Lilly (Cymbalta®) were blockbusters with significant investment in direct - to - consumer advertising • Despite FDA - approved drugs, FM remains an unmet need • Majority of patients do not respond or cannot tolerate therapy due to side effects 3 • Substantial off - label use of narcotic painkillers and prescription sleep aids 4 1 Phillips K & Clauw DJ, Best Pract Res Clin Rheumatol 2011;25:141; 2 American Chronic Pain Association (www.theacpa.org, 2019); 3 Market research by Frost & Sullivan, commissioned by Tonix , 2011; 4 Patient Trends: Fibromyalgia”, Decision Resources, 2011
© 2020 Tonix Pharmaceuticals Holding Corp. 8 Fibromyalgia Program Goals • TNX - 102 SL is a non - opioid, centrally acting analgesic • Potential to provide a new therapeutic option for the management of fibromyalgia • Treatment objective: restore functionality and quality of life • Potential to broadly improve symptoms with acceptable tolerability • Tonix’s proprietary sublingual formulation allows for dosing convenience (one pill taken daily at bedtime) • Designed to emphasize sleep properties of TNX - 102 SL while decreasing undesirable day time side effects
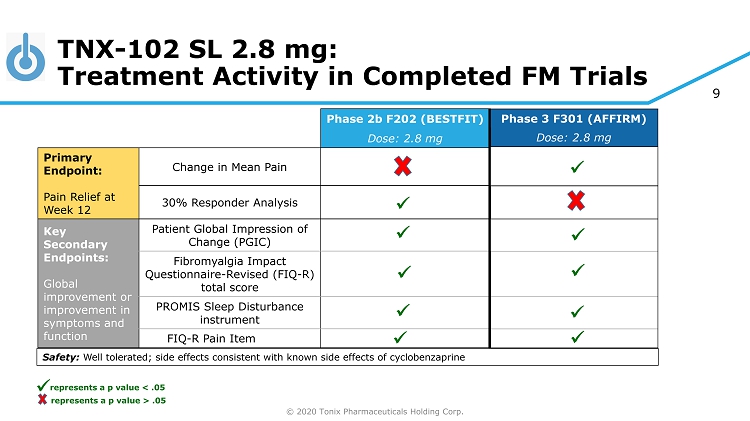
© 2020 Tonix Pharmaceuticals Holding Corp. 9 Phase 3 F301 (AFFIRM) Dose: 2.8 mg Phase 2b F202 (BESTFIT) Dose: 2.8 mg Primary Endpoint: Pain Relief at Week 12 Change in Mean Pain 30% Responder Analysis Key Secondary Endpoints: Global improvement or improvement in symptoms and function Patient Global Impression of Change (PGIC) Fibromyalgia Impact Questionnaire - Revised (FIQ - R) total score PROMIS Sleep Disturbance instrument FIQ - R Pain Item TNX - 102 SL 2.8 mg: Treatment Activity in Completed FM Trials x Safety: Well tolerated; side effects consistent with known side effects of cyclobenzaprine x x x x x x x x x represents a p value > .05 x represents a p value < .05
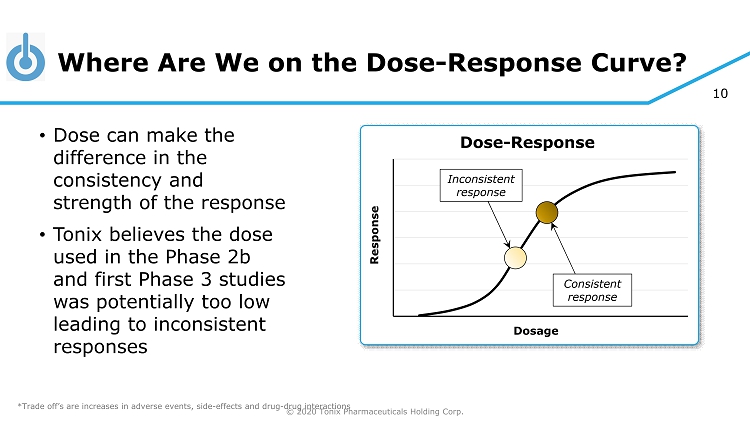
© 2020 Tonix Pharmaceuticals Holding Corp. 10 Where Are We on the Dose - Response Curve? • Dose can make the difference in the consistency and strength of the response • Tonix believes the dose used in the Phase 2b and first Phase 3 studies was potentially too low leading to inconsistent responses Response Dosage Dose - Response Inconsistent response Consistent response *Trade off’s are increases in adverse events, side - effects and drug - drug interactions
© 2020 Tonix Pharmaceuticals Holding Corp. 11 TNX - 102 SL: On - going Phase 3 Study in FM (F304/RELIEF ) © 2020 Tonix Pharmaceuticals Holding Corp. • Key changes to protocol from previous Phase 3 trial in FM • Exclusive use of higher dose of 5.6 mg (2 x 2.8 mg) • Primary endpoint: mean pain improvement (vs. responder analysis) • Potential pivotal efficacy study to support NDA approval • Long - term safety of 5.6 mg dose from PTSD studies expected to support FM NDA • Study is progressing ahead of schedule • Achieved 50% enrollment in April 2020
© 2020 Tonix Pharmaceuticals Holding Corp. 12 Fibromyalgia Next Steps • Upcoming milestones (before year end) • Phase 3 interim analysis results expected September 2020 • Topline Phase 3 results expected 4Q20 • TNX - 102 SL is a new, differentiated product candidate • Sleep quality mechanism of action • Established but unsatisfied patient population • 6 - 12 M U.S. patients • Widespread off - label opiate use
© 2020 Tonix Pharmaceuticals Holding Corp. 13 Opportunities to Expand to Other Indications Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (Depression) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

© 2020 Tonix Pharmaceuticals Holding Corp. 14 COVID - 19 Vaccine Landscape • We expect more than one vaccine will be approved by FDA • Different vaccines for different individuals • More than 125 vaccines in development • Diversity of approaches is important since protective immunity is not yet understood • Only ~4 live replicating vector systems in development • Tonix (horsepox), Merck (measles 1 - and VSV 2 - based), Zydus Cadila (measles - based) 1 Measles - based vaccine, acquisition of Themis, collaboration with Institute Pasteur 2 VSV = vesicular stomatitis virus; collaboration with IAVI = International AIDS Vaccine Initiative

© 2020 Tonix Pharmaceuticals Holding Corp. 15 Where Do We Go From Here? • Goal: Effective COVID - 19 vaccines • So we can return to work and school • Need #1 Quickly available vaccines • Even if they offer only temporary immunity -- several now in human trials • Need #2 Vaccines providing long - term immunity • Durable immunity for years • Blocking of forward transmission • Expect longer development and testing timelines
© 2020 Tonix Pharmaceuticals Holding Corp. 16 Live, Replicating Virus Vaccines for Other Infectious Diseases 1 • Long term, durable immunity • Stimulate T cells and provide years to decades of protection • Single administration, scalable manufacturing • Low dose is amplified by replication, mRNA and protein synthesis at vaccination site • Block forward transmission (infectivity) • Key to conferring herd immunity and protecting immunocompromised 1 For example, the eradication of smallpox, containment of measles, mumps, and rubella
© 2020 Tonix Pharmaceuticals Holding Corp. 17 TNX 1800: COVID - 19 Vaccine Engineered for Long - term Immunity • Based on viral vaccine developed more than 200 years ago by Edward Jenner for a smallpox vaccine • Eradicated smallpox • T cell eliciting immunity • Single dose immunity without adjuvants • Manufacturable in scale on existing systems • Glass - sparing packaging owing to small unit dose • One of 4 known live replicating virus vaccine vectors under development for COVID - 19 • Tonix, Merck, Zydus Cadila • Expected to provide T H 1 immunity
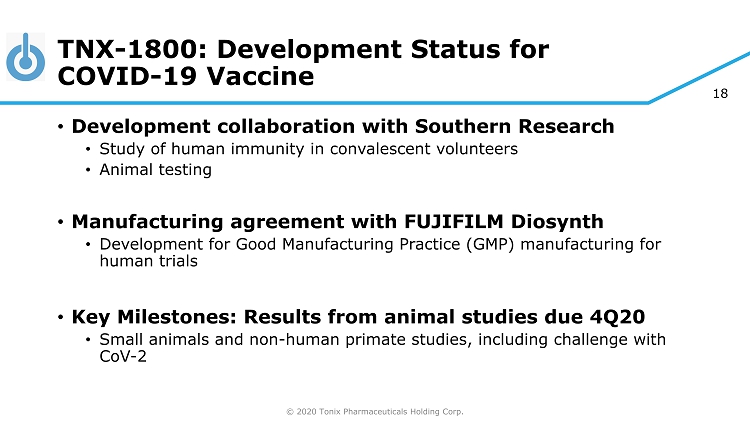
© 2020 Tonix Pharmaceuticals Holding Corp. 18 TNX - 1800: Development Status for COVID - 19 Vaccine • Development collaboration with Southern Research • Study of human immunity in convalescent volunteers • Animal testing • Manufacturing agreement with FUJIFILM Diosynth • Development for Good Manufacturing Practice (GMP) manufacturing for human trials • Key Milestones: Results from animal studies due 4Q20 • Small animals and non - human primate studies, including challenge with CoV - 2
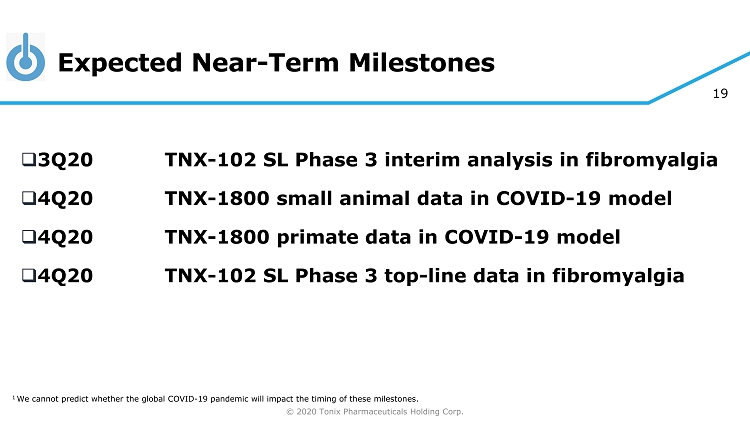
© 2020 Tonix Pharmaceuticals Holding Corp. 19 Expected Near - Term Milestones □ 3Q20 TNX - 102 SL Phase 3 interim analysis in fibromyalgia □ 4Q20 TNX - 1800 small animal data in COVID - 19 model □ 4Q20 TNX - 1800 primate data in COVID - 19 model □ 4Q20 TNX - 102 SL Phase 3 top - line data in fibromyalgia 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones.

© 2020 Tonix Pharmaceuticals Holding Corp. 20 Thank You!