
Tonix Pharmaceuticals Holdings Corp. 8-K
Exhibit 99.01

© 2020 Tonix Pharmaceuticals Holding Corp. 1 October 2020 NASDAQ: TNXP Version P0252 (Doc 0725) TNX - 1800: Horsepox - Vector Based Potential COVID - 19 Vaccine

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2019 , as filed with the Securities and Exchange Commission (the “SEC”) on March 24 , 2020 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Seth Lederman, MD Currently, CEO of Tonix Pharmaceuticals, Dr. Lederman began his career in research in the early days of the AIDS crisis and studied the entry of HIV into cells at the molecular and cell biological levels. Dr. Lederman is a co - inventor of the horsepox vaccine vector on which Tonix’s current COVID - 19 vaccine, TNX - 1800 is based. Previously, Dr. Lederman served as an Associate Professor at Columbia University from 1996 until 2017. He joined the faculty of Columbia University's College of Physicians and Surgeons in 1985, became Assistant Professor of Medicine in 1988, and Associate Professor with tenure in 1996 and Director of the Laboratory of Molecular Immunology in 1997. From 1988 to 2002, Dr. Lederman directed basic science research at Columbia in molecular immunology, infectious diseases and the development of therapeutics for autoimmune diseases. Dr. Lederman is author of numerous scientific articles, and inventor of technologies recognized by a number of issued patents. His fundamental work on the CD40 - Ligand (CD154) elucidated the molecular basis of T cell helper function and has led to the development of therapeutic candidates for autoimmune diseases and organ transplant rejection in collaboration with Biogen and UCB. In addition to his research, Dr. Lederman served as attending physician in the Edward Daniels Arthritis and Autoimmunity Clinic on the Medical Service at Columbia Presbyterian Hospital from 1988 - 1996. Dr. Lederman earned an AB from Princeton in Chemistry cum laude in 1979 and an MD from Columbia University's College of Physicians and Surgeons in 1983. Dr. Lederman trained in internal medicine and rheumatology at Columbia's Presbyterian Hospital. He was an NIH Physician - Scientist 1985 - 1990 at Columbia.
© 2020 Tonix Pharmaceuticals Holding Corp. 4 COVID - 19 Vaccine Landscape • More than 150 vaccines in development • Diversity of approaches is important since protective immunity is not yet understood • Technologies range from never tested before to 220 years old • Uncertainty exists around efficacy, durability and importantly, safety • Fastest developed vaccines benefit balance speed of development with potentially short - term antibody - based protection • We expect more than one vaccine will be approved by FDA • Different vaccines for different individuals • Live attenuated vector systems in development include: • Tonix (horsepox) 1 , Tonix (bovine parainfluenza) 1 , Merck (measles 2 - and VSV 3 - based), Zydus Cadila (measles - based) 1 TNX - 1800 (horsepox/Cov - 2 spike live virus vaccine) and TNX - 2300/TNX - 2600 (bovine parainfluenza virus/CoV - 2 spike live virus vacc ines) are at the pre - IND stage of development and are not approved for any indication 2 Measles - based vaccine, acquisition of Themis, collaboration with Institute Pasteur 3 VSV = vesicular stomatitis virus; collaboration with IAVI = International AIDS Vaccine Initiative
© 2020 Tonix Pharmaceuticals Holding Corp. 5 Live Attenuated Virus Vaccines for Other Infectious Diseases 1 • Long term, durable immunity • Stimulate T cells and provide years to decades of protection • Single administration, scalable manufacturing • Low dose is amplified by replication, mRNA and protein synthesis at vaccination site • Block forward transmission (infectivity) • Key to conferring herd immunity and protecting immunocompromised 1 For example, the eradication of smallpox, containment of measles, mumps, and rubella
© 2020 Tonix Pharmaceuticals Holding Corp. 6 TNX - 1800 1 : Potential COVID - 19 Vaccine Engineered for Long - term Immunity • Based on “vaccinia” vaccine developed more than 200 years ago by Dr. Edward Jenner to prevent smallpox • Eradicated smallpox (only viral disease ever eradicated) • Elicits durable (many decades) T cell immunity • Single dose protection without adjuvants • Manufacturable at scale • Minimal “cold chain” supply issues • Glass - sparing packaging owing to small unit dose • Genetic analysis of early vaccines indicates that Tonix’s “horsepox” is closely related to Edward Jenner’s “vaccinia” • Modern “vaccinia” evolved during the 220 years it was propagated by primitive methods – for over 120 years before “viruses” were identified 1 TNX - 1800 (horsepox/Cov - 2 spike live vaccine) is at the pre - IND stage of development
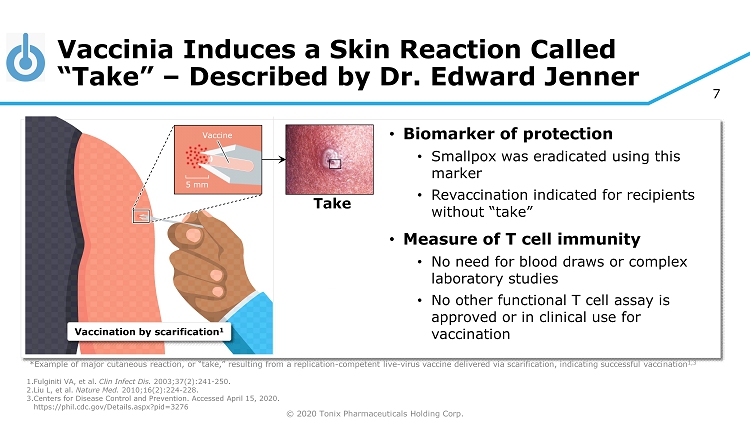
© 2020 Tonix Pharmaceuticals Holding Corp. 7 Vaccinia Induces a Skin Reaction Called “Take” – Described by Dr. Edward Jenner 1. Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2. Liu L, et al. Nature Med. 2010;16(2):224 - 228. 3. Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276 *Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine delivered via scarifi cation, indicating successful vaccination 1,3 5 mm Vaccine Vaccination by scarification 1 • Biomarker of protection • Smallpox was eradicated using this marker • Revaccination indicated for recipients without “take” • Measure of T cell immunity • No need for blood draws or complex laboratory studies • No other functional T cell assay is approved or in clinical use for vaccination Take
© 2020 Tonix Pharmaceuticals Holding Corp. 8 TNX - 1800 1 , a SARS - CoV - 2 Vaccine Candidate • Utilizes Tonix’s proprietary horsepox virus as a vector • Encodes a protein from SARS - CoV - 2, the cause of COVID - 19 • Developed in collaboration with University of Alberta, Canada • Animal testing with Southern Research Institute • Small animal and non - human primate testing data expected in 4Q20 • Manufacturing agreement with FUJIFILM Diosynth • Development for Good Manufacturing Practice (GMP) manufacturing for human trials • GMP 2 clinical supply expected to be ready for human trials in 2021 3 1 TNX - 1800 is at the pre - IND stage of development 2 Good Manufacturing Practice = GMP 3 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
© 2020 Tonix Pharmaceuticals Holding Corp. 9 Why Use a Horsepox Platform for a Vaccine? Horsepox can be engineered to express foreign genes • Lack of persistence or genomic integration in the host • Strong immunogenicity as a vaccine • Readily manufacture at scale • Live, attenuated vaccine – direct antigen presentation Potential advantages of horsepox over vaccinia • Maintains strong immunogenicity with potentially improved tolerability • Relative to non - replicating vaccinia, horsepox’s replication in human cells provides direct antigen presentation, which is expected to trigger a T cell immune response, by Class I Major Histocompatibility Complex (MHC) Antigens • Horsepox may behave differently than vaccinia as a vector, in part because of its different repertoire of genes that modulate immune responses and host range

© 2020 Tonix Pharmaceuticals Holding Corp. 10 TNX - 1800 Upcoming Milestones Southern Research studies will address two key questions: • Will vaccination of animals elicit an immune response to the S protein? • 4th Quarter 2020 – Non - human primate and small animal response results expected 1 • Will immune response protect animals against a challenge with SARS - CoV - 2 virus? • 4th Quarter 2020 – Non - human primate and small animal results expected 1 Detailed analysis of primates planned, including: • Major cutaneous reaction or “take” in primates • In vitro stimulation of T cells • Neutralizing antibodies 2 1 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
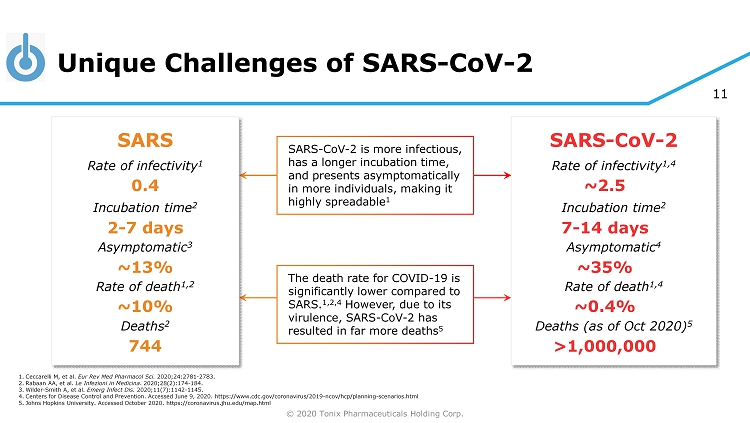
© 2020 Tonix Pharmaceuticals Holding Corp. 11 Unique Challenges of SARS - CoV - 2 SARS - CoV - 2 is more infectious, has a longer incubation time, and presents asymptomatically in more individuals, making it highly spreadable 1 The death rate for COVID - 19 is significantly lower compared to SARS. 1,2,4 However, due to its virulence, SARS - CoV - 2 has resulted in far more deaths 5 SARS Rate of infectivity 1 Incubation time 2 Asymptomatic 3 Rate of death 1,2 Deaths 2 0.4 2 - 7 days ~13% ~10% SARS - CoV - 2 Rate of infectivity 1,4 Incubation time 2 Asymptomatic 4 Rate of death 1,4 Deaths (as of Oct 2020) 5 ~2.5 7 - 14 days ~35% ~0.4% 1. Ceccarelli M, et al. Eur Rev Med Pharmacol Sci. 2020;24:2781 - 2783. 2. Rabaan AA, et al. Le Infezioni in Medicina . 2020;28(2):174 - 184. 3. Wilder - Smith A, et al. Emerg Infect Dis. 2020;11(7):1142 - 1145. 4. Centers for Disease Control and Prevention. Accessed June 9, 2020. https://www.cdc.gov/coronavirus/2019 - ncov/hcp/planning - scenar ios.html 5. Johns Hopkins University. Accessed October 2020. https://coronavirus.jhu.edu/map.html >1,000,000 744
© 2020 Tonix Pharmaceuticals Holding Corp. 12 Contrasting T cell and Antibody Immunity • T cell immunity • Durable or long - lived (many years) • Recognize fragments of pathogens on the surfaces of infected cells • Cannot recognize pathogens directly • Potential to clear viral infections (by killing infected cells) • Potential to block forward transmission (contagion) by infected people • Antibody immunity • Temporary or short - lived (typically 3 - 6 months) • Recognize pathogens directly • Potential to block viral entry (by recognizing pathogens) • Can only recognize virally infected cells that express viral surface proteins
© 2020 Tonix Pharmaceuticals Holding Corp. 13 What Is the Goal of Vaccination? • Vaccination instructs the immune system how to respond rapidly upon reinfection 1 - 3 • A typical infection is a race between the virus and the immune response • Vaccination gives the body a “head start” • Most vaccines against viruses protect against serious illness , not infection 2,3 • Different vaccines work in different ways • How an effective vaccine protects against disease depends on the nature of the virus 2,3 • Blocking forward transmission (spread of infection) is essential for public health 4 1. Centers for Disease Control and Prevention. Accessed June 9, 2020. https://www.cdc.gov/vaccines/pubs/pinkbook/prinvac.html 2. Plotkin SA. Vaccines. 2008;47(3):401 - 409. 3. Plotkin SA. Clin Vaccine Immunol. 2010;17(7):1055 - 1065. 4. Hardt K, et al. Vaccine. 2016;34(52):6691 - 6699.

© 2020 Tonix Pharmaceuticals Holding Corp. 14 COVID - 19: Reason for Optimism? • Most people recover, which suggests: • Vaccines can be designed that safely mimic infection • Herd immunity can be achieved by vaccination • Vaccine developers can learn from individuals who recover • Study their immunity in detail for a blueprint of a successful immune response • Potential to block forward transmission (contagion) by infected people • Comparison to HIV: protective immunity is unknown • No vaccine developed after 35 years

© 2020 Tonix Pharmaceuticals Holding Corp. 15 Where Do We Go From Here? • Goal: Effective COVID - 19 vaccines • So we can return to work and school • Need #1 Quickly available vaccines • Even if they offer only temporary immunity -- several now in human trials • Need #2 Vaccines providing long - term immunity • Durable immunity for years • Blocking of forward transmission • Expect longer development and testing timelines

© 2020 Tonix Pharmaceuticals Holding Corp. 16 Upcoming Milestones of TNX - 1800 Program 1 © 2020 Tonix Pharmaceuticals Holding Corp. • Results from small animal and non - human primate studies, including challenge with SARS - CoV - 2, due 4Q 2020 2 • 4 th Quarter 2020 – Small animal and primate immune response results expected 3 • 4 th Quarter 2020 – Small animal and primate CoV - 2 challenge results expected 3 • Phase 1 safety study in humans expected to be initiated in 2021 3 • Clinical trial quality or “GMP” 4 vaccine development and production partnered with FUJIFILM Diosynth 1 Investigational new drug and biologic, not approved for any indication 2 Collaboration with Southern Research 3 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones 4 Good Manufacturing Practice = GMP

© 2020 Tonix Pharmaceuticals Holding Corp. 17 Thank You!