
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.01

© 2020 Tonix Pharmaceuticals Holding Corp. 1 November 2020 Version P0253 11 - 10 - 20 (Doc 0726) Investor Presentation NASDAQ:TNXP

© 2020 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2019 , as filed with the Securities and Exchange Commission (the “SEC”) on March 24 , 2020 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
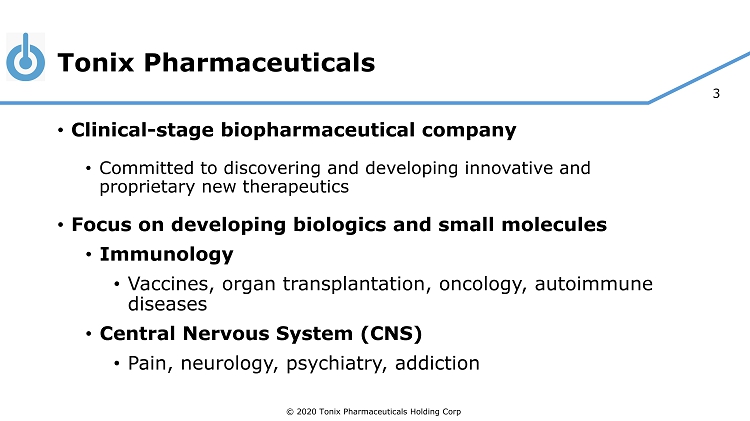
© 2020 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals © 2020 Tonix Pharmaceuticals Holding Corp . • Clinical - stage biopharmaceutical company • Committed to discovering and developing innovative and proprietary new therapeutics • Focus on developing biologics and small molecules • Immunology • Vaccines, organ transplantation, oncology, autoimmune diseases • Central Nervous System (CNS) • Pain, neurology, psychiatry, addiction
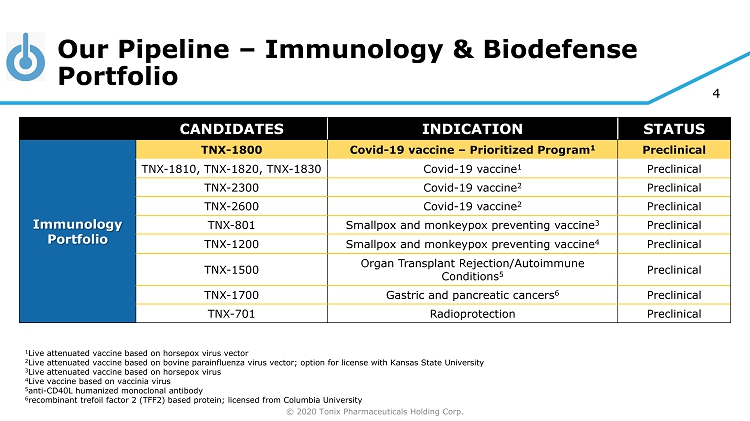
© 2020 Tonix Pharmaceuticals Holding Corp. 4 Our Pipeline – Immunology & Biodefense Portfolio CANDIDATES INDICATION STATUS Immunology Portfolio TNX - 1800 Covid - 19 vaccine – Prioritized Program 1 Preclinical TNX - 1810, TNX - 1820, TNX - 1830 Covid - 19 vaccine 1 Preclinical TNX - 2300 Covid - 19 vaccine 2 Preclinical TNX - 2600 Covid - 19 vaccine 2 Preclinical TNX - 801 Smallpox and monkeypox preventing vaccine 3 Preclinical TNX - 1200 Smallpox and monkeypox preventing vaccine 4 Preclinical TNX - 1500 Organ Transplant Rejection/Autoimmune Conditions 5 Preclinical TNX - 1700 Gastric and pancreatic cancers 6 Preclinical TNX - 701 Radioprotection Preclinical 1 Live attenuated vaccine based on horsepox virus vector 2 Live attenuated vaccine based on bovine parainfluenza virus vector; option for license with Kansas State University 3 Live attenuated vaccine based on horsepox virus 4 Live vaccine based on vaccinia virus 5 anti - CD40L humanized monoclonal antibody 6 recombinant trefoil factor 2 (TFF2) based protein; licensed from Columbia University
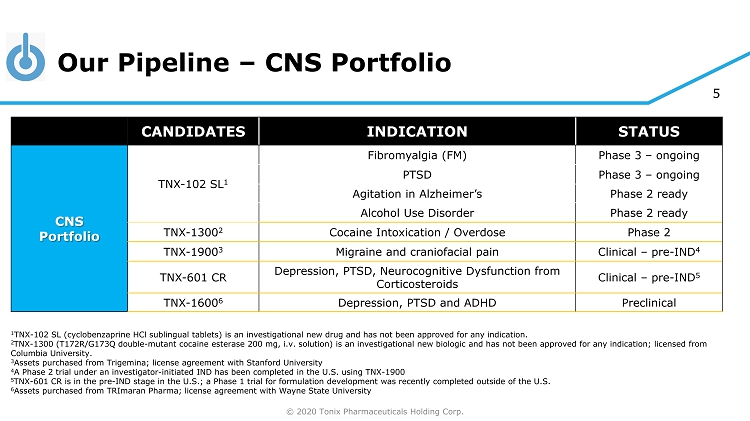
© 2020 Tonix Pharmaceuticals Holding Corp. 5 Our Pipeline – CNS Portfolio 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication. 2 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; licensed from Columbia University . 3 Assets purchased from Trigemina ; license agreement with Stanford University 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 5 TN X - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was recently completed outside of the U.S. 6 Assets purchased from TRImaran Pharma; license agreement with Wayne State University CANDIDATES INDICATION STATUS CNS Portfolio TNX - 102 SL 1 Fibromyalgia (FM) Phase 3 – ongoing PTSD Phase 3 – ongoing Agitation in Alzheimer’s Phase 2 ready Alcohol Use Disorder Phase 2 ready TNX - 1300 2 Cocaine Intoxication / Overdose Phase 2 TNX - 1900 3 Migraine and craniofacial pain Clinical – pre - IND 4 TNX - 601 CR Depression, PTSD, Neurocognitive Dysfunction from Corticosteroids Clinical – pre - IND 5 TNX - 1600 6 Depression, PTSD and ADHD Preclinical

© 2020 Tonix Pharmaceuticals Holding Corp. 6 COVID - 19 Vaccine Landscape • We expect more than one vaccine will be approved by FDA • Different vaccines for different individuals • More than 150 vaccines in development • Diversity of approaches is important since protective immunity is not yet understood • Technologies range from never tested before to 220 years old • Uncertainty exists around efficacy, durability and importantly, safety • Live attenuated vector systems in development include: • Tonix (horsepox), Tonix (bovine parainfluenza), Merck (measles 1 - and VSV 2 - based), Zydus Cadila (measles - based) 1 Measles - based vaccine, acquisition of Themis, collaboration with Institute Pasteur 2 VSV = vesicular stomatitis virus; collaboration with IAVI = International AIDS Vaccine Initiative
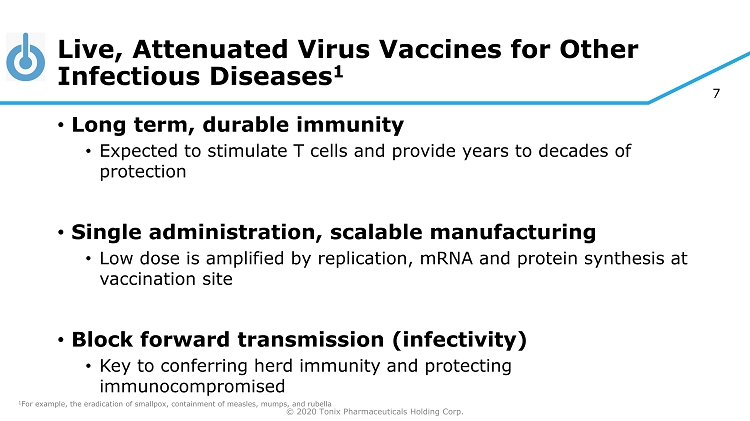
© 2020 Tonix Pharmaceuticals Holding Corp. 7 Live, Attenuated Virus Vaccines for Other Infectious Diseases 1 • Long term, durable immunity • Expected to stimulate T cells and provide years to decades of protection • Single administration, scalable manufacturing • Low dose is amplified by replication, mRNA and protein synthesis at vaccination site • Block forward transmission (infectivity) • Key to conferring herd immunity and protecting immunocompromised 1 For example, the eradication of smallpox, containment of measles, mumps, and rubella
© 2020 Tonix Pharmaceuticals Holding Corp. 8 TNX - 1800 1 : a COVID - 19 Vaccine Candidate • Utilizes Tonix’s proprietary horsepox virus as a vector • Encodes a protein from SARS - CoV - 2, the cause of COVID - 19 • Developed in collaboration with University of Alberta, Canada • Animal testing with Southern Research Institute • Small animal and non - human primate testing data expected in 4Q20 • Manufacturing agreement with FUJIFILM Diosynth • Development for Good Manufacturing Practice (GMP) manufacturing for human trials • GMP 2 clinical supply expected to be ready for human trials in 2021 3 1 TNX - 1800 (horsepox/Cov - 2 spike live vaccine) is at the pre - IND stage of development 2 Good Manufacturing Practice = GMP 3 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
© 2020 Tonix Pharmaceuticals Holding Corp. 9 TNX - 1800 1 : Engineered for Long - term Immunity • Based on “vaccinia” vaccine developed more than 200 years ago by Dr. Edward Jenner to prevent smallpox • Eradicated smallpox (only viral disease ever eradicated) • Elicits durable (many decades) T cell immunity • Single dose protection without adjuvants • Manufacturable at scale • Minimal “cold chain” supply issues • Glass - sparing packaging owing to small unit dose • Genetic analysis of early vaccines indicates that Tonix’s “horsepox” is closely related to Edward Jenner’s “vaccinia” • Modern “vaccinia” evolved during the 220 years it was propagated by primitive methods – for over 120 years before “viruses” were identified 1 TNX - 1800 (horsepox/Cov - 2 spike live vaccine) is at the pre - IND stage of development
© 2020 Tonix Pharmaceuticals Holding Corp. 10 Why Use a Horsepox Platform for a Vaccine? Horsepox can be engineered to express foreign genes • Lack of persistence or genomic integration in the host • Strong immunogenicity as a vaccine • Readily manufacture at scale • Live, attenuated vaccine – direct antigen presentation Potential advantages of horsepox over vaccinia • Maintains strong immunogenicity with potentially improved tolerability • Relative to non - replicating vaccinia, horsepox’s replication in human cells provides direct antigen presentation, which is expected to trigger a T cell immune response, by Class I Major Histocompatibility Complex (MHC) Antigens • Horsepox may behave differently than vaccinia as a vector, in part because of its different repertoire of genes that modulate immune responses and host range
© 2020 Tonix Pharmaceuticals Holding Corp. 11 TNX - 1800 is Based on a Horsepox Virus (HPXV) Vector Designed to Express SARS - CoV - 2 S Protein *TNX - 1800 is at the pre - IND stage of development Horsepox sHPXV ~200,000 Bp TNX - 1800 * rHPXV/SARS - CoV - 2S ~200,000 Bp Homologous Recombination
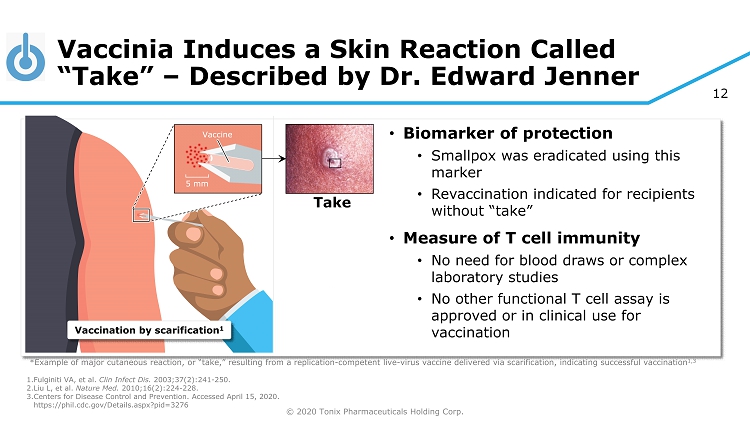
© 2020 Tonix Pharmaceuticals Holding Corp. 12 Vaccinia Induces a Skin Reaction Called “Take” – Described by Dr. Edward Jenner 1. Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2. Liu L, et al. Nature Med. 2010;16(2):224 - 228. 3. Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276 *Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine delivered via scarifi cation, indicating successful vaccination 1,3 5 mm Vaccine Vaccination by scarification 1 • Biomarker of protection • Smallpox was eradicated using this marker • Revaccination indicated for recipients without “take” • Measure of T cell immunity • No need for blood draws or complex laboratory studies • No other functional T cell assay is approved or in clinical use for vaccination Take
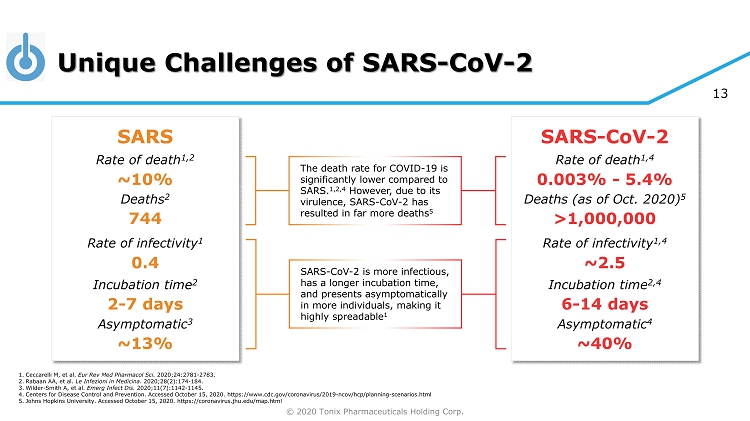
© 2020 Tonix Pharmaceuticals Holding Corp. 13 Unique Challenges of SARS - CoV - 2 SARS Rate of death 1,2 Deaths 2 Rate of infectivity 1 Incubation time 2 Asymptomatic 3 0.4 2 - 7 days ~13% ~10% SARS - CoV - 2 Rate of death 1,4 Deaths (as of Oct. 2020) 5 Rate of infectivity 1,4 Incubation time 2,4 Asymptomatic 4 ~2.5 6 - 14 days ~40% 0.003% - 5.4% 1. Ceccarelli M, et al. Eur Rev Med Pharmacol Sci. 2020;24:2781 - 2783. 2. Rabaan AA, et al. Le Infezioni in Medicina . 2020;28(2):174 - 184. 3. Wilder - Smith A, et al. Emerg Infect Dis. 2020;11(7):1142 - 1145. 4. Centers for Disease Control and Prevention. Accessed October 15, 2020. https://www.cdc.gov/coronavirus/2019 - ncov/hcp/planning - sc enarios.html 5. Johns Hopkins University. Accessed October 15, 2020. https://coronavirus.jhu.edu/map.html >1,000,000 744 The death rate for COVID - 19 is significantly lower compared to SARS. 1,2,4 However, due to its virulence, SARS - CoV - 2 has resulted in far more deaths 5 SARS - CoV - 2 is more infectious, has a longer incubation time, and presents asymptomatically in more individuals, making it highly spreadable 1
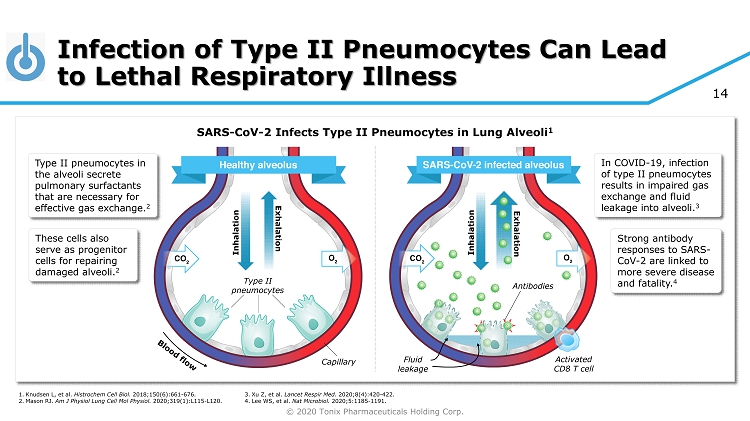
© 2020 Tonix Pharmaceuticals Holding Corp. 14 Infection of Type II Pneumocytes Can Lead to Lethal Respiratory Illness Capillary Type II pneumocytes Inhalation Exhalation Inhalation Exhalation Fluid leakage Activated CD8 T cell Antibodies Type II pneumocytes in the alveoli secrete pulmonary surfactants that are necessary for effective gas exchange. 2 SARS - CoV - 2 Infects Type II Pneumocytes in Lung Alveoli 1 These cells also serve as progenitor cells for repairing damaged alveoli. 2 In COVID - 19, infection of type II pneumocytes results in impaired gas exchange and fluid leakage into alveoli. 3 Strong antibody responses to SARS - CoV - 2 are linked to more severe disease and fatality. 4 1. Knudsen L, et al. Histrochem Cell Biol. 2018;150(6):661 - 676. 2. Mason RJ. Am J Physiol Lung Cell Mol Physiol. 2020;319(1):L115 - L120. 3. Xu Z, et al. Lancet Respir Med. 2020;8(4):420 - 422. 4. Lee WS, et al. Nat Microbiol. 2020;5:1185 - 1191.
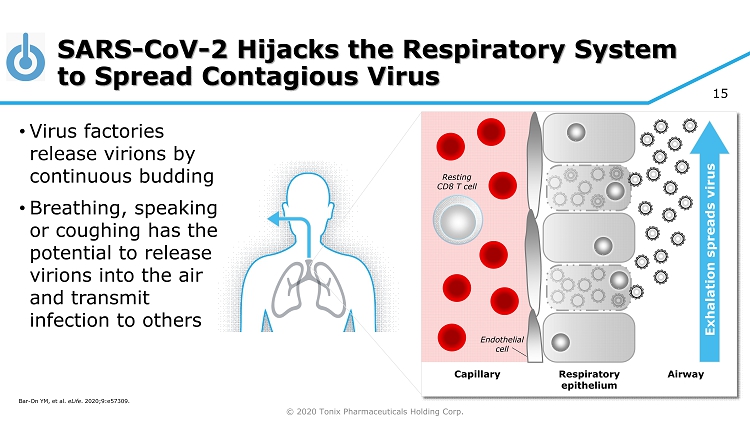
© 2020 Tonix Pharmaceuticals Holding Corp. 15 SARS - CoV - 2 Hijacks the Respiratory System to Spread Contagious Virus Respiratory epithelium Capillary Airway Resting CD8 T cell Endothelial cell Exhalation spreads virus • Virus factories release virions by continuous budding • Breathing, speaking or coughing has the potential to release virions into the air and transmit infection to others Bar - On YM, et al. eLife . 2020;9:e57309 .
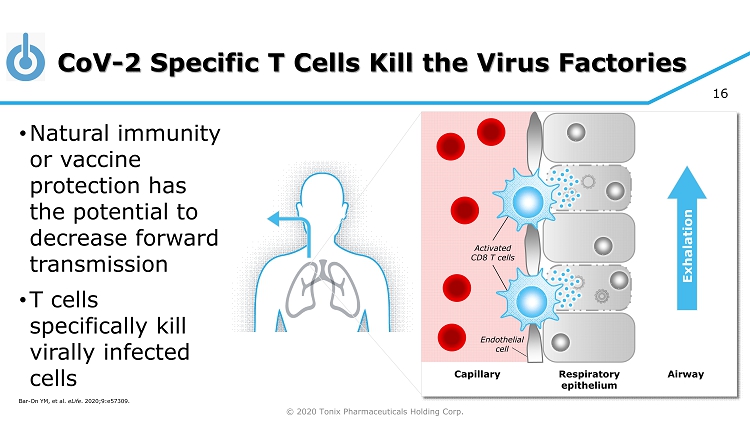
© 2020 Tonix Pharmaceuticals Holding Corp. 16 CoV - 2 Specific T Cells Kill the Virus Factories • Natural immunity or vaccine protection has the potential to decrease forward transmission • T cells specifically kill virally infected cells Bar - On YM, et al. eLife . 2020;9:e57309 . Respiratory epithelium Capillary Airway Endothelial cell Exhalation Activated CD8 T cells
© 2020 Tonix Pharmaceuticals Holding Corp. 17 Contrasting T cell and Antibody Immunity • T cell immunity • Durable or long - lived (many years) • Recognize fragments of pathogens on the surfaces of infected cells • Cannot recognize pathogens directly • Potential to clear viral infections (by killing infected cells) • Potential to block forward transmission (contagion) by infected people • Antibody immunity • Temporary or short - lived (typically 3 - 6 months) • Recognize pathogens directly • Potential to block viral entry (by recognizing pathogens) • Can only recognize virally infected cells that express viral surface proteins

© 2020 Tonix Pharmaceuticals Holding Corp. 18 TNX - 1800 Upcoming Milestones Southern Research studies will address two key questions: • Will vaccination of animals elicit an immune response to the S protein? • 4th Quarter 2020 – Non - human primate and small animal response results expected 1 • Will immune response protect animals against a challenge with SARS - CoV - 2 virus? • 1 st Quarter 2021 – Non - human primate and small animal results expected 1 Detailed analysis of primates planned, including: • Major cutaneous reaction or “take” in primates • In vitro stimulation of T cells • Neutralizing antibodies 2 1 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
© 2020 Tonix Pharmaceuticals Holding Corp. 19 2 nd SARS - CoV - 2 Vaccine Platform: Bovine Parainfluenza (BPI) Virus Collaboration with Kansas State University to develop a vaccine candidate for the prevention of COVID - 19 • Utilizes a novel live attenuated vaccine vector platform and the CD40 - ligand to stimulate T cell immunity • TNX - 2300 1 and TNX - 2600 1 drive expression of CoV - 2 spike and CD40 - L Live attenuated vaccines based on bovine parainfluenza virus 2 - 6 • Previously has been shown to be an effective antigen delivery vector in humans, notably well tolerated in infants and children • Vector is well suited for mucosal immunization using a nasal atomizer, but it can also be delivered parenterally Data from small animals to measure efficacy in challenge studies using SARS - COV - 2 are expected in the second quarter of 2021 1 Pre - IND stage of development; 2 Halle, AA et al. J Gen. Virology (2003) 84 :2153 – 2162; 3 Halle, AA et al. J Virology (2000) 74 (24): 11626 – 11635 ; 4 Karron RA et al. J Inf Dis (1995) 171: 1107 - 14; 5 Karron RA et al. Vaccine (2012) 30: 3975 – 3981; 6 Schmidt AC et al. J Virology (2001) 75(10): 4594 – 4603
© 2020 Tonix Pharmaceuticals Holding Corp. 20 Overview of TNX - 102 SL* Protectic ® proprietary formulation of cyclobenzaprine that supports sublingual administration Scientific Rationale for Protectic ® Formulation • Engenders unique pharmacokinetic and pharmacodynamic properties that emphasize sleep properties of cyclobenzaprine while minimizing undesirable properties • Potential therapeutic value in a constellation of disorders where sleep disturbances are: • Co - morbid • Involved in the onset, progression and severity of the disease ◊ ◊ *TNX - 102 SL is in clinical stage of development and not approved for any indication
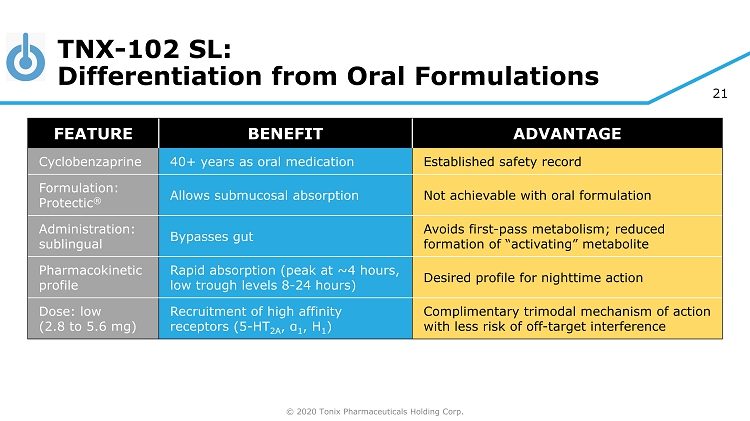
© 2020 Tonix Pharmaceuticals Holding Corp. 21 TNX - 102 SL: Differentiation from Oral Formulations FEATURE BENEFIT ADVANTAGE Cyclobenzaprine 40+ years as oral medication Established safety record Formulation: Protectic ® Allows submucosal absorption Not achievable with oral formulation Administration: sublingual Bypasses gut Avoids first - pass metabolism; reduced formation of “activating” metabolite Pharmacokinetic profile Rapid absorption (peak at ~4 hours, low trough levels 8 - 24 hours) Desired profile for nighttime action Dose: low (2.8 to 5.6 mg) Recruitment of high affinity receptors (5 - HT 2A , α 1 , H 1 ) Complimentary trimodal mechanism of action with less risk of off - target interference
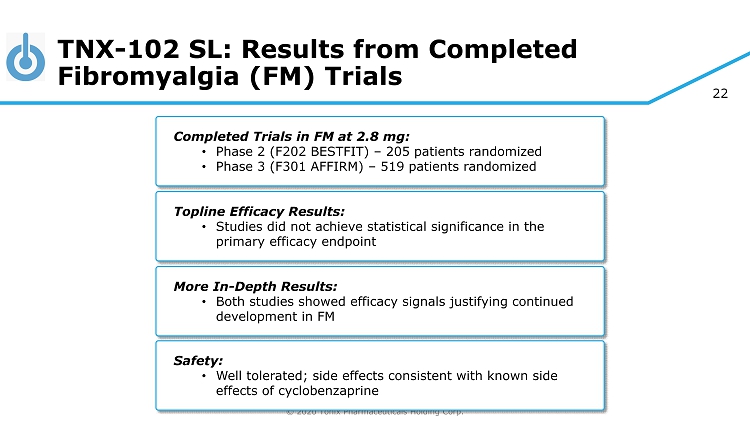
© 2020 Tonix Pharmaceuticals Holding Corp. 22 TNX - 102 SL: Results from Completed Fibromyalgia (FM) Trials Completed Trials in FM at 2.8 mg: • Phase 2 (F202 BESTFIT) – 205 patients randomized • Phase 3 (F301 AFFIRM) – 519 patients randomized Topline Efficacy Results: • Studies did not achieve statistical significance in the primary efficacy endpoint More In - Depth Results: • Both studies showed efficacy signals justifying continued development in FM Safety: • Well tolerated; side effects consistent with known side effects of cyclobenzaprine
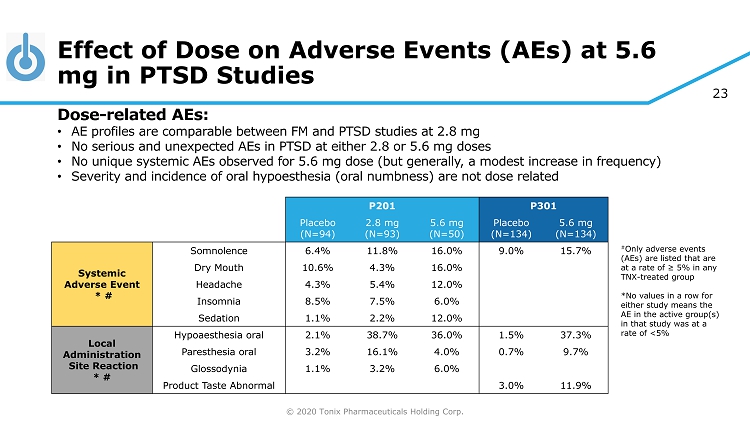
© 2020 Tonix Pharmaceuticals Holding Corp. 23 Effect of Dose on Adverse Events (AEs) at 5.6 mg in PTSD Studies # Only adverse events (AEs) are listed that are at a rate of ≥ 5% in any TNX - treated group *No values in a row for either study means the AE in the active group(s) in that study was at a rate of <5% P201 P301 Placebo (N=94) 2.8 mg (N=93) 5.6 mg (N=50) Placebo (N=134) 5.6 mg (N=134) Systemic Adverse Event * # Somnolence 6.4% 11.8% 16.0% 9.0% 15.7% Dry Mouth 10.6% 4.3% 16.0% Headache 4.3% 5.4% 12.0% Insomnia 8.5% 7.5% 6.0% Sedation 1.1% 2.2% 12.0% Local Administration Site Reaction * # Hypoaesthesia oral 2.1% 38.7% 36.0% 1.5% 37.3% Paresthesia oral 3.2% 16.1% 4.0% 0.7% 9.7% Glossodynia 1.1% 3.2% 6.0% Product Taste Abnormal 3.0% 11.9% Dose - related AEs: • AE profiles are comparable between FM and PTSD studies at 2.8 mg • No serious and unexpected AEs in PTSD at either 2.8 or 5.6 mg doses • No unique systemic AEs observed for 5.6 mg dose (but generally, a modest increase in frequency) • Severity and incidence of oral hypoesthesia (oral numbness) are not dose related
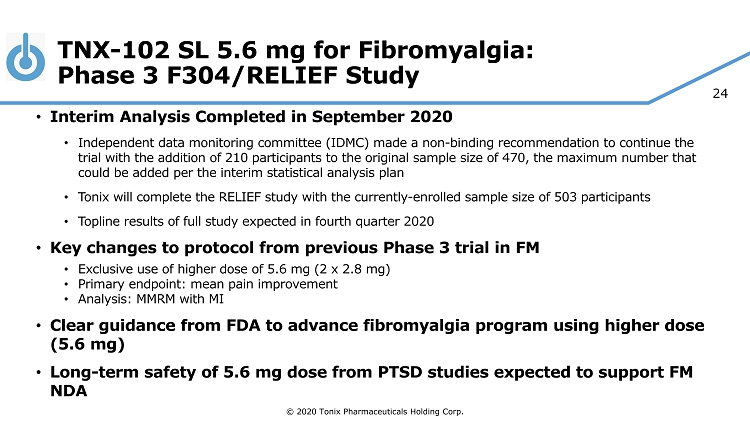
© 2020 Tonix Pharmaceuticals Holding Corp. 24 TNX - 102 SL 5.6 mg for Fibromyalgia: Phase 3 F304/RELIEF Study © 2020 Tonix Pharmaceuticals Holding Corp. • Interim Analysis Completed in September 2020 • Independent data monitoring committee (IDMC) made a non - binding recommendation to continue the trial with the addition of 210 participants to the original sample size of 470, the maximum number that could be added per the interim statistical analysis plan • Tonix will complete the RELIEF study with the currently - enrolled sample size of 503 participants • Topline results of full study expected in fourth quarter 2020 • Key changes to protocol from previous Phase 3 trial in FM • Exclusive use of higher dose of 5.6 mg (2 x 2.8 mg) • Primary endpoint: mean pain improvement • Analysis: MMRM with MI • Clear guidance from FDA to advance fibromyalgia program using higher dose (5.6 mg) • Long - term safety of 5.6 mg dose from PTSD studies expected to support FM NDA
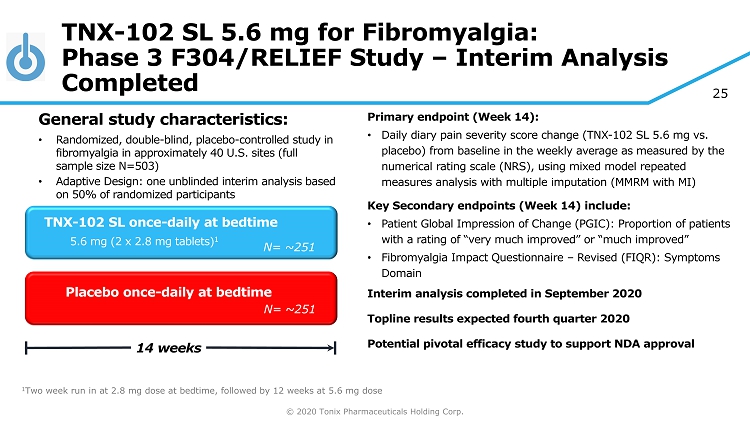
© 2020 Tonix Pharmaceuticals Holding Corp. 25 TNX - 102 SL 5.6 mg for Fibromyalgia: Phase 3 F304/RELIEF Study – Interim Analysis Completed Primary e ndpoint (Week 14) : • Daily diary pain severity score change (TNX - 102 SL 5.6 mg vs. placebo) from baseline in the weekly average as measured by the numerical rating scale (NRS), using mixed model repeated measures analysis with multiple imputation (MMRM with MI) Key Secondary e ndpoint s (Week 14) include: • Patient Global Impression of Change (PGIC): Proportion of patients with a rating of “very much improved” or “much improved” • Fibromyalgia Impact Questionnaire – Revised (FIQR): Symptoms Domain Interim analysis completed in September 2020 Topline results expected fourth quarter 2020 Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) 1 General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia in approximately 40 U.S. sites (full sample size N=503) • Adaptive Design: one unblinded interim analysis based on 50% of randomized participants 1 Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose N= ~251 N= 250 N= ~251
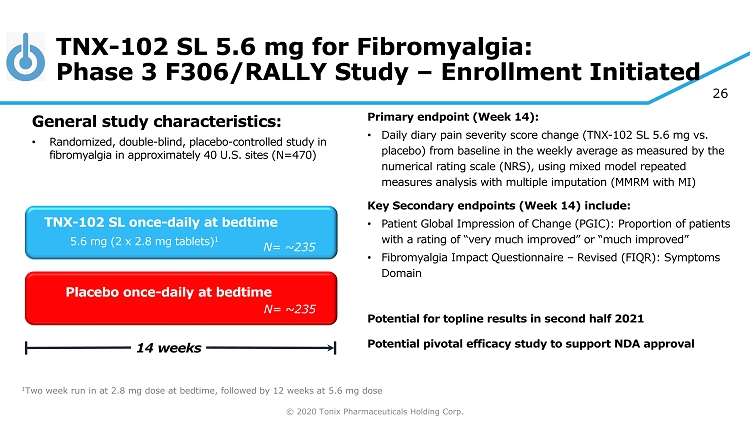
© 2020 Tonix Pharmaceuticals Holding Corp. 26 TNX - 102 SL 5.6 mg for Fibromyalgia: Phase 3 F306/RALLY Study – Enrollment Initiated Primary e ndpoint (Week 14) : • Daily diary pain severity score change (TNX - 102 SL 5.6 mg vs. placebo) from baseline in the weekly average as measured by the numerical rating scale (NRS), using mixed model repeated measures analysis with multiple imputation (MMRM with MI) Key Secondary e ndpoint s (Week 14) include: • Patient Global Impression of Change (PGIC): Proportion of patients with a rating of “very much improved” or “much improved” • Fibromyalgia Impact Questionnaire – Revised (FIQR): Symptoms Domain Potential for topline results in second half 2021 Potential pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) 1 General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia in approximately 40 U.S. sites (N=470) 1 Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose N= ~235 N= 250 N= ~235

© 2020 Tonix Pharmaceuticals Holding Corp. 27 TNX - 102 SL Intellectual Property – U.S. Protection expected until 2035 • United States Patent and Trademark Office (USPTO) issued United States Patent No. 9636408 in May 2017, Patent No. 9956188 in May 2018, Patent No. 10117936 in November 2018, Patent No. 10,357,465 in July 2019, and Patent No. 10736859 in August 2020 • European Patent Office (EPO) issued European Patent No. 2968992 in December 2019 (validated in 37 countries). Opposition filed in October 2020 by Hexal AG • China National Intellectual Property Administration issued Chinese Patent No. ZL 201480024011.1 in April 2019 • Japanese Patent Office (JPO) issued Japanese Patent No. 6310542 in March 2018, Patent No. 6614724 in November 2019, and Patent No. 6717902 in June 2020 • 10 granted patents (Indonesia, Saudi Arabia, New Zealand, Australia, Mexico, Taiwan, Israel, South Africa) • 31 patent applications pending (4 being allowed in U.S., China, Israel, South Africa) Composition of matter (eutectic): Protection expected to 2034/2035 • NZIPO issued New Zealand Patent No. 631144 in March 2017 and Patent No. 726488 in January 2019 • Taiwanese Intellectual Property Office issued Taiwanese Patent No. I590820 in July 2017, Patent No. I642429 in December 2018 and Patent No. I683660 in February 2020 • Australian Patent Office issued Australian Patent No. 2013274003 in October 2018 and Patent No. 2018241128 in September 2020 • JPO issued Japanese Patent No. 6259452 in December 2017 • 20 patent applications pending Composition of matter (sublingual): Protection expected to 2033
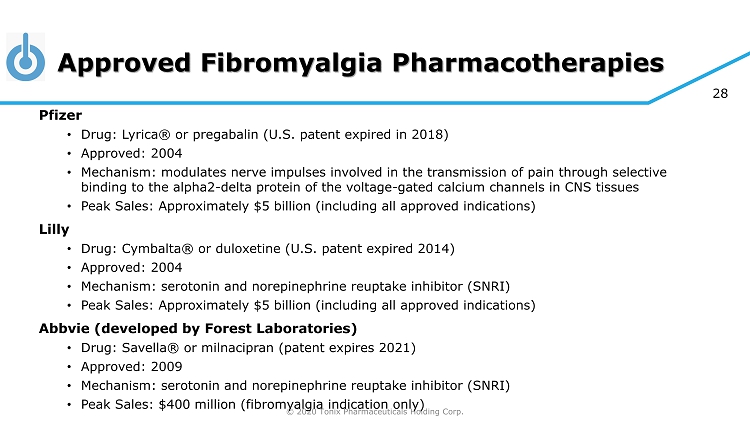
© 2020 Tonix Pharmaceuticals Holding Corp. 28 Approved Fibromyalgia Pharmacotherapies Pfizer • Drug: Lyrica® or pregabalin (U.S. patent expired in 2018) • Approved: 2004 • Mechanism: modulates nerve impulses involved in the transmission of pain through selective binding to the alpha2 - delta protein of the voltage - gated calcium channels in CNS tissues • Peak Sales: Approximately $5 billion (including all approved indications) Lilly • Drug: Cymbalta® or duloxetine (U.S. patent expired 2014) • Approved: 2004 • Mechanism: serotonin and norepinephrine reuptake inhibitor (SNRI) • Peak Sales: Approximately $5 billion (including all approved indications) Abbvie (developed by Forest Laboratories) • Drug: Savella ® or milnacipran (patent expires 2021) • Approved: 2009 • Mechanism: serotonin and norepinephrine reuptake inhibitor (SNRI) • Peak Sales: $400 million (fibromyalgia indication only)

© 2020 Tonix Pharmaceuticals Holding Corp. 29 Other Fibromyalgia Pharmacotherapies in Development in the U.S. Axsome Therapeutics - AXS - 14 • Drug: esreboxetine • Mechanism: Selective norepinephrine reuptake inhibitor • Developmental Stage: At least mid - Phase 3 (Phase 2 and Phase 3 trial positive*) Aptinyx - NYX - 2925 • Drug: ((2S, 3R) - 3 - hydroxy - 2 - ((R) - 5 - isobutyryl - 1 - oxo - 2,5 - diazaspiro(3.4)octan - 2 - yl)butanamide) • Mechanism: NMDA receptor modulator • Developmental Stage: Phase 2 study is “active, not recruiting” Teva - Ajovy ® • Drug: fremanezumab • Anti - CGRP antibody • Developmental Stage: Phase 2 proof - of - concept study “recruiting” *licensed from Pfizer, Jan 2020
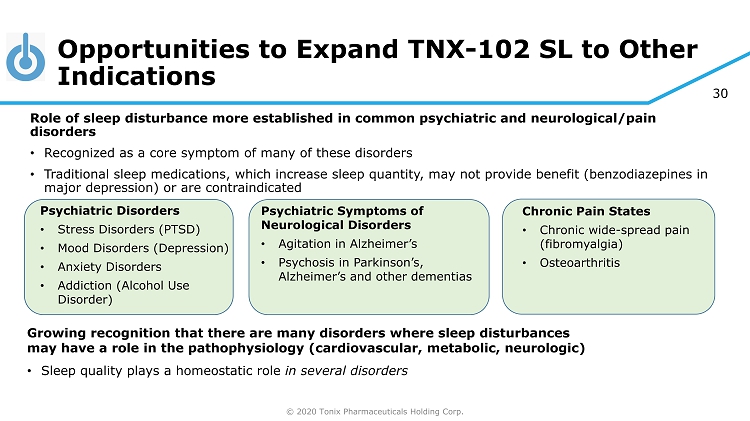
© 2020 Tonix Pharmaceuticals Holding Corp. 30 Opportunities to Expand TNX - 102 SL to Other Indications Growing recognition that there are many disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Sleep quality plays a homeostatic role in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders (Depression) • Anxiety Disorders • Addiction (Alcohol Use Disorder) Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias

© 2020 Tonix Pharmaceuticals Holding Corp. 31 TNX - 1300* for the Treatment of Cocaine Intoxication Recombinant protein that degrades cocaine in the bloodstream 1 • Double - mutant cocaine esterase ( CocE ) • CocE was identified in a bacterium ( Rhodococcus ) that use cocaine as its sole source of carbon and nitrogen and that grow in soil surrounding coca plants 2 • CocE catalyzes the breakdown of cocaine into metabolites ecgonine methyl ester and benzoic acid Phase 2 study comp leted by Reckitt Benckiser (TNX - 1300 was formerly RBP - 8000) 3 • Volunteer cocaine abusers received cocaine 50 mg i.v. infusion over 10 minutes • TNX - 1300 given one minute after completion of cocaine infusion • Rapidly reversed the physiologic effects of cocaine; cocaine plasma exposures dropped by 90% within two minutes • Well tolerated with the most frequently reported adverse events being gastrointestinal disorders ( including dry mouth, nausea); nervous systems disorders (including headache, dizziness) and skin and subcutaneous tissue disorders (including hyperhidrosis , dermatitis) *TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication. 1 Gao D et al, Mol Pharmacol . 2009. 75(2):318 - 23. 2 Bresler MM et al, Appl Environ Microbiol . 2000. 66(3):904 - 8. 3 Nasser AF et al, J Addict Dis . 2014;33(4):289 - 302. 31
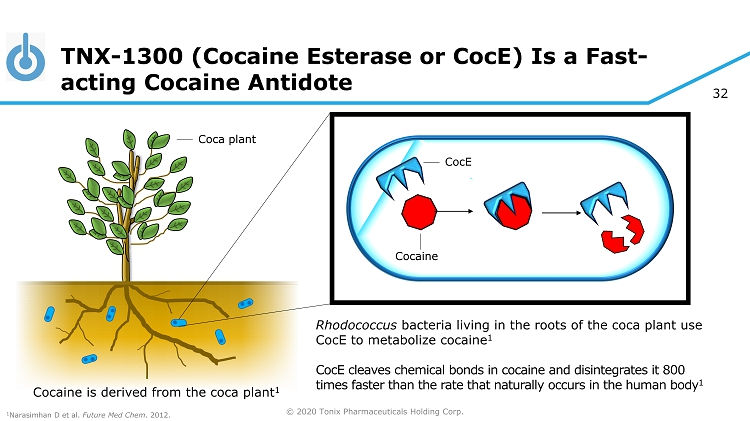
© 2020 Tonix Pharmaceuticals Holding Corp. 32 TNX - 1300 (Cocaine Esterase or CocE ) Is a Fast - acting Cocaine Antidote CocE Rhodococcus bacteria living in the roots of the coca plant use CocE to metabolize cocaine 1 CocE cleaves chemical bonds in cocaine and disintegrates it 800 times faster than the rate that naturally occurs in the human body 1 Cocaine Cocaine is derived from the coca plant 1 1 Narasimhan D et al. Future Med Chem . 2012. Coca plant
© 2020 Tonix Pharmaceuticals Holding Corp. 33 TNX - 1300 Development Plan 33 • Targeting to initiate a Phase 2 open - label, randomized pilot study of TNX - 1300 in the first quarter of 2021 • Emergency department (ED) setting with patients coming in for treatment of cocaine and/or polysubstance intoxication • Objectives • Primary: To evaluate the safety of TNX - 1300 in the ED setting • Secondary: • To evaluate TNX - 1300 in the management of cardiovascular (CV) and other signs and symptoms associated with cocaine intoxication compared to usual care (UC) alone • To demonstrate reduction of plasma cocaine, cocaethylene , and ecgonine methyl ester levels after TNX - 1300 administration and compare cocaine and cocaethylene levels of TNX - 1300 group to those in UC alone

© 2020 Tonix Pharmaceuticals Holding Corp. 34 TNX - 1900 for the Treatment of Migraine and Craniofacial Pain – Overview Novel intranasal oxytocin formulation being developed as a prophylactic treatment for chronic migraine • Based on a propriety formulation of oxytocin*, a naturally occurring human hormone that acts as a neurotransmitter in the brain Clinical and preliminary research has shown that low oxytocin levels in the body can lead to increase in headache frequency, and that increased oxytocin levels can relieve headaches • Certain other chronic pain conditions are also associated with decreased oxytocin levels Oxytocin when delivered via the nasal route, results in enhanced binding of oxytocin to receptors on neurons in the trigeminal system, inhibiting transmission of pain signals Intranasal oxytocin has been shown in animals that it can also block CGRP release, a pathway known to be critical to the pathogenesis of migraine attacks. * Oxytocin is approved by the U.S. Food and Drug Administration (FDA) as Pitocin ® , an intravenous infusion or intramuscular injection drug, for use in pregnant women to induce labor. An intranasal form of oxytocin was marketed by Novartis to assist in nursing as Syntocinon ® , but the product was withdrawn and the New Drug Application (NDA) has been discontinued.
© 2020 Tonix Pharmaceuticals Holding Corp. 35 TNX - 1900 for the Treatment of Migraine – Prevalence One billion individuals worldwide suffer from migraines (~14% of population) 1 Migraine is the second leading cause of years lived with disability 1 In U.S., the estimated cost of all migraine headaches was $78 billion in 2014 2 • Approximately 30% of those costs ($23 billion) were direct medical costs Chronic migraine (≥ 15 headaches / month ) effects about 1 - 2% of individuals 3 • 75 - 150 million individuals worldwide • 3 - 7 million in the U.S. CGRP antibodies are the only migraine specific prophylaxis drugs approved in decades • Requires parenteral administration (systemic effects on peripheral CGRP pathways) • Long term safety concerns with prolonged systemic blockade of CGRP receptor 4 1 GBD 2016 Headache Collaborators, Global, regional, and national burden of migraine and tension - type headache, 1990 – 2016: a syste matic analysis for the Global Burden of Disease Study 2016, Lancet Neurol 2018; 17: 954 – 76 2 Gooch, C. L., et al., The Burden of Neurological Disease in the United States: A Summary Report and Call to Action. Ann Neuro l. 2017; 81:479 - 484 3 Natoli et al., Global prevalence of chronic migraine: a systematic review, Cephalagia , 2010, 30:599 - 609 4 Robbins, At Stake: The Possible Long - Term Side Effects of CGRP Antagonists, https://www.practicalpainmanagement.com/pain/headache/stake - possible - long - term - side - effects - cgrp - antagonists , accessed November 8, 2020.

© 2020 Tonix Pharmaceuticals Holding Corp. 36 TNX - 1900 for the Treatment of Migraine – Mechanism of Action Preclinical research showed that nasally applied TNX - 1900 selectively inhibits the activity of trigeminal pain - sensing nerve cells and blocks the release of CGRP • TNX - 1900 is believed to interrupt pain signals at the trigeminal ganglia by suppressing electrical impulses, a potentially different activity than drugs that just block CGRP Migraine attacks are caused, in part, by the release of CGRP from pain - sensing nerve cells that are part of the trigeminal system • The CGRP binds to receptors on other nerve cells and starts a cascade of events that eventually results in a severe headache. This, in turn, reduces various kinds of trigeminal nerve associated pain and prevents CGRP from acting at receptors in the central nervous system that are involved in migraine. We believe targeted delivery of oxytocin could translate into selective blockade of CGRP release in the trigeminal ganglion and not throughout the body, which could be a potential safety advantage over systemic CGRP inhibition • In addition, daily dosing is more quickly reversible, in contrast to monthly or quarterly dosing, giving physicians and their patients greater control
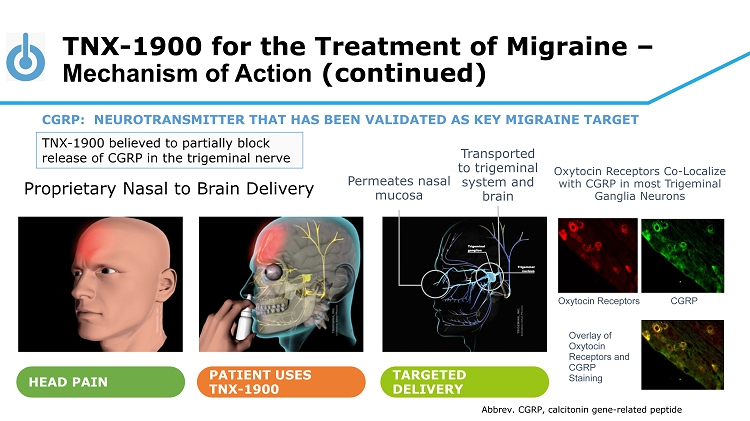
TNX - 1900 for the Treatment of Migraine – Mechanism of Action (continued) HEAD PAIN PATIENT USES TNX - 1900 TARGETED DELIVERY Permeates nasal mucosa Transported to trigeminal system and brain Proprietary Nasal to Brain Delivery CGRP: NEUROTRANSMITTER THAT HAS BEEN VALIDATED AS KEY MIGRAINE TARGET TNX - 1900 believed to partially block release of CGRP in the trigeminal nerve Oxytocin Receptors CGRP Oxytocin Receptors Co - Localize with CGRP in most Trigeminal Ganglia Neurons Overlay of Oxytocin Receptors and CGRP Staining Abbrev. CGRP, calcitonin gene - related peptide
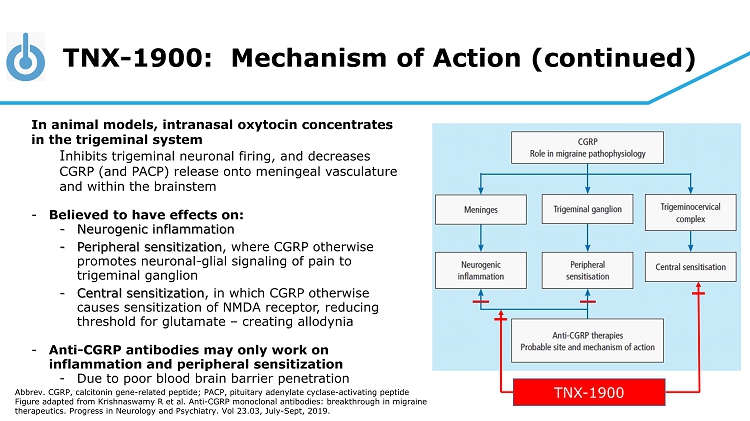
TNX - 1900: Mechanism of Action (continued) In animal models, intranasal oxytocin concentrates in the trigeminal system I nhibits trigeminal neuronal firing, and decreases CGRP (and PACP) release onto meningeal vasculature and within the brainstem - Believed to have effects on: - Neurogenic inflammation - Peripheral sensitization , where CGRP otherwise promotes neuronal - glial signaling of pain to trigeminal ganglion - Central sensitization , in which CGRP otherwise causes sensitization of NMDA receptor, reducing threshold for glutamate – creating allodynia - Anti - CGRP antibodies may only work on inflammation and peripheral sensitization - Due to poor blood brain barrier penetration Abbrev. CGRP, calcitonin gene - related peptide; PACP, pituitary adenylate cyclase - activating peptide Figure adapted from Krishnaswamy R et al. Anti - CGRP monoclonal antibodies: breakthrough in migraine therapeutics. Progress in Neurology and Psychiatry. Vol 23.03, July - Sept, 2019. TNX - 1900
© 2020 Tonix Pharmaceuticals Holding Corp. 39 TNX - 1900 for the Treatment of Migraine – Development Status In June 2020, Tonix acquired a proprietary formulation of nasal oxytocin solution for intranasal delivery from Trigemina Also acquired migraine and pain treatment technologies of Trigemina , Inc. and assumed license for some of technologies from Stanford University A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 Completed by Trigemina prior to acquisition Tonix intends to submit an IND application for this program to the FDA in the first quarter of 2021 Targeting start of a Phase 2 study of TNX - 1900 for the prophylactic treatment of chronic migraine in the U.S. in the second quarter of 2021 • Primary endpoint expected to be mean change in number of migraine headache days from the last 28 days of baseline to the last 28 days of treatment in each treatment group
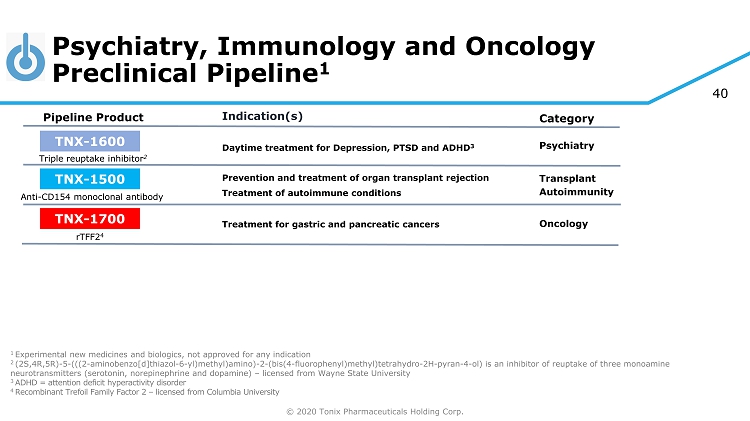
© 2020 Tonix Pharmaceuticals Holding Corp. 40 Psychiatry, Immunology and Oncology Preclinical Pipeline 1 1 Experimental new medicines and biologics, not approved for any indication 2 (2S,4R,5R) - 5 - (((2 - aminobenzo[d]thiazol - 6 - yl)methyl)amino) - 2 - (bis(4 - fluorophenyl)methyl)tetrahydro - 2H - pyran - 4 - ol) is an inhibitor of reuptake of three monoamine neurotransmitters (serotonin, norepinephrine and dopamine) – licensed from Wayne State University 3 ADHD = attention deficit hyperactivity disorder 4 R ecombinant Trefoil Family Factor 2 – licensed from Columbia University Category Pipeline Product Indication(s) Triple reuptake inhibitor 2 TNX - 1600 Daytime treatment for Depression, PTSD and ADHD 3 Psychiatry TNX - 1500 Anti - CD154 monoclonal antibody Prevention and treatment of organ transplant rejection Autoimmunity Transplant TNX - 1700 rTFF2 4 Treatment for gastric and pancreatic cancers Oncology Treatment of autoimmune conditions
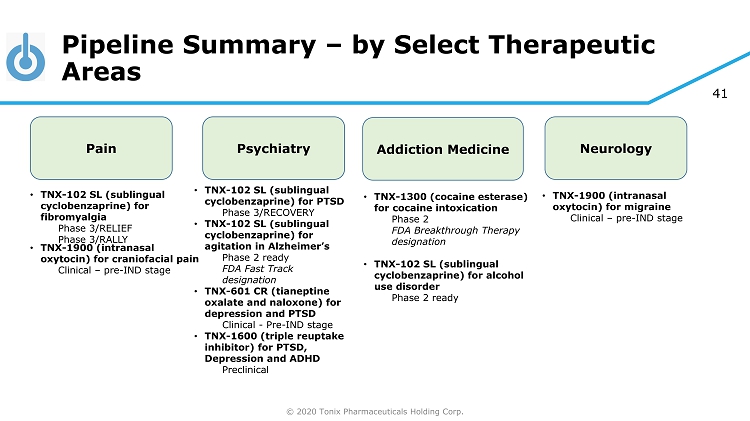
© 2020 Tonix Pharmaceuticals Holding Corp. 41 Pipeline Summary – by Select Therapeutic Areas Pain Psychiatry Addiction Medicine • TNX - 102 SL (sublingual cyclobenzaprine) for fibromyalgia Phase 3/RELIEF Phase 3/RALLY • TNX - 102 SL (sublingual cyclobenzaprine) for PTSD Phase 3/RECOVERY • TNX - 102 SL (sublingual cyclobenzaprine) for agitation in Alzheimer’s Phase 2 ready FDA Fast Track designation • TNX - 601 CR (tianeptine oxalate and naloxone) for depression and PTSD Clinical - Pre - IND stage • TNX - 1600 (triple reuptake inhibitor) for PTSD, Depression and ADHD Preclinical • TNX - 1300 (cocaine esterase) for cocaine intoxication Phase 2 FDA Breakthrough Therapy designation • TNX - 102 SL (sublingual cyclobenzaprine) for alcohol use disorder Phase 2 ready Neurology • TNX - 1900 (intranasal oxytocin) for migraine Clinical – pre - IND stage • TNX - 1900 (intranasal oxytocin) for craniofacial pain Clinical – pre - IND stage
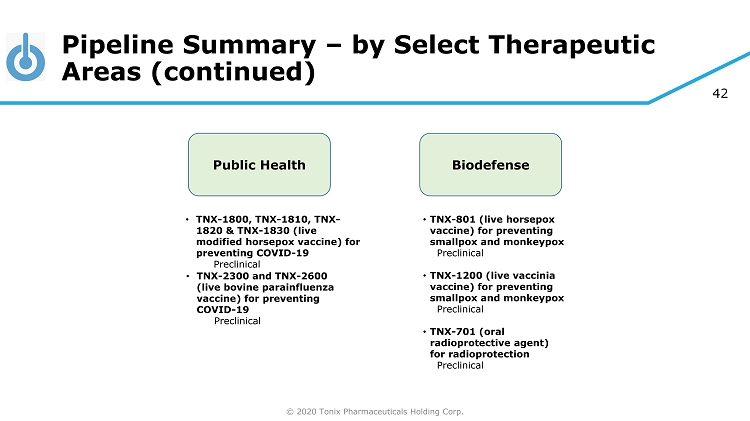
© 2020 Tonix Pharmaceuticals Holding Corp. 42 Pipeline Summary – by Select Therapeutic Areas (continued) Biodefense • TNX - 801 (live horsepox vaccine) for preventing smallpox and monkeypox Preclinical • TNX - 1200 (live vaccinia vaccine) for preventing smallpox and monkeypox Preclinical • TNX - 701 (oral radioprotective agent) for radioprotection Preclinical Public Health • TNX - 1800, TNX - 1810, TNX - 1820 & TNX - 1830 (live modified horsepox vaccine) for preventing COVID - 19 Preclinical • TNX - 2300 and TNX - 2600 (live bovine parainfluenza vaccine) for preventing COVID - 19 Preclinical

© 2020 Tonix Pharmaceuticals Holding Corp. 43 Milestones – Recently Completed and Upcoming 1 □ September 2020 Interim analysis of TNX - 102 SL Phase 3 F304/RELIEF study in fibromyalgia completed □ 4 th Quarter 2020 Topline data from TNX - 102 SL Phase 3 F304/RELIEF study in fibromyalgia expected □ 4 th Quarter 2020 Small animal and non - human primate immune response data from TNX - 1800 in COVID - 19 models expected □ 2021 Initiation of Phase 1 safety study of TNX - 1800 for COVID - 19 expected □ 1 st Quarter 2021 Small animal and non - human primate efficacy data from TNX - 1800 in COVID - 19 models expected □ 1 st Quarter 2021 Initiate Phase 2 open - label safety study of TNX - 1300 in ED setting □ 1 st Quarter 2021 Submit IND application for TNX - 1900 for the treatment of migraine □ 2 nd Quarter 2021 Initiate Phase 2 study of TNX - 1900 for the treatment of migraine □ 2 nd Quarter 2021 Small animal efficacy data from TNX - 2300 in COVID - 19 models expected □ 2 nd Half 2021 Topline data from TNX - 102 SL Phase 3 F306/RALLY study in fibromyalgia expected x 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones.

© 2020 Tonix Pharmaceuticals Holding Corp. 44 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger , CPA Chief Financial Officer

© 2020 Tonix Pharmaceuticals Holding Corp. 45 Thank You!