
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2021 Tonix Pharmaceuticals Holding Corp. 1 April 2021 Investor Presentation NASDAQ:TNXP Version P0288 4 - 19 - 2021 (Doc 0821)

© 2021 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations ; our need for additional financing ; delays and uncertainties caused by the global COVID - 19 pandemic ; substantial competition ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2020 , as filed with the Securities and Exchange Commission (the “SEC”) on March 15 , 2021 , and periodic reports and current reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .

© 2021 Tonix Pharmaceuticals Holding Corp. 3 Tonix Pharmaceuticals Who We Are – Mission And Purpose Clinical - stage biopharmaceutical company that invents and develops medicines to help patients manage the central nervous system (CNS) and immunology diseases. “Advancing science to improve patient care and public health”
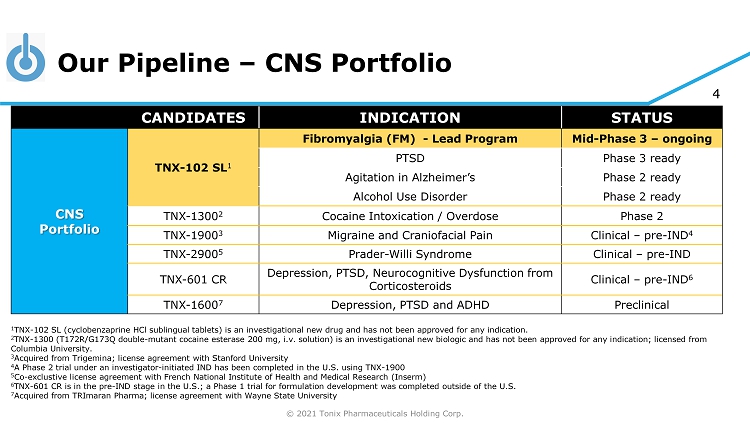
© 2021 Tonix Pharmaceuticals Holding Corp. 4 Our Pipeline – CNS Portfolio CANDIDATES INDICATION STATUS CNS Portfolio TNX - 102 SL 1 Fibromyalgia (FM) - Lead Program Mid - Phase 3 – ongoing PTSD Phase 3 ready Agitation in Alzheimer’s Phase 2 ready Alcohol Use Disorder Phase 2 ready TNX - 1300 2 Cocaine Intoxication / Overdose Phase 2 TNX - 1900 3 Migraine and Craniofacial Pain Clinical – pre - IND 4 TNX - 2900 5 Prader - Willi Syndrome Clinical – pre - IND TNX - 601 CR Depression, PTSD, Neurocognitive Dysfunction from Corticosteroids Clinical – pre - IND 6 TNX - 1600 7 Depression, PTSD and ADHD Preclinical 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and has not been approved for any indication. 2 TNX - 1300 (T172R/G173Q double - mutant cocaine esterase 200 mg, i.v. solution) is an investigational new biologic and has not been approved for any indication; licensed from Columbia University . 3 Acquired from Trigemina ; license agreement with Stanford University 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 5 Co - exclustive license agreement with French National Institute of Health and Medical Research ( Inserm ) 6 TN X - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was completed outside of the U.S. 7 Acquired from TRImaran Pharma; license agreement with Wayne State University
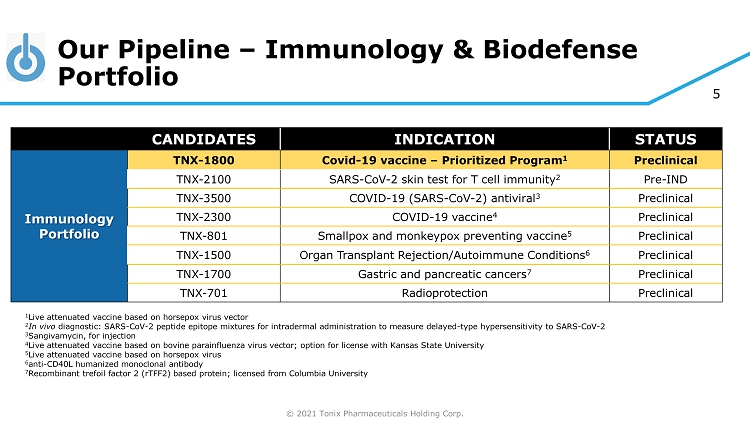
© 2021 Tonix Pharmaceuticals Holding Corp. 5 Our Pipeline – Immunology & Biodefense Portfolio CANDIDATES INDICATION STATUS Immunology Portfolio TNX - 1800 Covid - 19 vaccine – Prioritized Program 1 Preclinical TNX - 2100 SARS - CoV - 2 skin test for T cell immunity 2 Pre - IND TNX - 3500 COVID - 19 (SARS - CoV - 2) antiviral 3 Preclinical TNX - 2300 COVID - 19 vaccine 4 Preclinical TNX - 801 Smallpox and monkeypox preventing vaccine 5 Preclinical TNX - 1500 Organ Transplant Rejection/Autoimmune Conditions 6 Preclinical TNX - 1700 Gastric and pancreatic cancers 7 Preclinical TNX - 701 Radioprotection Preclinical 1 Live attenuated vaccine based on horsepox virus vector 2 In vivo diagnostic: SARS - CoV - 2 peptide epitope mixtures for intradermal administration to measure delayed - type hypersensitivity to SARS - CoV - 2 3 Sangivamycin, for injection 4 Live attenuated vaccine based on bovine parainfluenza virus vector; option for license with Kansas State University 5 Live attenuated vaccine based on horsepox virus 6 anti - CD40L humanized monoclonal antibody 7 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University
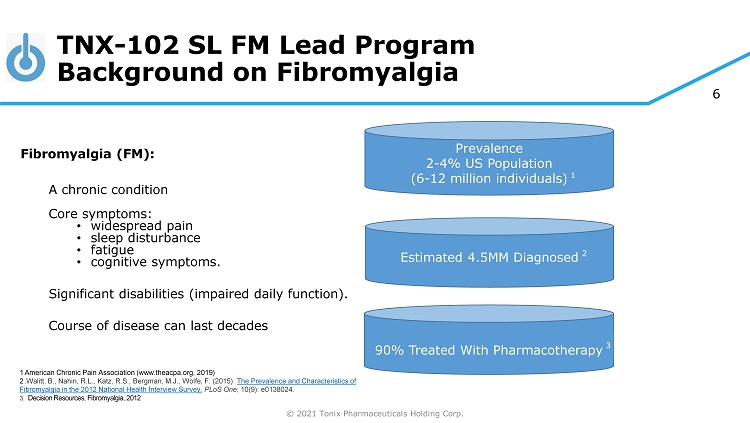
© 2021 Tonix Pharmaceuticals Holding Corp. 6 TNX - 102 SL FM Lead Program Background on Fibromyalgia Fibromyalgia (FM): A chronic condition Core symptoms: • widespread pain • sleep disturbance • fatigue • cognitive symptoms. Significant disabilities (impaired daily function). Course of disease can last decades 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 . Walitt, B., Nahin, R.L., Katz, R.S., Bergman, M.J., Wolfe, F. (2015). The Prevalence and Characteristics of Fibromyalgia in the 2012 National Health Interview Survey. PLoS One; 10(9): e0138024. 3. Decision Resources, Fibromyalgia, 2012 Prevalence 2 - 4% US Population (6 - 12 million individuals) 1 90% Treated With Pharmacotherapy 3 Estimated 4.5MM Diagnosed 2
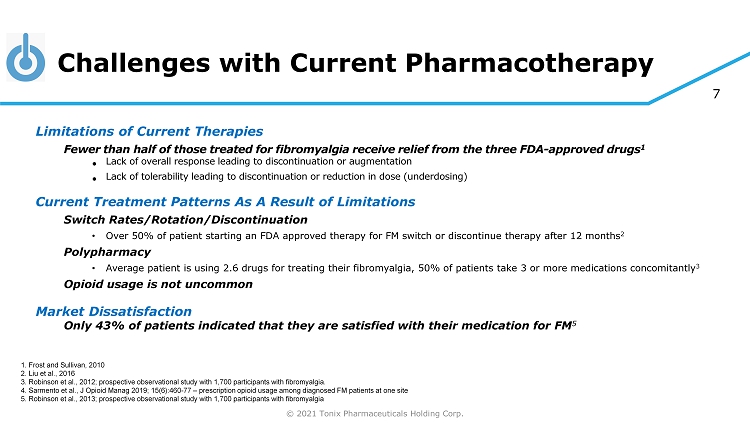
© 2021 Tonix Pharmaceuticals Holding Corp. 7 Challenges with Current Pharmacotherapy Limitations of Current Therapies Fewer than half of those treated for fibromyalgia receive relief from the three FDA - approved drugs 1 • Lack of overall response leading to discontinuation or augmentation • Lack of tolerability leading to discontinuation or reduction in dose (underdosing) Current Treatment Patterns As A Result of Limitations Switch Rates/Rotation/Discontinuation • Over 50% of patient starting an FDA approved therapy for FM switch or discontinue therapy after 12 months 2 Polypharmacy • Average patient is using 2.6 drugs for treating their fibromyalgia, 50% of patients take 3 or more medications concomitantly 3 Opioid usage is not uncommon Market Dissatisfaction Only 43% of patients indicated that they are satisfied with their medication for FM 5 1. Frost and Sullivan, 2010 2. Liu et al., 2016 3. Robinson et al., 2012; prospective observational study with 1,700 participants with fibromyalgia. 4. Sarmento et al., J Opioid Manag 2019; 15(6):460 - 77 – prescription opioid usage among diagnosed FM patients at one site 5. Robinson et al., 2013; prospective observational study with 1,700 participants with fibromyalgia
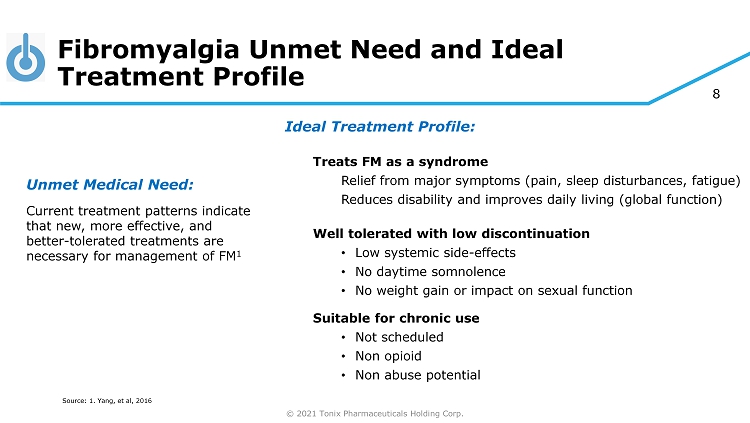
© 2021 Tonix Pharmaceuticals Holding Corp. 8 Fibromyalgia Unmet Need and Ideal Treatment Profile Unmet Medical Need: Current treatment patterns indicate that new, more effective, and better - tolerated treatments are necessary for management of FM 1 Source: 1. Yang, et al, 2016 Ideal Treatment Profile: Treats FM as a syndrome Relief from major symptoms (pain, sleep disturbances, fatigue) Reduces disability and improves daily living (global function) Well tolerated with low discontinuation • Low systemic side - effects • No daytime somnolence • No weight gain or impact on sexual function Suitable for chronic use • Not scheduled • Non opioid • Non abuse potential

© 2021 Tonix Pharmaceuticals Holding Corp. 9 TNX - 102 SL: Engineered to Treat FM This unique formulation of cyclobenzaprine has been designed to optimize delivery and absorption, while minimizing the potential residual effects of oral formulations of cyclobenzaprine. Innovative and proprietary Protectic ® delivery technology • Overcomes mucosal absorption barrier • Allows sublingual (SL) administration to achieves relevant systemic drug exposure • Stable SL tablet formulation • Benefits of sublingual delivery • Rapid drug exposure following nighttime administration • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite No recognized abuse or dependency concerns
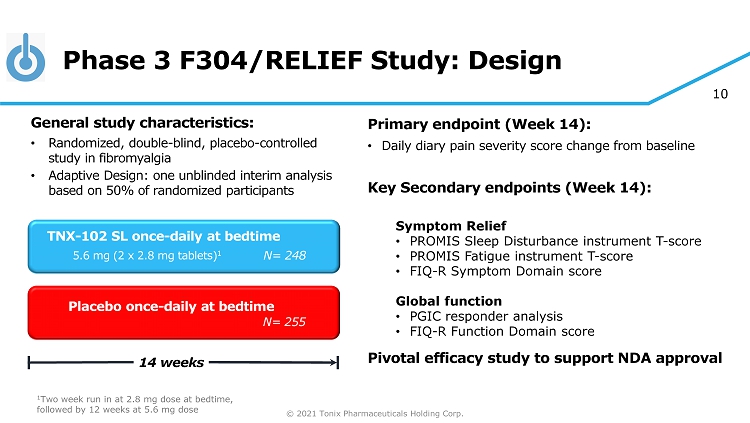
© 2021 Tonix Pharmaceuticals Holding Corp. 10 Phase 3 F304/RELIEF Study: Design Primary e ndpoint (Week 14) : • Daily diary pain severity score change from baseline Key Secondary e ndpoint s (Week 14): Symptom Relief • PROMIS Sleep Disturbance instrument T - score • PROMIS Fatigue instrument T - score • FIQ - R Symptom Domain score Global function • PGIC responder analysis • FIQ - R Function Domain score Pivotal efficacy study to support NDA approval Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) 1 General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia • Adaptive Design: one unblinded interim analysis based on 50% of randomized participants 1 Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose N= 255 N= 248
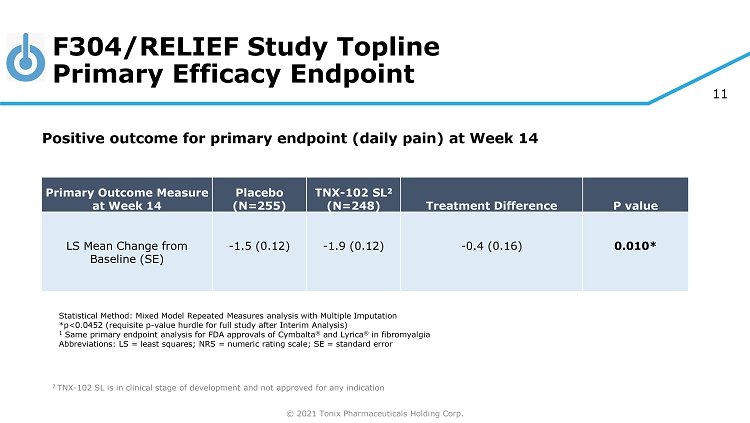
© 2021 Tonix Pharmaceuticals Holding Corp. 11 F304/RELIEF Study Topline Primary Efficacy Endpoint ◊ ◊ 2 TNX - 102 SL is in clinical stage of development and not approved for any indication Primary Outcome Measure at Week 14 Placebo (N=255) TNX - 102 SL 2 (N=248) Treatment Difference P value LS Mean Change from Baseline (SE) - 1.5 (0.12) - 1.9 (0.12) - 0.4 (0.16) 0.010* Statistical Method: Mixed Model Repeated Measures analysis with Multiple Imputation *p<0.0452 (requisite p - value hurdle for full study after Interim Analysis) 1 Same primary endpoint analysis for FDA approvals of Cymbalta ® and Lyrica ® in fibromyalgia Abbreviations: LS = least squares; NRS = numeric rating scale; SE = standard error Positive outcome for primary endpoint (d aily pain) at Week 14
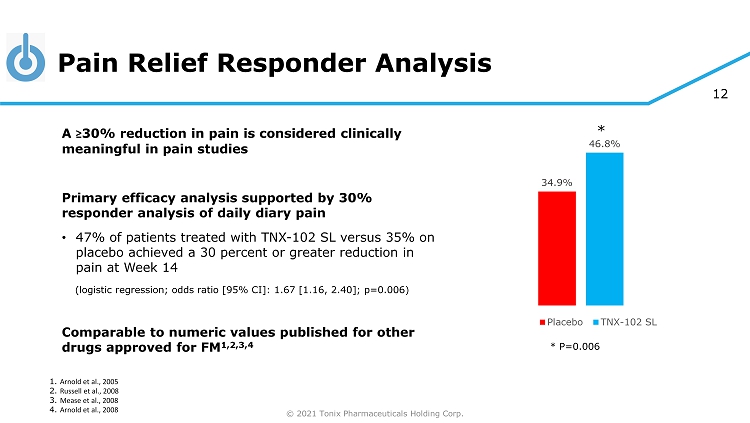
© 2021 Tonix Pharmaceuticals Holding Corp. 12 Pain Relief Responder Analysis 34.9% 46.8% Placebo TNX-102 SL 1. Arnold et al., 2005 2. Russell et al., 2008 3. Mease et al., 2008 4. Arnold et al., 2008 A ≥ 30% reduction in pain is considered clinically meaningful in pain studies Primary efficacy analysis supported by 30% responder analysis of daily diary pain • 47% of patients treated with TNX - 102 SL versus 35% on placebo achieved a 30 percent or greater reduction in pain at Week 14 (logistic regression; odds ratio [95% CI]: 1.67 [1.16, 2.40]; p=0.006) Comparable to numeric values published for other drugs approved for FM 1,2,3,4 * P=0.006 *
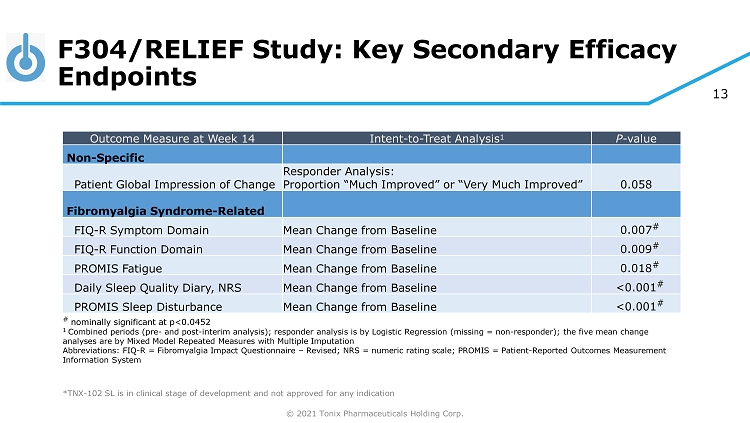
© 2021 Tonix Pharmaceuticals Holding Corp. 13 F304/RELIEF Study: Key Secondary Efficacy Endpoints ◊ ◊ *TNX - 102 SL is in clinical stage of development and not approved for any indication Outcome Measure at Week 14 Intent - to - Treat Analysis 1 P - value Non - Specific Patient Global Impression of Change Responder Analysis: Proportion “Much Improved” or “Very Much Improved” 0.058 Fibromyalgia Syndrome - Related FIQ - R Symptom Domain Mean Change from Baseline 0.007 # FIQ - R Function Domain Mean Change from Baseline 0.009 # PROMIS Fatigue Mean Change from Baseline 0.018 # Daily Sleep Quality Diary, NRS Mean Change from Baseline <0.001 # PROMIS Sleep Disturbance Mean Change from Baseline <0.001 # # nominally significant at p<0.0452 1 Combined periods (pre - and post - interim analysis); responder analysis is by Logistic Regression (missing = non - responder); the f ive mean change analyses are by Mixed Model Repeated Measures with Multiple Imputation Abbreviations: FIQ - R = Fibromyalgia Impact Questionnaire – Revised; NRS = numeric rating scale; PROMIS = Patient - Reported Outcomes Measurement Information System
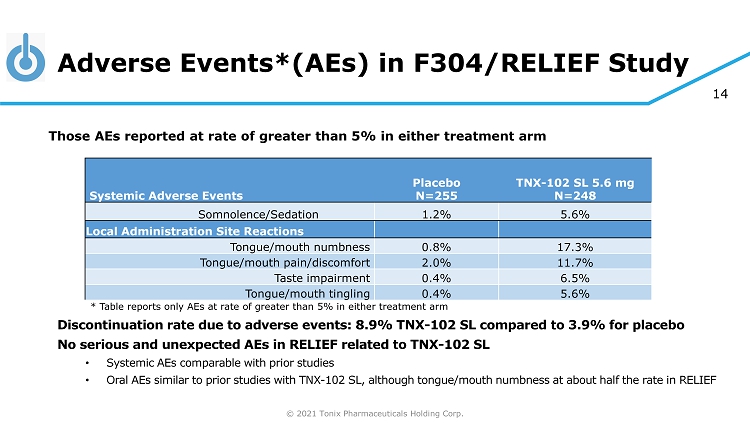
© 2021 Tonix Pharmaceuticals Holding Corp. 14 Adverse Events*(AEs) in F304/RELIEF Study Discontinuation rate due to adverse events: 8.9% TNX - 102 SL compared to 3.9% for placebo No serious and unexpected AEs in RELIEF related to TNX - 102 SL • Systemic AEs comparable with prior studies • Oral AEs similar to prior studies with TNX - 102 SL, although tongue/mouth numbness at about half the rate in RELIEF Those AEs reported at rate of greater than 5% in either treatment arm Systemic Adverse Events Placebo N=255 TNX - 102 SL 5.6 mg N=248 Somnolence/Sedation 1.2% 5.6% Local Administration Site Reactions Tongue/mouth numbness 0.8% 17.3% Tongue/mouth pain/discomfort 2.0% 11.7% Taste impairment 0.4% 6.5% Tongue/mouth tingling 0.4% 5.6% * Table reports only AEs at rate of greater than 5% in either treatment arm
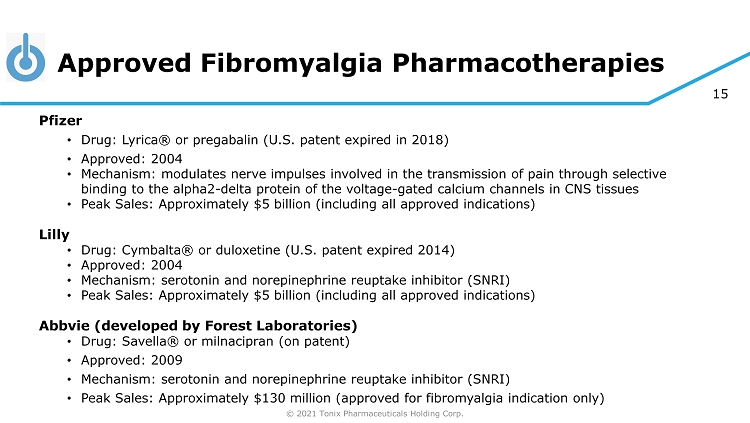
© 2021 Tonix Pharmaceuticals Holding Corp. 15 Approved Fibromyalgia Pharmacotherapies Pfizer • Drug: Lyrica® or pregabalin (U.S. patent expired in 2018) • Approved: 2004 • Mechanism: modulates nerve impulses involved in the transmission of pain through selective binding to the alpha2 - delta protein of the voltage - gated calcium channels in CNS tissues • Peak Sales: Approximately $5 billion (including all approved indications) Lilly • Drug: Cymbalta® or duloxetine (U.S. patent expired 2014) • Approved: 2004 • Mechanism: serotonin and norepinephrine reuptake inhibitor (SNRI) • Peak Sales: Approximately $5 billion (including all approved indications) Abbvie (developed by Forest Laboratories) • Drug: Savella ® or milnacipran (on patent) • Approved: 2009 • Mechanism: serotonin and norepinephrine reuptake inhibitor (SNRI) • Peak Sales: Approximately $130 million (approved for fibromyalgia indication only)

© 2021 Tonix Pharmaceuticals Holding Corp. 16 TNX - 102 SL Intellectual Property – U.S. Protection expected until 2035 • United States Patent and Trademark Office (USPTO) issued United States Patent No. 9636408 in May 2017, Patent No. 9956188 in May 2018, Patent No. 10117936 in November 2018, and Patent No. 10864175 on December 2020 • European Patent Office (EPO) issued European Patent No. 2968992 in December 2019 (validated in 37 countries). Opposition filed in October 2020 by Hexal AG • China National Intellectual Property Administration issued Chinese Patent No. ZL 201480024011.1 in April 2019 • Japanese Patent Office (JPO) issued Japanese Patent No. 6310542 in March 2018 • 8 granted patents (Indonesia, Saudi Arabia, New Zealand, Australia, Mexico, Taiwan, Israel, South Africa) • 11 patent applications pending (1 being allowed in Canada) Composition of matter (eutectic): Protection expected to 2034/2035 • New Zealand Intellectual Property Office issued New Zealand Patent No. 631144 in March 2017 and Patent No. 726488 in January 2019 • Taiwanese Intellectual Property Office issued Taiwanese Patent No. I590820 in July 2017, Patent No. I642429 in December 2018 and Patent No. I683660 in February 2020 • Australian Patent Office issued Australian Patent No. 2013274003 in October 2018 and Patent No. 2018241128 in September 2020 • JPO issued Japanese Patent No. 6259452 in December 2017 • 20 patent applications pending (1 being allowed in Mexico) Composition of matter (sublingual): Protection expected to 2033

© 2021 Tonix Pharmaceuticals Holding Corp. 17 TNX - 102 SL for FM: Next Steps 2 nd Phase 3 study, RALLY (F306) • Same protocol design as RELIEF study but with 200 more patients • Enrollment began in September 2020 • Interim cohort recruited in March 2021 • Interim analysis results expected in 3 rd quarter 2021 1 • Topline results expected in 4 th quarter of 2021 Following positive results from RALLY, an NDA could potentially be filed in 2022 • Long term safety exposure studies completed in PTSD patients expected to support the FM NDA • GMP manufacturing processes mature and 36 - month stability established 1 Pending agreement from FDA on statistical analysis plan

© 2021 Tonix Pharmaceuticals Holding Corp. 18 COVID - 19 Vaccines with Emergency Use Authorization (EUA): Still Uncertainty Durability of protection • Are vaccinated people protected one year later? • Need for annual vaccinations with mRNA vaccines? • Durable protection is associated with T cell response Detecting and mitigating vaccine failure • Need a strategy for identifying individuals at risk after vaccination and second line vaccines Protection against forward transmission • Highly contagious nature of CoV - 2 is a major problem driving pandemic No biomarker of protection • No test to establish protection from vaccination Current and future variants • Unknown effectiveness of existing vaccines
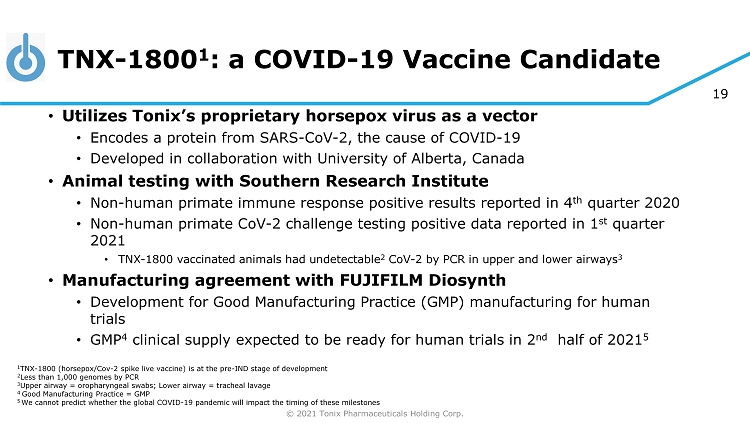
© 2021 Tonix Pharmaceuticals Holding Corp. 19 TNX - 1800 1 : a COVID - 19 Vaccine Candidate • Utilizes Tonix’s proprietary horsepox virus as a vector • Encodes a protein from SARS - CoV - 2, the cause of COVID - 19 • Developed in collaboration with University of Alberta, Canada • Animal testing with Southern Research Institute • Non - human primate immune response positive results reported in 4 th quarter 2020 • Non - human primate CoV - 2 challenge testing positive data reported in 1 st quarter 2021 • TNX - 1800 vaccinated animals had undetectable 2 CoV - 2 by PCR in upper and lower airways 3 • Manufacturing agreement with FUJIFILM Diosynth • Development for Good Manufacturing Practice (GMP) manufacturing for human trials • GMP 4 clinical supply expected to be ready for human trials in 2 nd half of 2021 5 1 TNX - 1800 (horsepox/Cov - 2 spike live vaccine) is at the pre - IND stage of development 2 Less than 1,000 genomes by PCR 3 Upper airway = oropharyngeal swabs; Lower airway = tracheal lavage 4 Good Manufacturing Practice = GMP 5 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones
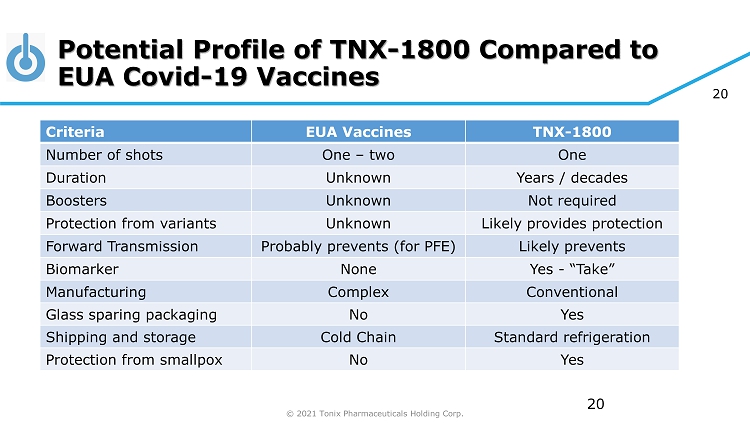
© 2021 Tonix Pharmaceuticals Holding Corp. 20 Potential Profile of TNX - 1800 Compared to EUA Covid - 19 Vaccines 20 Criteria EUA Vaccines TNX - 1800 Number of shots One – two One Duration Unknown Years / decades Boosters Unknown Not required Protection from variants Unknown Likely provides protection Forward Transmission Probably prevents (for PFE) Likely prevents Biomarker None Yes - “Take” Manufacturing Complex Conventional Glass sparing packaging No Yes Shipping and storage Cold Chain Standard refrigeration Protection from smallpox No Yes
© 2021 Tonix Pharmaceuticals Holding Corp. 21 TNX - 3500 1 : SARS - CoV - 2 Antiviral for the Treatment of COVID - 19 TNX - 3500 (sangivamycin) – potential monotherapy antiviral 2 • Licensed from OyaGen , April 2021 • Demonstrated broad spectrum antiviral (nanomolar activity against SARS - CoV - 2, MERS, Ebola, and Lassa) • Demonstrated human tolerability for chronic dosing from US National Cancer Institute studies 3 • 65 times more potent than remdesivir in inhibiting SARS - CoV - 2 in cell culture infectivity studies (dose to achieve IC 90 ) 4 Potential COVID - 19 combination therapy with remdesivir • TNX - 3500 antiviral effect is additive when combined with remdesivir and reduces the amount of each drug necessary for an IC 90 • Combination therapies for other viruses have reduced the emergence of drug resistant viral strains Development plans • 2 nd quarter 2021: Plan to initiate animal pharmacokinetic and efficacy studies • 2 nd half 2021: Plan to submit IND 1 TNX - 3500 is in the pre - IND stage of development and has not been approved for any indication. 2 Bennett, RP et al., Viruses . 2020 13(1):52. doi : 10.3390/v13010052. 3 Cavins JA et al., Cancer Chemotherapy Reports . 1967. 51(4) 4 Data on file, live virus BSL - 4 testing conducted by NIAID in collaboration with OyaGen
© 2021 Tonix Pharmaceuticals Holding Corp. 22 TNX - 2100 1 : Potential Skin Test to Measure SARS - CoV - 2 Exposure and T Cell Immunity TNX - 2100 (SARS - CoV - 2 epitope peptide mixtures for intradermal administration) • Designed to elicit delayed - type hypersensitivity (DTH) in individuals who have been exposed to SARS - CoV - 2 or who have been successfully vaccinated • Potential to measure the presence and strength of functional in vivo T cell immunity Potentially scalable test for widespread use • Adaptive Biotech’s T Detect Œ COVID received FDA EUA – based on genetic analysis of T cell receptors • Other tests 2 for T cell immunity to SARS - CoV - 2 require specialized laboratories and are not amenable to standardization Development plans • 2 nd quarter 2021: Plan to submit IND based on FDA feedback • 2 nd half 2021: Plan to initiate clinical testing pending approval of IND 1 TNX - 2100 is in the pre - IND stage of development and has not been approved for any indication. 2 Intracellular cytokine staining (ICS) measured by flow cytometry after in vitro stimulation of purified peripheral blood mono nuc lear cells

© 2021 Tonix Pharmaceuticals Holding Corp. 23 TNX - 1300: Cocaine Esterase (CocE) CocE is the most potent known catalyst for cocaine degradation • Natural bacterial CocE is unstable at body temperature Thermostable bacterial CocE (active for ~6 hours at body temperature) • Targeted mutations stabilize CocE • Natural bacterial CocE is unstable at body temperature Phase 2 open - label safety study of TNX - 1300 in emergency department setting for cocaine intoxication ) • Initiation of enrollment anticipated 2 nd quarter 2021
© 2021 Tonix Pharmaceuticals Holding Corp. 24 TNX - 1900 (Intranasal Potentiated Oxytocin) for the Treatment of Migraine Intranasal oxytocin(OT) has potential utility in treating migraine 1 • Intranasal ( i.n. ) OT reaches the trigeminal ganglion • Preclinical evidence of OT blocking CGRP release and suppressing pain transmission • CGRP antagonists and antibodies approved for the treatment of migraine • Association of low oxytocin levels during and preceding migraine episodes TNX - 1900 is an intranasal formulation of magnesium and OT • Magnesium is known to potentiate the binding of oxytocin to its receptor 2 Submission of IND application in 3 rd quarter 2021 and initiation of Phase 2 study for treatment of chronic migraine anticipated in 3 rd quarter 2021 1. Tzabazis et al., 22017 2. Antoni and Chadio, 1989

© 2021 Tonix Pharmaceuticals Holding Corp. 25 TNX - 2900 ( i.n. Potentiated OT) for the Treatment of Prader - Willi Syndrome Prader - Willi syndrome is the most common genetic cause of life - threatening childhood obesity 1 • Results in lack of suckling in infants and, in children and adults, severe hyperphagia, an overriding physiological drive to eat, leading to severe obesity and other complications associated with significant mortality • No approved treatment for either the suckling deficit in babies or the obesity and hyperphagia in older children associated with Prader - Willi syndrome. • Orphan disease occurring in approximately one in 15,000 births Intranasal OT has been shown to improve suckling in newborn animals but also suppresses feeding behaviors in adult animal models • Tonix’s patented potentiated oxytocin formulation is believed to increase specificity for OT receptors relative to vasopressin receptors Tonix intends to submit applications to the FDA for Orphan Drug and Fast Track designations for TNX - 2900 1 Foundation for Prader - Willi Research (fpwr.org).

© 2021 Tonix Pharmaceuticals Holding Corp. 26 TNX - 601 CR* (Tianeptine Oxalate and Naloxone HCl Controlled Release) Tablets for the Treatment of Major Depressive Disorder (MDD) Proprietary new controlled release formulation for once - daily dosing • Expect to open IND with a Human Abuse Potential study pending IND clearance • Pending toxicology results, expect to start Phase 2 study in 4Q 2021 • Suitability for once - daily dosing established in Phase 1 pharmacokinetic study, completed outside of the U.S. • Well tolerated in study and side effects were consistent with the known safety profile of tianeptine sodium • Tianeptine sodium immediate release is approved and marketed outside of the U.S. for three times a day dosing for the treatment of depression • Once - daily dosing for TNX - 601 CR believed to have an adherence advantage over three times a day dosing with tianeptine sodium Proprietary new oxalate salt with improved pharmaceutical properties • Tianeptine oxalate is crystalline, while tianeptine sodium is amorphous Issued patents directed to tianeptine and tianeptine oxalate • Composition of Matter: Issued US patent directed to oxalate salt, U.S. Patent No. 10,449,203 and 10,946,027 • Method of Use: Issued European patent directed to methods of treating cognitive impairment associated with corticosteroid treatment, European Patent No. 3246031 *TNX - 601 CR (tianeptine oxalate and naloxone HCl controlled release tablets) is in the pre - IND stage in the U.S. and has not bee n approved for any indication.
© 2021 Tonix Pharmaceuticals Holding Corp. 27 TNX - 601 CR : A Potential Treatment for Depression TNX - 601 CR’s proposed mechanism of action is completely distinct from any approved antidepressant in the U.S. • Antidepressant activity is believed to relate to indirect modulation of the glutamatergic system • Known to modulate AMPA receptor trafficking and to promote synaptic plasticity in the hippocampus under conditions of stress or corticosteroid use. • Tianeptine sodium is reported to have prominent anti - anxiety effects in depression with a low incidence of sexual side effects • TNX - 601 CR leverages the established efficacy and safety of tianeptine sodium IR as a treatment for depression outside of the U.S. Significant interest and need for new treatments, particularly for medicines that modulate the glutamatergic system • Majority suffering from depression do not have an adequate response to initial antidepressant therapy • Recently Spravato ® ( esketamine ) a glutamine system modulator was approved for the treatment of depression with Breakthrough Therapy designation
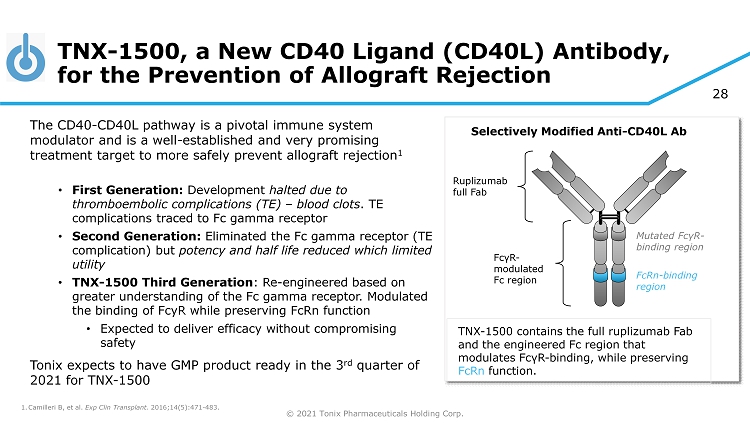
© 2021 Tonix Pharmaceuticals Holding Corp. 28 TNX - 1500, a New CD40 Ligand (CD40L) Antibody, for the Prevention of Allograft Rejection The CD40 - CD40L pathway is a pivotal immune system modulator and is a well - established and very promising treatment target to more safely prevent allograft rejection 1 • First Generation: Development halted due to thromboembolic complications (TE) – blood clots . TE complications traced to Fc gamma receptor • Second Generation: Eliminated the Fc gamma receptor (TE complication) but potency and half life reduced which limited utility • TNX - 1500 Third Generation : Re - engineered based on greater understanding of the Fc gamma receptor. Modulated the binding of FcyR while preserving FcRn function • Expected to deliver efficacy without compromising safety Tonix expects to have GMP product ready in the 3 rd quarter of 2021 for TNX - 1500 1. Camilleri B, et al. Exp Clin Transplant. 2016;14(5):471 - 483. Ruplizumab full Fab Selectively Modified Anti - CD40L Ab TNX - 1500 contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region
© 2021 Tonix Pharmaceuticals Holding Corp. 29 Milestones – Upcoming 1 Data 3 rd Quarter 2021 Interim analysis of TNX - 102 SL Phase 3 F306/RALLY study in fibromyalgia expected 4 th Quarter 2021 Topline data from TNX - 102 SL Phase 3 F306/RALLY study in fibromyalgia expected IND Submissions – 4 expected to be submitted this year □ 2 nd Quarter 2021 Submission of IND application for TNX - 2100 for SARS - CoV - 2 skin test expected □ 3 rd Quarter 2021 Submission of IND application for TNX - 1900 for the treatment of migraine expected □ 3 rd Quarter 2021 Submission of IND for TNX - 601 CR expected □ 2 nd Half 2021 Submission of IND for TNX - 3500 expected Clinical Trial Initiations – 5 expected to be initiated this year □ 2 nd Quarter 2021 Initiation of Phase 2 OL safety study of TNX - 1300 in ED setting for cocaine intoxication expected □ 3 rd Quarter 2021 Initiation of Phase 2 study of TNX - 1900 for the treatment of migraine expected □ 4 th Quarter 2021 Initiation of Phase 2 study of TNX - 601 for the treatment of major depressive disorder expected □ 2 nd Half 2021 Initiation of Phase 1 safety study of TNX - 1800 for COVID - 19 expected □ 2 nd Half 2021 Initiation of clinical trials for TNX - 2100 SARS - CoV - 2 skin test expected 1 We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones.

© 2021 Tonix Pharmaceuticals Holding Corp. 30 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger , CPA Chief Financial Officer

© 2021 Tonix Pharmaceuticals Holding Corp. 31 Thank You!