
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.3

© 2020 Tonix Pharmaceuticals Holding Corp. 1 August 2020 NASDAQ: TNXP Version P0240 (Doc 0691) COVID - 19 Vaccines: Challenges and Opportunities

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2019 , as filed with the Securities and Exchange Commission (the “SEC”) on March 24 , 2020 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Seth Lederman, MD Currently, CEO of Tonix Pharmaceuticals, Dr. Lederman began his career in research in the early days of the AIDS crisis and studied the entry of HIV into cells at the molecular and cell biological levels. Dr. Lederman is a co - inventor of the horsepox vaccine vector on which Tonix’s current COVID - 19 vaccine, TNX - 1800 is based. Previously, Dr. Lederman served as an Associate Professor at Columbia University from 1996 until 2017. He joined the faculty of Columbia University's College of Physicians and Surgeons in 1985, became Assistant Professor of Medicine in 1988, and Associate Professor with tenure in 1996 and Director of the Laboratory of Molecular Immunology in 1997. From 1988 to 2002, Dr. Lederman directed basic science research at Columbia in molecular immunology, infectious diseases and the development of therapeutics for autoimmune diseases. Dr. Lederman is author of numerous scientific articles, and inventor of technologies recognized by a number of issued patents. His fundamental work on the CD40 - Ligand (CD154) elucidated the molecular basis of T cell helper function and has led to the development of therapeutic candidates for autoimmune diseases and organ transplant rejection in collaboration with Biogen and UCB. In addition to his research, Dr. Lederman served as attending physician in the Edward Daniels Arthritis and Autoimmunity Clinic on the Medical Service at Columbia Presbyterian Hospital from 1988 - 1996. Dr. Lederman earned an AB from Princeton in Chemistry cum laude in 1979 and an MD from Columbia University's College of Physicians and Surgeons in 1983. Dr. Lederman trained in internal medicine and rheumatology at Columbia's Presbyterian Hospital. He was an NIH Physician - Scientist 1985 - 1990 at Columbia.
© 2020 Tonix Pharmaceuticals Holding Corp. 4 What Is the Goal of Vaccination? • Vaccination instructs the immune system how to respond rapidly upon reinfection 1 - 3 • A typical infection is a race between the virus and the immune response • Vaccination gives the body a “head start” • Most vaccines against viruses protect against serious illness , not infection 2,3 • Different vaccines work in different ways • How an effective vaccine protects against disease depends on the nature of the virus 2,3 • Blocking forward transmission (spread of infection) is essential for public health 4 1. Centers for Disease Control and Prevention. Accessed June 9, 2020. https://www.cdc.gov/vaccines/pubs/pinkbook/prinvac.html 2. Plotkin SA. Vaccines. 2008;47(3):401 - 409. 3. Plotkin SA. Clin Vaccine Immunol. 2010;17(7):1055 - 1065. 4. Hardt K, et al. Vaccine. 2016;34(52):6691 - 6699.
© 2020 Tonix Pharmaceuticals Holding Corp. 5 Immune Memory • Recognizes the offending pathogen or toxin • T cells recognize fragments of pathogens on the surfaces of infected cells • Antibodies recognize the pathogens directly • Recalls the type of immune response elicited • For better: Protective responses are repeated on re - exposure • For worse: Non - productive responses are repeated ( e.g., allergic responses become established patterns ) • Vaccines are designed to teach the body both recognition and a protective type of immune response
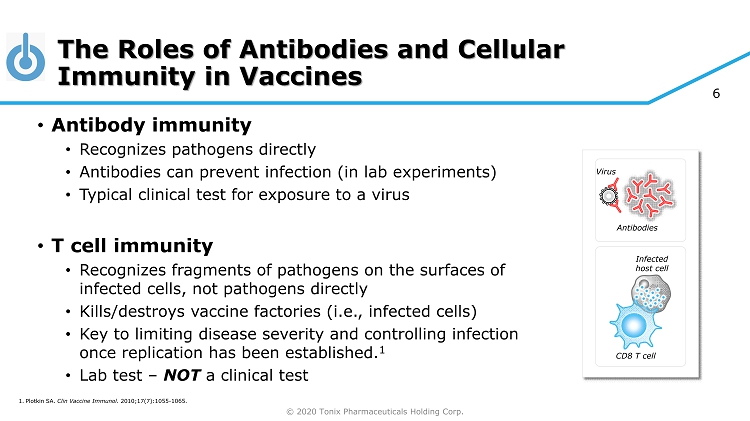
© 2020 Tonix Pharmaceuticals Holding Corp. 6 The Roles of Antibodies and Cellular Immunity in Vaccines • Antibody immunity • Recognizes pathogens directly • Antibodies can prevent infection (in lab experiments) • Typical clinical test for exposure to a virus • T cell immunity • Recognizes fragments of pathogens on the surfaces of infected cells, not pathogens directly • Kills/destroys vaccine factories (i.e., infected cells) • Key to limiting disease severity and controlling infection once replication has been established. 1 • Lab test – NOT a clinical test Antibodies CD8 T cell Infected host cell 1. Plotkin SA. Clin Vaccine Immunol. 2010;17(7):1055 - 1065. Virus
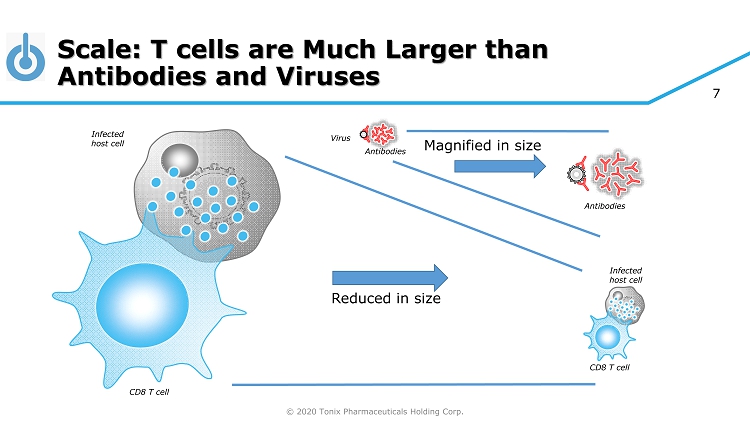
© 2020 Tonix Pharmaceuticals Holding Corp. 7 Scale: T cells are Much Larger than Antibodies and Viruses Antibodies CD8 T cell Infected host cell CD8 T cell Reduced in size Magnified in size Antibodies Infected host cell Virus
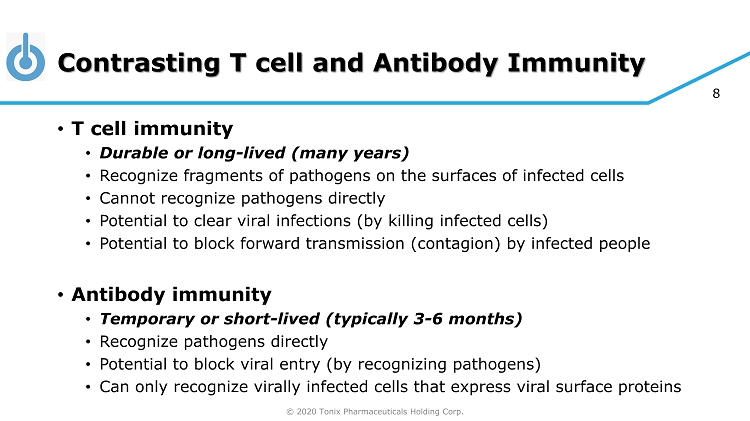
© 2020 Tonix Pharmaceuticals Holding Corp. 8 Contrasting T cell and Antibody Immunity • T cell immunity • Durable or long - lived (many years) • Recognize fragments of pathogens on the surfaces of infected cells • Cannot recognize pathogens directly • Potential to clear viral infections (by killing infected cells) • Potential to block forward transmission (contagion) by infected people • Antibody immunity • Temporary or short - lived (typically 3 - 6 months) • Recognize pathogens directly • Potential to block viral entry (by recognizing pathogens) • Can only recognize virally infected cells that express viral surface proteins
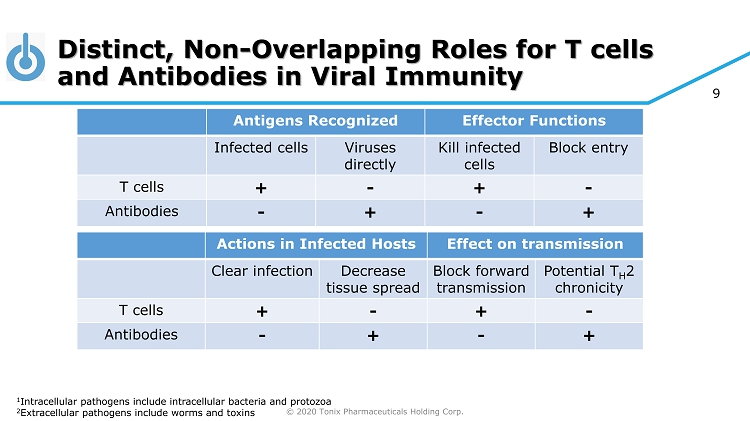
© 2020 Tonix Pharmaceuticals Holding Corp. 9 Distinct, Non - Overlapping Roles for T cells and Antibodies in Viral Immunity Antigens Recognized Effector Functions Infected cells Viruses directly Kill infected cells Block entry T cells + - + - Antibodies - + - + 1 Intracellular pathogens include intracellular bacteria and protozoa 2 Extracellular pathogens include worms and toxins Actions in Infected Hosts Effect on transmission Clear infection Decrease tissue spread Block forward transmission Potential T H 2 chronicity T cells + - + - Antibodies - + - +
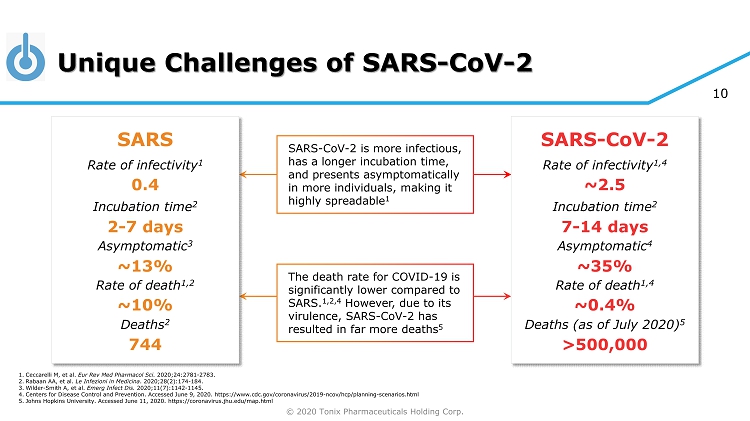
© 2020 Tonix Pharmaceuticals Holding Corp. 10 Unique Challenges of SARS - CoV - 2 SARS - CoV - 2 is more infectious, has a longer incubation time, and presents asymptomatically in more individuals, making it highly spreadable 1 The death rate for COVID - 19 is significantly lower compared to SARS. 1,2,4 However, due to its virulence, SARS - CoV - 2 has resulted in far more deaths 5 SARS Rate of infectivity 1 Incubation time 2 Asymptomatic 3 Rate of death 1,2 Deaths 2 0.4 2 - 7 days ~13% ~10% SARS - CoV - 2 Rate of infectivity 1,4 Incubation time 2 Asymptomatic 4 Rate of death 1,4 Deaths (as of July 2020) 5 ~2.5 7 - 14 days ~35% ~0.4% 1. Ceccarelli M, et al. Eur Rev Med Pharmacol Sci. 2020;24:2781 - 2783. 2. Rabaan AA, et al. Le Infezioni in Medicina . 2020;28(2):174 - 184. 3. Wilder - Smith A, et al. Emerg Infect Dis. 2020;11(7):1142 - 1145. 4. Centers for Disease Control and Prevention. Accessed June 9, 2020. https://www.cdc.gov/coronavirus/2019 - ncov/hcp/planning - scenar ios.html 5. Johns Hopkins University. Accessed June 11, 2020. https://coronavirus.jhu.edu/map.html >500,000 744

© 2020 Tonix Pharmaceuticals Holding Corp. 11 COVID - 19: Reason for Optimism? • Most people recover, which suggests: • Vaccines can be designed that safely mimic infection • Herd immunity can be achieved by vaccination • Vaccine developers can learn from individuals who recover • Study their immunity in detail for a blueprint of a successful immune response • Potential to block forward transmission (contagion) by infected people • Comparison to HIV: protective immunity is unknown • No vaccine developed after 35 years
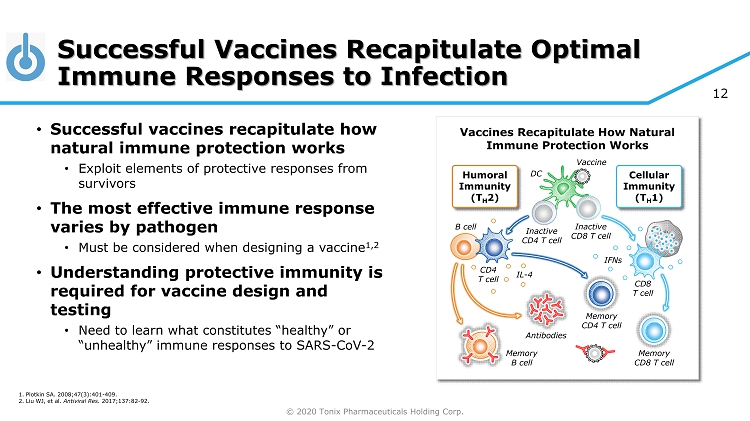
© 2020 Tonix Pharmaceuticals Holding Corp. 12 Humoral Immunity (T H 2) Inactive CD8 T cell DC Memory CD4 T cell Memory CD8 T cell CD8 T cell Cellular Immunity (T H 1) Memory B cell Antibodies CD4 T cell B cell IFNs IL - 4 Vaccine Inactive CD4 T cell Successful Vaccines Recapitulate Optimal Immune Responses to Infection • Successful vaccines recapitulate how natural immune protection works • Exploit elements of protective responses from survivors • The most effective immune response varies by pathogen • Must be considered when designing a vaccine 1,2 • Understanding protective immunity is required for vaccine design and testing • Need to learn what constitutes “healthy” or “unhealthy” immune responses to SARS - CoV - 2 Vaccines Recapitulate How Natural Immune Protection Works 1. Plotkin SA. 2008;47(3):401 - 409. 2. Liu WJ, et al. Antiviral Res. 2017;137:82 - 92.
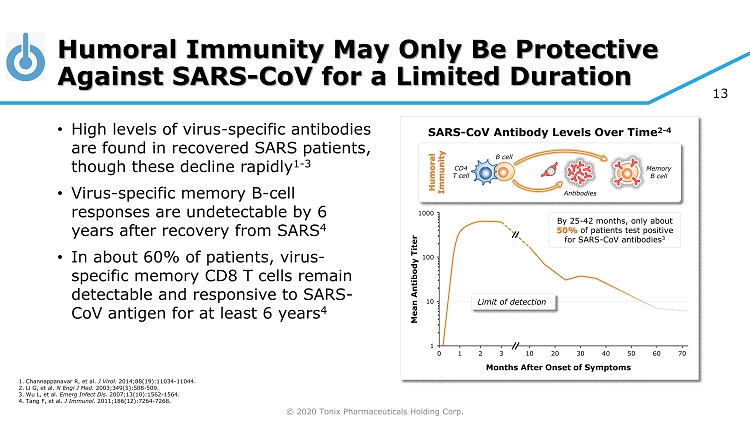
© 2020 Tonix Pharmaceuticals Holding Corp. 13 Humoral Immunity May Only Be Protective Against SARS - CoV for a Limited Duration • High levels of virus - specific antibodies are found in recovered SARS patients, though these decline rapidly 1 - 3 • Virus - specific memory B - cell responses are undetectable by 6 years after recovery from SARS 4 • In about 60% of patients, virus - specific memory CD8 T cells remain detectable and responsive to SARS - CoV antigen for at least 6 years 4 SARS - CoV Antibody Levels Over Time 2 - 4 Months After Onset of Symptoms By 25 - 42 months, only about 50% of patients test positive for SARS - CoV antibodies 3 Limit of detection 0 1 2 3 10 20 30 70 60 50 40 Mean Antibody Titer 1 10 100 1000 1. Channappanavar R, et al. J Virol. 2014;88(19):11034 - 11044. 2. Li G, et al. N Engl J Med. 2003;349(5):508 - 509. 3. Wu L, et al. Emerg Infect Dis. 2007;13(10):1562 - 1564. 4. Tang F, et al. J Immunol. 2011;186(12):7264 - 7268. Memory B cell CD4 T cell B cell Antibodies Humoral Immunity
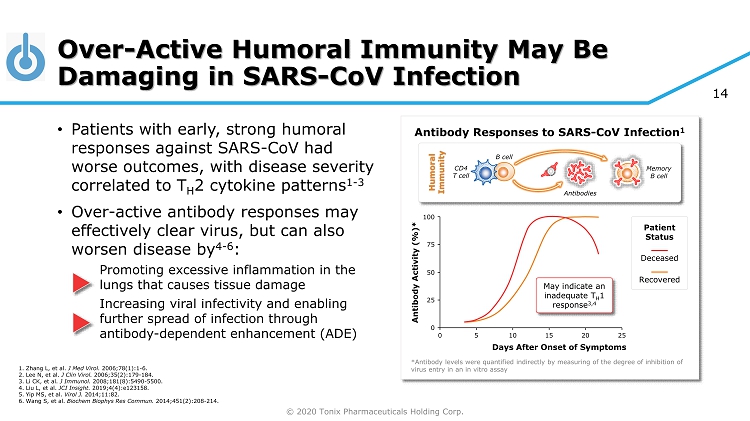
© 2020 Tonix Pharmaceuticals Holding Corp. 14 100 Over - Active Humoral Immunity May Be Damaging in SARS - CoV Infection • Patients with early, strong humoral responses against SARS - CoV had worse outcomes, with disease severity correlated to T H 2 cytokine patterns 1 - 3 • Over - active antibody responses may effectively clear virus, but can also worsen disease by 4 - 6 : Promoting excessive inflammation in the lungs that causes tissue damage Increasing viral infectivity and enabling further spread of infection through antibody - dependent enhancement (ADE) Antibody Responses to SARS - CoV Infection 1 Days After Onset of Symptoms 0 5 10 15 20 25 0 *Antibody levels were quantified indirectly by measuring of the degree of inhibition of virus entry in an in vitro assay Patient Status Deceased Recovered 75 50 25 Antibody Activity (%)* May indicate an inadequate T H 1 response 3,4 1. Zhang L, et al. J Med Virol. 2006;78(1):1 - 6. 2. Lee N, et al. J Clin Virol. 2006;35(2):179 - 184. 3. Li CK, et al. J Immunol. 2008;181(8):5490 - 5500. 4. Liu L, et al. JCI Insight. 2019;4(4):e123158. 5. Yip MS, et al. Virol J. 2014;11:82. 6. Wang S, et al. Biochem Biophys Res Commun. 2014;451(2):208 - 214. Memory B cell CD4 T cell B cell Antibodies Humoral Immunity
© 2020 Tonix Pharmaceuticals Holding Corp. 15 Live, Replicating Virus Vaccines for Other Infectious Diseases 1 • Long term, durable immunity • Stimulate T cells and provide years to decades of protection • Single administration, scalable manufacturing • Low dose is amplified by replication, mRNA and protein synthesis at vaccination site • Block forward transmission (infectivity) • Key to conferring herd immunity and protecting immunocompromised 1 For example, the eradication of smallpox, containment of measles, mumps, and rubella
© 2020 Tonix Pharmaceuticals Holding Corp. 16 Live Replicating Vaccines Induce T Helper 1 (T H 1) Polarization of Immunity Initial exposure to a vaccine or pathogen pushes immunity towards one of two mutually exclusive responses 1 • T H 1 is T cell predominant – “cell mediated immunity” • Effective against viruses, intracellular bacteria and protozoa • T H 2 is antibody predominant – “humoral immunity” • Effective against bacteria and other extracellular parasites including worms 1 Note: The CoV - 2 antibody test is expected to be “positive” in either T H 1 or T H 2 responses
© 2020 Tonix Pharmaceuticals Holding Corp. 17 T H 1 Polarization is Typically Effective Against Viruses FDA’s guidance on COVID - 19 vaccines recognizes T H 1 responses 1 • T H 1 inducing vaccines may go to First - in - Human studies without certain animal testing An inappropriate T H 2 immune response to a virus can lead to bad outcomes • Serious disease • Chronic, persistent infection and inflammation 1 FDA guidance for Industry “Development and Licensure of Vaccines to Prevent COVID - 19” – July 2020 - Page 8, Section D. – 4 th bullet point: “COVID - 19 vaccine candidates with immunogenicity data demonstrating high neutralizing antibody titers and Th1 - type T cell polarization may be allowed to proceed to [First in human] FIH trials without first completing postvaccination challenge st udies in appropriate animal models, provided adequate risk mitigation strategies are put in place in the FIH trials” https://www.fda.gov/media/139638/download .
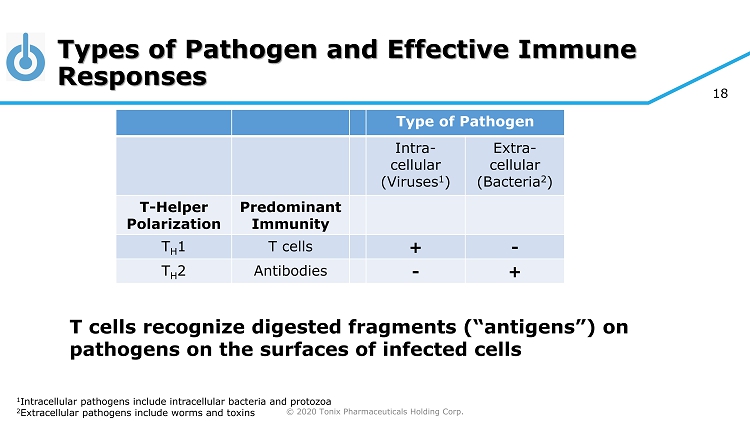
© 2020 Tonix Pharmaceuticals Holding Corp. 18 Types of Pathogen and Effective Immune Responses Type of Pathogen Intra - cellular (Viruses 1 ) Extra - cellular (Bacteria 2 ) T - Helper Polarization Predominant Immunity T H 1 T cells + - T H 2 Antibodies - + 1 Intracellular pathogens include intracellular bacteria and protozoa 2 Extracellular pathogens include worms and toxins T cells recognize digested fragments (“antigens”) on pathogens on the surfaces of infected cells
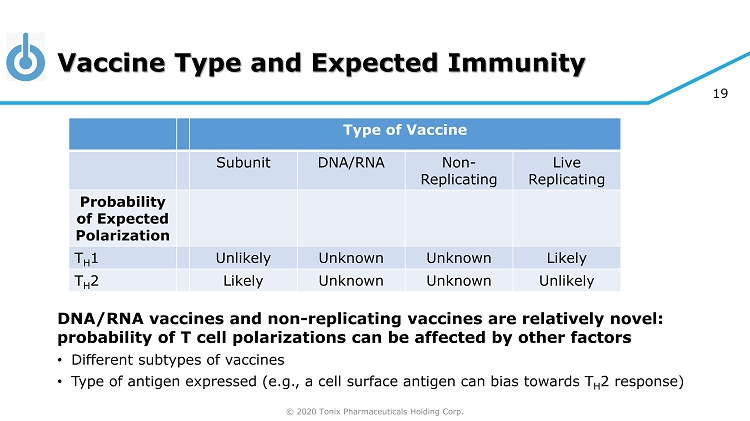
© 2020 Tonix Pharmaceuticals Holding Corp. 19 Vaccine Type and Expected Immunity Type of Vaccine Subunit DNA/RNA Non - Replicating Live Replicating Probability of Expected Polarization T H 1 Unlikely Unknown Unknown Likely T H 2 Likely Unknown Unknown Unlikely DNA/RNA vaccines and non - replicating vaccines are relatively novel: probability of T cell polarizations can be affected by other factors • Different subtypes of vaccines • Type of antigen expressed (e.g., a cell surface antigen can bias towards T H 2 response)

© 2020 Tonix Pharmaceuticals Holding Corp. 20 COVID - 19 Vaccine Landscape • We expect more than one vaccine will be approved by FDA • Different vaccines for different individuals • More than 150 vaccines in development • Diversity of approaches is important since protective immunity is not yet understood • Technologies range from never tested before to 220 years old • Live attenuated vector systems in development include: • Tonix (horsepox), Tonix (bovine parainfluenza), Merck (measles 1 - and VSV 2 - based), Zydus Cadila (measles - based) 1 Measles - based vaccine, acquisition of Themis, collaboration with Institute Pasteur 2 VSV = vesicular stomatitis virus; collaboration with IAVI = International AIDS Vaccine Initiative

© 2020 Tonix Pharmaceuticals Holding Corp. 21 Where Do We Go From Here? • Goal: Effective COVID - 19 vaccines • So we can return to work and school • Need #1 Quickly available vaccines • Even if they offer only temporary immunity -- several now in human trials • Need #2 Vaccines providing long - term immunity • Durable immunity for years • Blocking of forward transmission • Expect longer development and testing timelines
© 2020 Tonix Pharmaceuticals Holding Corp. 22 TNX - 1800 1 : COVID - 19 Vaccine Engineered for Long - term Immunity • Based on viral vaccine developed more than 200 years ago by Edward Jenner for a smallpox vaccine • Eradicated smallpox • T cell eliciting immunity • Single dose immunity without adjuvants • Manufacturable in scale on existing systems • Glass - sparing packaging owing to small unit dose 1 TNX - 1800 is at the pre - IND stage of development

© 2020 Tonix Pharmaceuticals Holding Corp. 23 Thank You!