Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01

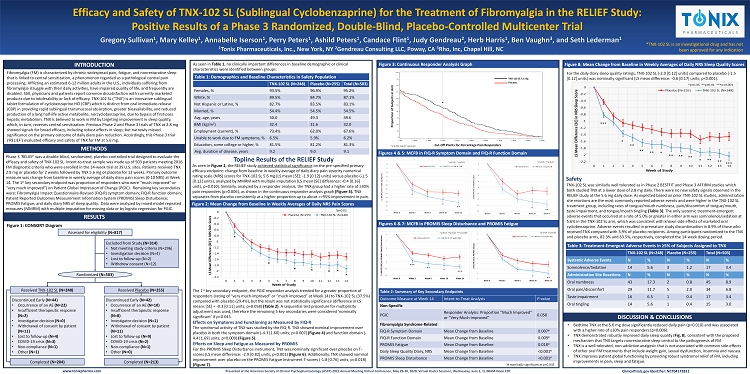
RESULTS DISCUSSION & CONCLUSIONS Figures 6 & 7: MCFB in PROMIS Sleep Disturbance and PROMIS Fatigue Figures 4 & 5: MCFB in FIQ - R Symptom Domain and FIQ - R Function Domain Figure 2: Mean Change from Baseline in Weekly Averages of Daily NRS Pain Scores Figure 3: Continuous Responder Analysis Graph Figure 8: Mean Change from Baseline in Weekly Averages of Daily NRS Sleep Quality Scores Efficacy and Safety of TNX - 102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia in the RELIEF Study: Positive Results of a Phase 3 Randomized, Double - Blind, Placebo - Controlled Multicenter Trial Gregory Sullivan 1 , Mary Kelley 1 , Annabelle Iserson 1 , Perry Peters 1 , Ashild Peters 1 , Candace Flint 1 , Judy Gendreau 2 , Herb Harris 1 , Ben Vaughn 3 , and Seth Lederman 1 1 Tonix Pharmaceuticals, Inc., New York, NY 2 Gendreau Consulting LLC, Poway, CA 3 Rho, Inc, Chapel Hill, NC INTRODUCTION Presented at the American Society of Clinical Psychopharmacology (ASCP) 2021 Annual Meeting Virtual Conference, May 29 - 30, 2020; Virtual Poster Session I, Wednesday, June 2, 11:00AM - Noon EDT www.tonixpharma.com *TNX - 102 SL is an investigational drug and has not been approved for any indication METHODS Fibromyalgia (FM) is characterized by chronic widespread pain, fatigue, and nonrestorative sleep that is linked to central sensitization, a phenomenon regarded as a pathological central pain processing. Afflicting an estimated 6 - 12 million adults in the U.S., individuals suffering from fibromyalgia struggle with their daily activities, have impaired quality of life, and frequently are disabled. Still, physicians and patients report common dissatisfaction with currently marketed products due to intolerability or lack of efficacy. TNX - 102 SL (‘TNX’) is an innovative sublingual tablet formulation of cyclobenzaprine HCl (CBP) which is distinct from oral immediate - release (CBP) in providing rapid sublingual transmucosal absorption, greater bioavailability, and reduced production of a long half - life active metabolite, norcyclobenzaprine, due to bypass of first - pass hepatic metabolism. TNX is believed to work in FM by targeting improvement in sleep quality, which, in turn, reverses central sensitization. Previous Phase 2 and Phase 3 trials of TNX at 2.8 mg showed signals for broad efficacy, including robust effects in sleep, but narrowly missed significance on the primary outcome of daily diary pain reduction. Accordingly, this Phase 3 trial (‘RELIEF’) evaluated efficacy and safety of TNX for FM at 5.6 mg. Phase 3 ‘RELIEF’ was a double - blind, randomized, placebo - controlled trial designed to evaluate the efficacy and safety of TNX - 102 SL. Intent - to - treat sample was made up of 503 patients meeting 2016 FM diagnostic criteria who were enrolled in the 14 - week trial at 39 U.S. sites. Patients received TNX 2.8 mg or placebo for 2 weeks followed by TNX 5.6 mg or placebo for 12 weeks. Primary outcome measure was change from baseline in weekly average of daily diary pain scores (0 - 10 NRS) at Week 14. The 1 st key secondary endpoint was proportion of responders who were “much improved” or “very much improved”) on Patient Global Impression of Change (PGIC). Remaining key secondaries were: Fibromyalgia Impact Questionnaire - Revised (FIQ - R) symptom domain; FIQ - R function domain; Patient Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance; PROMIS Fatigue; and daily diary NRS of sleep quality. Data were analyzed by mixed model repeated measures (MMRM) with multiple imputation for missing data or by logistic regression for PGIC. • Bedtime TNX at the 5.6 mg dose significantly reduced daily pain (p=0.010) and was associated with a higher rate of ≥30% pain responders (p=0.006). • TNX demonstrated robustly improved daily sleep quality ( Fig. 8 ), consistent with the proposed mechanism that TNX targets nonrestorative sleep central to the pathogenesis of FM • TNX is a well tolerated, non - addictive analgesic that is not associated with common side - effects of other oral FM treatments that include weight gain, sexual dysfunction, insomnia and nausea. • TNX improves patient global functioning by providing robust syndromal relief of FM, including improvements in pain, sleep and fatigue. Table 1: Demographics and Baseline Characteristics in Safety Population TNX - 102 SL (N=248) Placebo (N=255) Total (N=503) Females, % 93.5% 96.9% 95.2% White, % 89.5% 84.7% 87.1% Not Hispanic or Latino, % 82.7% 83.5% 83.1% Married, % 54.4% 54.5% 54.5% Avg. age, years 50.0 49.3 49.6 BMI (kg/m 2 ) 32.4 31.6 32.0 Employment (current), % 73.4% 62.0% 67.6% Unable to work due to FM symptoms, % 6.5% 5.9% 6.2% Education, some college or higher, % 81.5% 81.2% 81.3% Avg. duration of disease, years 9.2 9.0 9.1 Figure 1: CONSORT Diagram TNX - 102 SL was similarly well tolerated as in Phase 2 BESTFIT and Phase 3 AFFIRM studies which both studied TNX at a lower dose of 2.8 mg daily. There were no new safety signals observed in the RELIEF study at the 5.6 mg daily dose. As expected based on prior TNX - 102 SL studies, administration site reactions are the most commonly reported adverse events and were higher in the TNX - 102 SL treatment group, including rates of tongue/mouth numbness, pain/discomfort of tongue/mouth, taste impairment, and tongue/mouth tingling (Table 3) . The only systemic treatment - emergent adverse events that occurred at a rate of 5.0% or greater in either arm was somnolence/sedation at 5.6% in the TNX - 102 SL arm, which was consistent with known side effects of marketed oral cyclobenzaprine. Adverse events resulted in premature study discontinuation in 8.9% of those who received TNX compared with 3.9% of placebo recipients. Among participants randomized to the TNX and placebo arms, 82.3% and 83.5%, respectively, completed the 14 - week dosing period. Assessed for eligibility (N= 817 ) Excluded from Study (N= 314 ) • Not meeting study criteria (N=296) • Investigation decision (N=4) • Lost to follow - up (N=2) • Withdrew consent (N=12) Randomized (N=503) Received TNX - 102 SL (N=248) Received Placebo (N=255) Discontinued Early (N=44) • Occurrence of an AE (N=22) • Insufficient therapeutic response (N=2) • Investigator dec ision (N=0) • Withdrawal of consent by patient (N=11) • Lost to follow - up (N=4) • COVID - 19 crisis (N=3) • Non - compliance (N=1) • Other (N=1) Discontinued Early (N=42) • Occurrence of an AE (N=10) • Insufficient therapeutic response (N=8) • Investigator decision (N=1) • Withdrawal of consent by patient (N=11) • Lost to follow - up (N=9) • COVID - 19 crisis (N=2) • Non - compliance (N=1) • Other (N=0) Completed (N=204) Completed (N=213) The 1 st key secondary endpoint, the PGIC responder analysis trended for a greater proportion of responders (rating of “very much improved” or “much improved” at Week 14) to TNX - 102 SL (37.5%) compared with placebo (29.4%), but the result was not statistically significance (difference in LS means [SE] = - 0.3 [0.11] units; p=0.058) (Table 2) . A sequential test procedure for multiplicity adjustment was used, therefore the remaining 5 key secondaries were considered ‘nominally significant’ if p<0.045. Safety As seen in Figure 2 , the RELIEF study achieved statistical significance on the pre - specified primary efficacy endpoint: change from baseline in weekly average of daily diary pain severity numerical rating scale (NRS) scores for TNX - 102 SL 5.6 mg (LS mean [SE]: - 1.9 [0.12] units) versus placebo ( - 1.5 [0.12] units), analyzed by MMRM with multiple imputation (LS mean [SE] difference: - 0.4 [0.16] units, p=0.010). Similarly, analyzed by a responder analysis, the TNX group had a higher rate of ≥30% pain responders (p=0.006), as shown in the continuous responder analysis graph (Figure 3). TNX separates from placebo consistently at a higher proportion up to about >=95% improvement in pain. Topline Results of the RELIEF Study For the PROMIS Sleep Disturbance instrument, TNX was nominally significant over placebo on T - scores (LS mean difference: - 2.9 [0.82] units; p<0.001) (Figure 6) . Additionally, TNX showed nominal improvement over placebo on the PROMIS Fatigue instrument T - scores ( - 1.8 [0.76] units; p=0.018) (Figure 7) . The syndromal activity of TNX was studied by the FIQ - R. TNX showed nominal improvement over placebo in both the symptom domain ( - 4.3 [1.60] units; p=0.007) (Figure 4) and function domain ( - 4.4 [1.69] units; p=0.009) (Figure 5) . Effects on Sleep and Fatigue as Measured by PROMIS Effects on Symptoms and Functioning as Measured by FIQ - R * * ** *p<0.05 **p<0.01 For the daily diary sleep quality ratings, TNX - 102 SL ( - 2.0 [0.12] units) compared to placebo ( - 1.5 [0.12] units) was nominally significant (LS mean difference: - 0.6 [0.17] units; p<0.001). *p<0.05 **p<0.01 * ** Cut - Off Points for Percentage Pain Responders Proportion of Subjects in Arm Placebo TNX - 102 SL 5.6 mg Table 2: Summary of Key Secondary Endpoints Outcome Measure at Week 14 Intent - to - Treat Analysis P - value Non - Specific PGIC Responder Analysis: Proportion “Much Improved” or “Very Much Improved” 0.058 Fibromyalgia Syndrome - Related FIQ - R Symptom Domain Mean Change from Baseline 0.007 # FIQ - R Function Domain Mean Change from Baseline 0.009 # PROMIS Fatigue Mean Change from Baseline 0.018 # Daily Sleep Quality Diary, NRS Mean Change from Baseline <0.001 # PROMIS Sleep Disturbance Mean Change from Baseline <0.001 # # nominally significant at p<0.045 Table 3: Treatment - Emergent Adverse Events in ≥5% of Subjects Assigned to TNX TNX - 102 SL (N=248) Placebo (N=255) Total (N=503) Systemic Adverse Events N % N % N % Somnolence/Sedation 14 5.6 3 1.2 17 3.4 Administration Site Reactions N % N % N % Oral numbness 43 17.3 2 0.8 45 8.9 Oral pain/discomfort 29 11.7 5 2.0 34 6.8 Taste impairment 16 6.5 1 0.4 17 3.4 Oral tingling 14 5.6 1 0.4 15 3.0 *p<0.05 **p<0.01 * ** *** ** * *** *p<0.05 **p<0.01 ***p<0.001 *p=0.006, logistic regression * ClinicalTrials.gov Identifier: NCT04172831 *** * ** ** ** * ** ** ** * ** ** *** As seen in Table 1 , no clinically important differences in baseline demographic or clinical characteristics were identified between groups.