Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.01

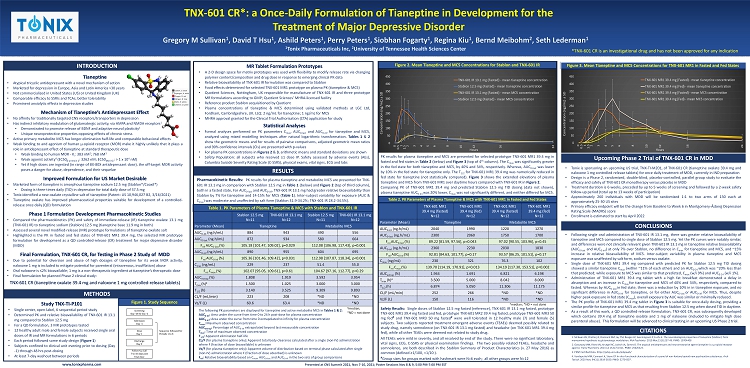
TNX - 601 CR*: a Once - Daily Formulation of Tianeptine in Development for the Treatment of Major Depressive Disorder Gregory M Sullivan 1 , David T Hsu 1 , Ashild Peters 1 , Perry Peters 1 , Siobhan Fogarty 1 , Regina Kiu 1 , Bernd Meibohm 2 , Seth Lederman 1 1 Tonix Pharmaceuticals Inc, 2 University of Tennessee Health Sciences Center *TNX - 601 CR is an investigational drug and has not been approved for any indication INTRODUCTION Tianeptine • A typical tricyclic antidepressant with a novel mechanism of action • M arketed for depression in Europe, Asia and Latin America > 30 years • Not commercialized in United States (US) or United Kingdom (UK) • Comparable efficacy to SSRIs and TCAs ; better tolerability • P rominent anxiolytic effects in depression studies Mechanism of Tianeptine’s Antidepressant Effect • No affinity for traditionally targeted CNS receptors/transporters in depression • Has i ndirect inhibitory modulation of glutamatergic activity, via AMPA and NMDA receptors 1 • Demonstrated to promote release of BDNF and adaptive neural plasticity 1 • Unique n europrotective properties opposing effects of chronic stress • Active primary metabolite MC 5 has longer elimination half - life and comparable behavioral effects • W eak binding to and agonism of human µ - opioid receptor (MOR) make it highly unlikely that it plays a role in antidepressant effect of tianeptine at standard therapeutic dose • Weak binding to human MOR - K i : 383 nM 2 ; 768 nM 3 • W eak agonist activity 4 (EC 50 β - arrestin 2 : 3262 nM ; EC 50 Mini - Gi : > 1 x 10 4 nM ) • Yet if high doses are ingested (in range of 8 X - 80 X antidepressant dose), the off - target MOR activity poses a danger for abuse, dependence, and their sequelae Improved Formulation for US Market Desirable • Marketed form of tianeptine is amorphous tianeptine sodium 12 . 5 mg (Stablon®/ Coaxil ®) • Dosing is three - times daily (TID) in depression for total daily dose of 37 . 5 mg • Tonix identified a new oxalate crystalline salt of tianeptine (Patent : US 10 , 946 , 027 B 2 , 3 / 16 / 2021 ) • Tianeptine oxalate has improved pharmaceutical properties suitable for development of a controlled - release once daily ( QD) formulation Phase 1 Formulation Development Pharmacokinetic Studies • C ompared the pharmacokinetics (PK) and safety of immediate - release (IR) tianeptine oxalate 13 . 1 mg (TNX - 601 IR) to tianeptine sodium (Stablon) 12 . 5 mg (tianeptine base 11 . 9 mg in both) • Assessed several novel modified - release (MR) prototype formulations of tianeptine oxalate salt • Highlighted is the PK in fasted and fed states of TNX - 601 MR 1 39 . 4 mg, the selected MR prototype formulation for development as a QD controlled - release (CR) treatment for major depressive disorder (MDD) Final Formulation, TNX - 601 CR, for Testing in Phase 2 Study of MDD • Due to potential for diversion and abuse of high dosages of tianeptine for its weak MOR activity, naloxone 1 mg is included to mitigate potential for parenteral (intravenous, insufflation) abuse • Oral naloxone is ≤ 2 % bioavailable ; 1 mg is a non - therapeutic ingredient at tianeptine’s therapeutic dose • Final formulation for planned Phase 2 clinical study: TNX - 601 CR (tianeptine oxalate 39.4 mg and naloxone 1 mg controlled - release tablets) METHODS www.tonixpharma.com Figure 1. Study Sequence Study TNX - TI - P101 • S ingle center, open - label, 6 sequential period study • Determined PK and relative bioavailability of TNX - 601 IR 13 . 1 mg compared to Stablon 12 . 5 mg • For a QD formulation, 3 MR prototypes tested • 12 healthy adult male and female subjects received single oral doses of IR and MR formulations in 6 periods • Each period followed same study design ( Figure 1 ) • Subjects confined to clinical unit evening prior to dosing (Day - 1 ) through 48 hrs post - dosing • At least 7 - day washout between periods MR Tablet Formulation Prototypes • A 2 - D design space for m atrix prototypes was used with flexibility to modify release rate via changing polymer content/composition and drug dose in response to emerging clinical PK data • R elative bioavailability of TNX - 601 IR formulation was compared to Stablon • Food effects determined for selected TNX - 601 MR 1 prototype on plasma PK (tianeptine & MC 5 ) • Quotient Sciences, Nottingham, UK responsible for manufacture of TNX - 601 IR and three prototype MR formulations according to GMP ; Quotient Sciences’ MHRA - licensed facility • Reference product Stablon acquisitioned by Quotient • P lasma concentrations of tianeptine & MC 5 determined using validated methods at LGC Ltd, Fordham, Cambridgeshire , UK . LLQ : 2 ng/mL for tianeptine ; 1 ng/mL for MC 5 • MHRA approval granted for the Clinical Trial Authorisation (CTA) application for study Statistical Analyses • Formal analyses performed on PK parameters C max , AUC 0 - last and AUC 0 - inf for tianeptine and MC 5 , analyzed using mixed modelling techniques after natural logarithmic transformation . Tables 1 & 2 show the geometric means and for results of pairwise comparisons, a djusted geometric mean ratios and 90 % confidence intervals (CIs) are presented with p - values • For plasma PK concentrations in Figures 2 & 3 , arithmetic means and standard deviations are shown • S afety Population : all subjects who received ≥ 1 dose IP . Safety assessed by adverse events (AEs), Columbia Suicide Severity Rating Scale (C - SSRS), physical exams, vital signs, ECG and labs Table 1. PK Parameters of Plasma Tianeptine & MC5 with Stablon and TNX - 601 IR The following PK parameters are displayed for tianeptine and active metabolite MC5 in Tables 1 & 2 : AUC 0 - 24 : Area under the curve from time 0 to 24 h post - dose for plasma concentration AUC 0 - inf : Area under the curve from time 0 extrapolated to infinity for plasma concentration C max : Maximum observed concentration AUC extrap : Percentage of AUC 0 - inf extrapolated beyond last measurable concentration T max : Time of maximum observed concentration T 1/2 : Apparent elimination half - life CL/F (for plasma tianeptine only) : Apparent total body clearance calculated after a single (non - IV) administration where F (fraction of dose bioavailable) is unknown Vz /F (for plasma tianeptine only) : Apparent volume of distribution based on terminal phase calculated after single (non - IV) administration where F (fraction of dose absorbed) is unknown F rel : Relative bioavailability based on C max , AUC 0 - last and AUC 0 - inf in the two sets of group comparisons Pharmacokinetic Results : PK results for plasma tianeptine and metabolite MC 5 are presented for TNX - 601 IR 13 . 1 mg in comparison with Stablon 12 . 5 mg in Table 1 (below) and Figure 2 (top of third column), both in a fasted state . For AUC 0 - last and AUC 0 - inf , TNX - 601 IR 13 . 1 mg had greater relative bioavailability than Stablon by 7 % for tianeptine and 14 - 15 % for MC 5 ( Table 1 ) . Inter - subject variability for exposure (AUC & C max ) was moderate and unaffected by salt form (Stablon 31 . 9 - 34 . 2 % ; TNX - 601 IR 28 . 2 - 34 . 5 % ) . Stablon 12.5 mg N=11 TNX - 601 IR 13.1 mg N=12 Stablon 12.5 mg N=11 TNX - 601 IR 13.1 mg N=12 Parameter (Mean) Tianeptine Metabolite MC5 AUC 0 - 24 ( ng.h /mL) 884 943 490 556 AUC 0 - last ( ng.h /mL) 872 934 580 664 F rel AUC 0 - last (%) 105.18 [101.47, 109.02], p=0.029 112.08 [106.98, 117.43], p=0.001 AUC 0 - inf ( ng.h /mL) 890 955 604 695 F rel AUC 0 - inf (%) 105.36 [101.46, 109.42], p=0.031 112.98 [107.87, 118.34], p<0.001 C max (ng/mL) 229 237 51.4 55.0 F rel C max (%) 102.07 [95.05, 109.61], p=0.61 104.67 [97.16, 112.77], p=0.29 AUC extrap (%) 1.808 1.919 3.592 3.954 T max (h) a 1.500 1.025 3.000 3.000 T 1/2 (h) 3.140 3.525 9.309 9.893 CL/F (mL/min) 223 208 *ND *ND Vz /F (L) 60.6 63.4 *ND *ND RESULTS Presented at CNS Summit 2021, Nov 7 - 10, 2021; Poster Sessions Nov 8 & 9, 5:00 PM - 7:00 PM EST Figure 2. Mean Tianeptine and MC5 Concentrations for Stablon and TNX - 601 IR PK results for plasma tianeptine and MC 5 are presented for selected prototype TNX - 601 MR 1 39 . 4 mg in fasted and fed states in Table 2 (below) and Figure 3 (top of 4 th column) . The C max was significantly greater in the fed state for both tianeptine and MC 5 , by 40 % and 34 % , respectively . Whereas, AUC 0 - last was lower by 10 % in the fed state for tianeptine only . The T 1 / 2 for TNX - 601 MR 1 39 . 4 mg was numerically reduced in fed state for tianeptine (not statistically compared) . Figure 3 shows the extended elevations of plasma tianeptine and MC 5 from TNX - 601 MR 1 over daytime hours compared with TNX - 601 IR in Figure 2 . Comparing PK of TNX - 601 MR 1 39 . 4 mg and predicted Stablon 12 . 5 mg TID dosing (data not shown), plasma tianeptine AUC 0 - 24 was 20 % lower, C max was not significantly different, and neither differed for MC 5 . Table 2. PK Parameters of Plasma Tianeptine & MC5 with TNX - 601 MR1 in Fasted and Fed States TNX - 601 MR1 39.4 mg (fasted) N=12 TNX - 601 MR1 39.4 mg (fed) N=12 TNX - 601 MR1 39.4 mg (fasted) N=12 TNX - 601 MR1 39.4 mg (fed) N=12 Parameter (Mean) Tianeptine Metabolite MC5 AUC 0 - 24 ( ng.h /mL) 2040 1990 1220 1270 AUC 0 - last ( ng.h /mL) 2300 2060 1750 1700 F rel AUC 0 - last (%) 89.22 [81.59, 97.56], p=0.043 97.02 [90.55, 103.96], p=0.45 AUC 0 - inf ( ng.h /mL) 2360 2230 2030 1830 F rel AUC 0 - inf (%) 92.81 [84.63, 101.77], p=0.17 93.57 [86.25, 101.51], p=0.17 C max (ng/mL) 230 321 76.3 102 F rel C max (%) 139.70 [114.19, 170.91], p=0.013 134.19 [117.30, 153.51], p=0.002 AUC extrap (%) 1.944 1.691 6.821 6.198 T max (h) a 3.500 5.000 8.042 8.000 T 1/2 (h) 6.874 5.050 11.306 11.175 CL/F (mL/min) 252 266 *ND *ND Vz /F (L) 150 116 *ND *ND Safety Results : Single doses of Stablon 12 . 5 mg fasted (reference), TNX - 601 IR 13 . 1 mg fasted, prototype TNX - 601 MR 1 39 . 4 mg fasted and fed, prototype TNX - 601 MR 2 39 . 4 mg fasted, prototype TNX - 601 MR 3 50 mg fed # and TNX - 601 MR 3 50 mg fasted # were well tolerated in 12 healthy male ( 7 ) and female ( 5 ) subjects . Two subjects reported treatment emergent adverse events (TEAEs) deemed possibly related to study drug, namely somnolence (on TNX - 601 IR 13 . 1 mg fasted) and headache (on TNX - 601 MR 1 39 . 4 mg fed), while all other TEAEs were deemed not related to study drug . All TEAEs were mild in severity, and all resolved by end of the study . There were no significant laboratory, vital signs, ECG, C - SSRS or physical examination findings . The two possibly related TEAEs, headache and somnolence, are both described in the Stablon Summary of Product Characteristics (v . 27 May 2016 ) as common (defined ≥ 1 / 100 , < 1 / 10 ) . # Group sizes for groups marked with hashmark were N= 6 each ; all other groups were N= 12 Upcoming Phase 2 Trial of TNX - 601 CR in MDD CONCLUSIONS • Following single oral administration of TNX - 601 IR 13 . 1 mg, there was greater relative bioavailability of tianeptine and MC 5 compared to single dose of Stablon 12 . 5 mg . Yet the PK curves were notably similar, and differences were not clinically relevant given TNX - 601 IR 13 . 1 mg in tianeptine relative bioavailability (AUC 0 - last and AUC 0 - inf ) at only 7 % over Stablon , no difference in C max for tianeptine and MC 5 , and ~ 15 % increase in relative bioavailability of MC 5 . Inter - subject variability in plasma tianeptine and MC 5 exposure was unaffected by salt form, sodium versus oxalate . • S ingle dose of TNX - 601 MR 1 39 . 4 mg compared with predicted PK for Stablon 12 . 5 mg TID dosing showed a similar tianeptine C max (within ~ 11 % of each other) and an AUC 0 - 24 which was ~ 20 % less than that predicted ; while exposure to MC 5 was similar to that predicted, C max (w/ i 9 % ) and AUC 0 - 24 (w/ i 1 % ) . • Administration of TNX - 601 MR 1 39 . 4 mg tablet with a high fat breakfast demonstrated a delay in absorption and an increase in C max for t ianeptine and MC 5 of 40 % and 34 % , respectively, compared to fasted . Whereas by AUC 0 - last in f ed state, there was a reduction by 10 % in in tianeptine exposure, and no significant difference in AUC 0 - inf for tianeptine, or for either AUC 0 - last or AUC 0 - inf for MC 5 . Thus, despite higher peak exposure in fed state ( C max ), overall exposure by AUC was similar or minimally reduced . • The PK profile of TNX - 601 MR 1 39 . 4 mg tablet in Figure 3 is suitable for once - daily dosing, providing a similar profile for tianeptine and MC 5 as that resulting from Stablon 12 . 5 mg when dosed TID in daytime . • As a result of this work, a QD controlled - release formulation, TNX - 601 CR, was subsequently developed which contains 39 . 4 mg of tianeptine oxalate and 1 mg of naloxone (included to mitigate high dose parenteral abuse) . This formulation will be advanced to clinical testing in an upcoming US Phase 2 trial . • Tonix is sponsoring an upcoming US trial, TNX - TI - M 201 , of TNX - 601 CR (tianeptine oxalate 39 . 4 mg and naloxone 1 mg controlled - release tablets) for once daily treatment of MDD, currently in IND preparation • Design is a Phase 2 , randomized, double - blind, placebo - controlled, parallel group study to evaluate the efficacy and safety of TNX - 601 CR monotherapy versus placebo in MDD • Treatment duration is 6 weeks, preceded by up to 5 weeks of screening and followed by a 2 - week safety follow - up period (total up to 13 weeks of participation) • Approximately 260 individuals with MDD will be randomized 1 : 1 to two arms of 130 each at approximately 25 - 30 US sites • Primary efficacy endpoint will be the change from Baseline to Week 6 in Montgomery - Åsberg Depression Rating Scale (MADRS) score • Enrollment is estimated to start by April 2022 CITATIONS 1. McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P, Fuchs E. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry . 2010 Mar;15(3):237 - 49. PMID: 19704408 2. Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D. The atypical antidepressant and neurorestorative agent tianeptine is a μ - opioid receptor agonist. Transl Psychiatry . 2014 Jul 15;4(7):e411. PMID: 25026323. 3. PDSP Certified Data. https://pdsp.unc.edu/pdspweb/ 4. Vandeputte MM, Cannaert A, Stove CP. In vitro functional characterization of a panel of non - fentanyl opioid new psychoactive substances. Arch Toxicol . 2020 Nov; 94(11):3819 - 3830. PMID: 32734307. a median; *ND = not done a median; *ND = not done Figure 3. Mean Tianeptine and MC5 Concentrations for TNX - 601 MR1 in Fasted and Fed States