
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2021 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P0330 November 23, 2021 (Doc 0937)

2 © 2021 Tonix Pharmaceuticals Holding Corp. CAUTIONARY NOTE ON FORWARD - LOOKING STATEMENTS Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2020, as filed with the Securities and Exchange Commission (the “SEC”) on March 15, 2021, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2021 Tonix Pharmaceuticals Holding Corp. ADVANCING THE SCIENCE AND UNDERSTANDING OF DISEASES by developing innovative therapies that improve population health by focusing on unmet needs in patient care WHAT WE DO Using our integrated development engine, we advance innovative programs across multiple therapeutic areas into the clinic while maximizing asset potential OUR MISSION OUR STRATEGY
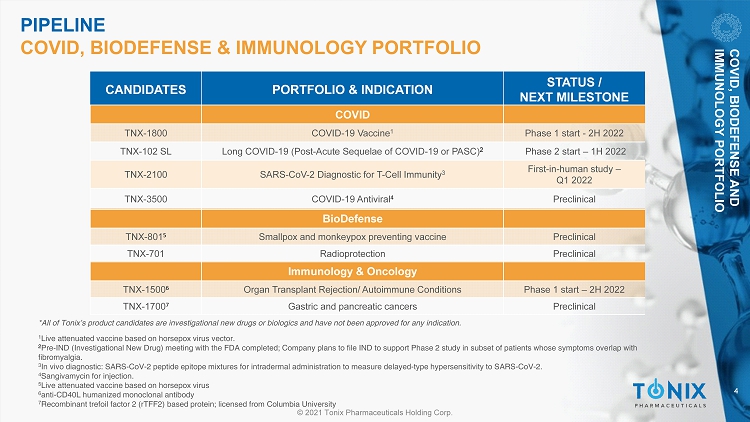
4 © 2021 Tonix Pharmaceuticals Holding Corp. *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Live attenuated vaccine based on horsepox virus vector. 2 Pre - IND (Investigational New Drug) meeting with the FDA completed; Company plans to file IND to support Phase 2 study in subset of patients whose symptoms overlap with fibromyalgia. 3 In vivo diagnostic: SARS - CoV - 2 peptide epitope mixtures for intradermal administration to measure delayed - type hypersensitivity to SARS - CoV - 2. 4 Sangivamycin for injection. 5 Live attenuated vaccine based on horsepox virus 6 anti - CD40L humanized monoclonal antibody 7 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University PIPELINE COVID, BIODEFENSE & IMMUNOLOGY PORTFOLIO CANDIDATES PORTFOLIO & INDICATION STATUS / NEXT MILESTONE Immunology & Oncology TNX - 1500 6 Organ Transplant Rejection/ Autoimmune Conditions Phase 1 start – 2H 2022 TNX - 1700 7 Gastric and pancreatic cancers Preclinical BioDefense TNX - 801 5 Smallpox and monkeypox preventing vaccine Preclinical TNX - 701 Radioprotection Preclinical COVID TNX - 1800 COVID - 19 Vaccine 1 Phase 1 start - 2H 2022 TNX - 102 SL Long COVID - 19 (Post - Acute Sequelae of COVID - 19 or PASC) 2 Phase 2 start – 1H 2022 TNX - 2100 SARS - CoV - 2 Diagnostic for T - Cell Immunity 3 First - in - human study – Q1 2022 TNX - 3500 COVID - 19 Antiviral 4 Preclinical COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
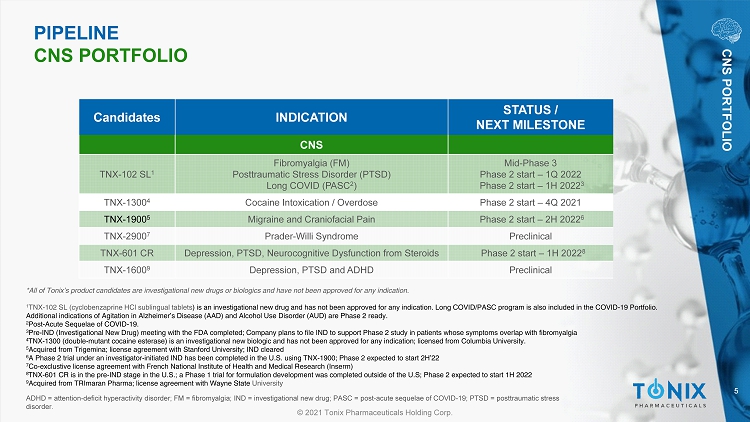
5 © 2021 Tonix Pharmaceuticals Holding Corp. ADHD = attention - deficit hyperactivity disorder; FM = fibromyalgia; IND = investigational new drug; PASC = post - acute sequelae o f COVID - 19; PTSD = posttraumatic stress disorder. Candidates INDICATION STATUS / NEXT MILESTONE CNS TNX - 102 SL 1 Fibromyalgia (FM) Posttraumatic Stress Disorder (PTSD) Long COVID (PASC 2 ) Mid - Phase 3 Phase 2 start – 1Q 2022 Phase 2 start – 1H 2022 3 TNX - 1300 4 Cocaine Intoxication / Overdose Phase 2 start – 4Q 2021 TNX - 1900 5 Migraine and Craniofacial Pain Phase 2 start – 2H 2022 6 TNX - 2900 7 Prader - Willi Syndrome Preclinical TNX - 601 CR Depression, PTSD, Neurocognitive Dysfunction from Steroids Phase 2 start – 1H 2022 8 TNX - 1600 9 Depression, PTSD and ADHD Preclinical PIPELINE CNS PORTFOLIO CNS PORTFOLIO *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets ) is an investigational new drug and has not been approved for any indication. Long COVID/PASC program is also included in th e C OVID - 19 Portfolio. Additional indications of Agitation in A lzheimer’s Disease (AAD) and Alcohol Use D isorder (AUD) are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 Pre - IND (Investigational New Drug) meeting with the FDA completed; Company plans to file IND to support Phase 2 study in patient s whose symptoms overlap with fibromyalgia 4 TNX - 1300 (double - mutant cocaine esterase) is an investigational new biologic and has not been approved for any indication; licensed from Columbia University . 5 Acquired from Trigemina ; license agreement with Stanford University; IND cleared 6 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900; Phase 2 expected to start 2H’ 22 7 Co - exclustive license agreement with French National Institute of Health and Medical Research ( Inserm ) 8 TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was completed outside of the U.S; Ph ase 2 expected to start 1H 2022 9 Acquired from TRImaran Pharma; license agreement with Wayne State University

© 2021 Tonix Pharmaceuticals Holding Corp. KEY CANDIDATES: COVID, BIODEFENSE & IMMUNOLOGY
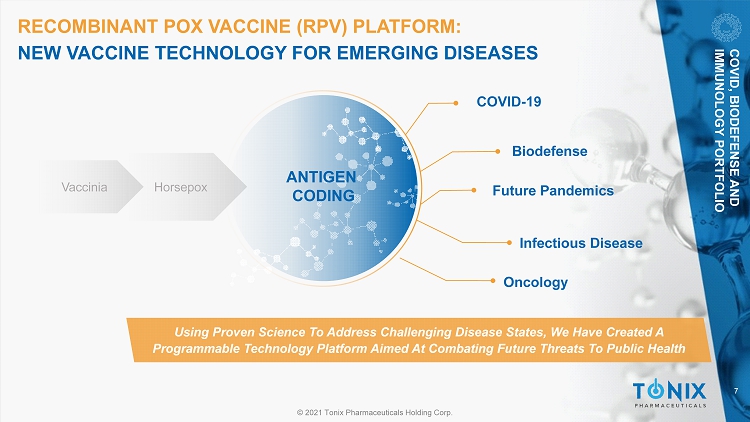
7 © 2021 Tonix Pharmaceuticals Holding Corp. RECOMBINANT POX VACCINE (RPV) PLATFORM: NEW VACCINE TECHNOLOGY FOR EMERGING DISEASES COVID - 19 Future Pandemics Infectious Disease Biodefense Using Proven Science To Address Challenging Disease States, We Have Created A Programmable Technology Platform Aimed At Combating Future Threats To Public Health Vaccinia Horsepox ANTIGEN CODING COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO Oncology
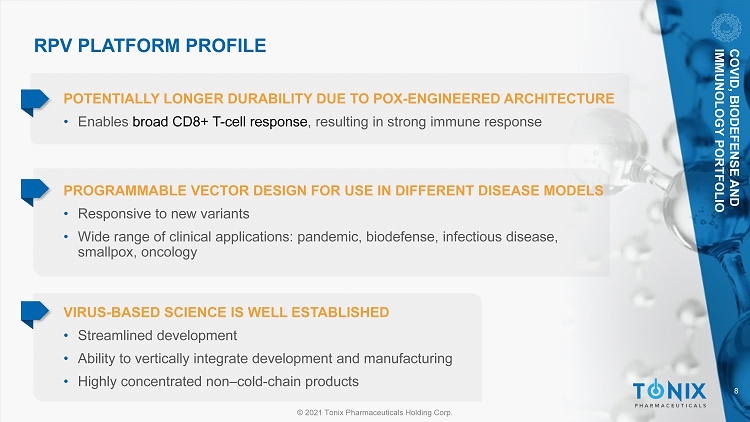
8 © 2021 Tonix Pharmaceuticals Holding Corp. VIRUS - BASED SCIENCE IS WELL ESTABLISHED • Streamlined development • Ability to vertically integrate development and manufacturing • Highly concentrated non – cold - chain products RPV PLATFORM PROFILE POTENTIALLY LONGER DURABILITY DUE TO POX - ENGINEERED ARCHITECTURE • Enables broad CD8+ T - cell response , resulting in strong immune response PROGRAMMABLE VECTOR DESIGN FOR USE IN DIFFERENT DISEASE MODELS • Responsive to new variants • Wide range of clinical applications: pandemic, biodefense, infectious disease, smallpox, oncology COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
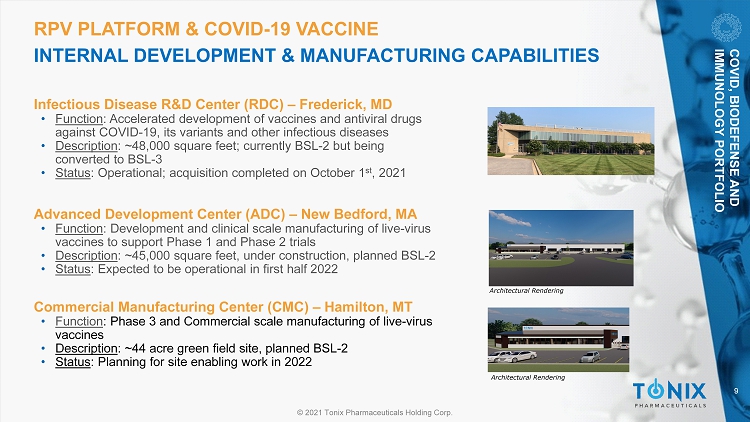
9 © 2021 Tonix Pharmaceuticals Holding Corp. RPV PLATFORM & COVID - 19 VACCINE INTERNAL DEVELOPMENT & MANUFACTURING CAPABILITIES Infectious Disease R&D Center (RDC) – Frederick, MD • Function : Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description : ~48,000 square feet; currently BSL - 2 but being converted to BSL - 3 • Status : Operational; acquisition completed on October 1 st , 2021 Advanced Development Center (ADC) – New Bedford, MA • Function : Development and clinical scale manufacturing of live - virus vaccines to support Phase 1 and Phase 2 trials • Description : ~45,000 square feet, under construction, planned BSL - 2 • Status : Expected to be operational in first half 2022 Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of live - virus vaccines • Description : ~44 acre green field site, planned BSL - 2 • Status : Planning for site enabling work in 2022 Architectural Rendering Architectural Rendering COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
10 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1800 COVID - 19 VACCINE RPV PLATFORM DEVELOPMENT PROGRAM ESTABLISHES RPV PLATFORM • Encodes a protein from SARS - CoV - 2, the cause of COVID - 19 • Provides a novel, variant - reflexive alternative to mRNA products ANIMAL TESTING WITH SOUTHERN RESEARCH INSTITUTE • Non - human primate immune response: positive results reported in Q4 2020 • Non - human primate CoV - 2 challenge testing: positive data reported in Q1 2021 MANUFACTURING AGREEMENT WITH FUJIFILM DIOSYNTH • Development for GMP manufacturing for human trials • GMP clinical supply expected to be ready for human trials in 2H 2022 DEVELOPMENT PROGRAM Market Entry: COVID - 19 Vaccine Additional Indications: Future Pandemic, Infectious Disease, Smallpox, Cancer Status: Preclinical Next Steps: 2H 2022 Initiate Phase 1 Study COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO Patents Filed
11 © 2021 Tonix Pharmaceuticals Holding Corp. RPV PLATFORM: WHAT MAKES TNX - 1800 DIFFERENT FROM MRNA VACCINES CRITERIA mRNA VACCINES TNX - 1800* Number of shots Two One Duration 8 months Years / decades Boosters Recommended Likely not required Protection from variants Variants Expected Forward transmission Unknown for variants like delta Likely prevents Biomarker None Yes – “Take” Manufacturing Complex Conventional Glass - sparing packaging No Yes Shipping and storage Cold chain Standard refrigeration Protection from smallpox No Yes COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO * Characterizations of TNX - 1800 show in table represent expectations.
12 © 2021 Tonix Pharmaceuticals Holding Corp. IMPORTANCE OF TESTING PROTECTIVE IMMUNITY • Personalized approach to determine need for vaccine boosters • More cost effective • Reduces risk with unnecessary vaccination • One - size - fits - all booster strategy is expensive and likely unsustainable US TRENDS IN COVID - 19 VACCINE BOOSTER DEVELOPMENT CURRENT US GOVERNMENT STANCE IS BOOSTERS MAY BE NEEDED POST - PFIZER OR MODERNA VACCINATION 1 • CDC, FDA, White House, COVID - 19 Response Team stated that immunity wanes and booster vaccines should be used in certain cases • FDA has authorized and CDC approved a single booster shot of the Pfizer - BioNTech and Moderna COVID - 19 vaccines for Americans age 18 and older, six months after second dose • FDA has authorized a single booster shot of the J&J vaccine for everyone who received the initial J&J vaccine two or more months ago COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO 1 www.cdc.gov/media/releases/2021/s0818 - covid - 19 - booster - shots.html
13 © 2021 Tonix Pharmaceuticals Holding Corp. TWO TYPES OF IMMUNITY • Antibodies – can be measured in a blood test, but anti - SARS - CoV - 2 antibodies are not predictive of protection • T cell – can be measured in a blood test, but requires sophisticated lab, unknown if predictive ASSESSING ANTI - SARS - COV - 2 PROTECTIVE IMMUNITY FUNCTIONAL T CELL IMMUNITY • in vivo – classic skin test – correlation with protection under investigation 2,3 NEUTRALIZING ANTIBODIES – APPEAR TO CORRELATE WITH PROTECTION 1 • Not part of standard antibody tests • Requires culture of antibodies with live SARS - CoV - 2; possibly “pseudo - type” assays 1 Krammer, F. (2021) Nature Medicine. 27:1145 – 1153. https://www.nature.com/articles/s41591 - 021 - 01432 - 4.pdf 2 Barrios, Y et al. Clinical Immunol. (2021) 226:108730 3 Barrios, Y et al. Vaccines (2021) 9:575 COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
14 © 2021 Tonix Pharmaceuticals Holding Corp. MEASURES THE PRESENCE AND STRENGTH OF FUNCTIONAL IN VIVO T - CELL IMMUNITY • Designed to elicit delayed - type hypersensitivity in individuals who have been exposed to SARS - CoV - 2 or successfully vaccinated • SARS - CoV - 2 epitope peptide mixtures for intradermal administration (Skin Test) TNX - 2100*: SARS - COV - 2 DIAGNOSTIC TO MEASURE T - CELL IMMUNITY *TNX - 2100 is in the pre - IND stage of development and has not been approved for any indication. † Intracellular cytokine staining (ICS) measured by flow cytometry after in vitro stimulation of purified peripheral blood mono nuc lear cells. DEVELOPMENT PLANS • Q1 2022: Plan to initiate first - in - human clinical testing • Patents filed POTENTIALLY SCALABLE FOR WIDESPREAD USE • Many tests † for T - cell immunity to SARS - CoV - 2 require specialized laboratories and are not amendable to standardization • Adaptive Biotech’s T Detect Œ COVID - 19 test received FDA EUA based on genetic analysis of T - cell receptors COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
15 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 3500*: COVID - 19 ANTIVIRAL TREATMENT SANGIVAMYCIN PROFILE New variants heighten need for therapeutics NIH Treatment Guidelines for COVID - 19 are mixed on use of remdesivir Potential monotherapy antiviral 1 • 65 times more potent than remdesivir in inhibiting SARS - CoV - 2 in cell culture infectivity studies (dose to achieve IC 90 ) Potential combination therapy with remdesivir 1 • TNX - 3500 antiviral effect is additive when combined with remdesivir and reduces the amount of each drug necessary for an IC 90 • Combination therapies for other viruses have reduced the emergence of drug resistant viral strains DEVELOPMENT PROGRAM Market Entry: COVID - 19 Antiviral Additional Indications: MERS, Ebola, Lassa, Oncology Status: Preclinical Next Steps: Q4 2021 Initiate Animal Studies Patents Filed *TNX - 3500 is in the pre - IND stage of development and has not been approved for any indication. 1. Bennett RP et al. Viruses . 2020;13(1):52. doi: 10.3390/v13010052. 2. Data on file, live virus BSL - 4 testing conducted by NIAID in collaboration with OyaGen. MERS = Middle East Respiratory Syndrome; NIH = National Institutes of Health; PK = pharmacokinetics. COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO 1 Bennett, RP et al. JCI Insight . 2021 in press preview 10.1172/jci.insight.153165
16 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: LONG COVID (PASC) CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS PROFILE Long COVID or Post - acute Sequelae of COVID - 19 (PASC 1 ) – What is it? • Symptoms can include fatigue, sleep disorders, pain, fevers, shortness of breath, cognitive impairment described as “brain fog”, gastrointestinal symptoms, anxiety, and depression 2 • Can persist for months and can range in severity from mild to incapacitating • Occurs in 30% of recovered COVID - 19 patients • Typically associated with moderate or severe COVID - 19, Long COVID can occur after mild COVID - 19 or even after asymptomatic SARS - CoV - 2 infection To address the urgent need for PASC therapies, Congress awarded the National Institutes of Health $1.15 billion to study Long COVID. 3 DEVELOPMENT PROGRAM Market Entry : Long COVID (PASC) Status: Clinical – pre - IND; FDA minutes from pre - IND meeting received in Q3 2021 Next Steps: Start Phase 2 study for treating subset of Long COVID patients whose symptoms overlap with fibromyalgia in 1H 2022 Patents Issued 1 Feb. 24, 2021 - White House COVID - 19 Response Team press briefing; Feb 25, 2021 - policy brief from the World Health Organizatio n on long COVID 2 Nalbandian, Ani, et al. "Post - acute COVID - 19 syndrome." Nature Medicine (2021): 1 - 15. 3 The NIH provision of Title III Health and Human Services, Division M -- Coronavirus Response and Relief Supplemental Appropriation s Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260. COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
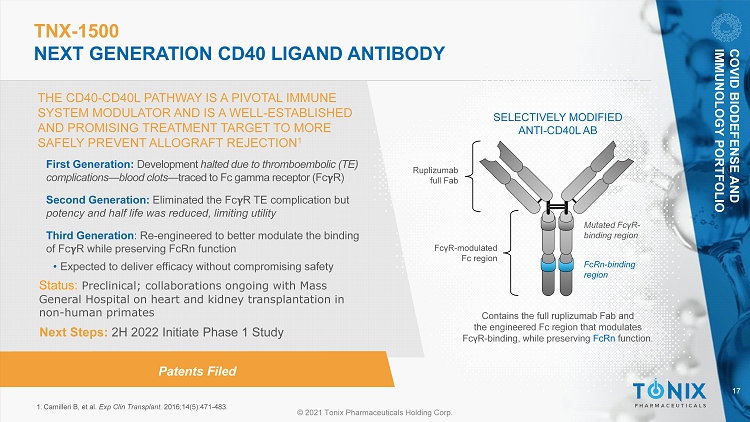
17 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1500 NEXT GENERATION CD40 LIGAND ANTIBODY THE CD40 - CD40L PATHWAY IS A PIVOTAL IMMUNE SYSTEM MODULATOR AND IS A WELL - ESTABLISHED AND PROMISING TREATMENT TARGET TO MORE SAFELY PREVENT ALLOGRAFT REJECTION 1 1. Camilleri B, et al. Exp Clin Transplant. 2016;14(5):471 - 483. SELECTIVELY MODIFIED ANTI - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region Patents Filed First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation : Re - engineered to better modulate the binding of Fc R while preserving FcRn function • Expected to deliver efficacy without compromising safety Status: Preclinical; collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates COVID BIODEFENSE AND IMMUNOLOGY PORTFOLIO Next Steps: 2H 2022 Initiate Phase 1 Study

© 2021 Tonix Pharmaceuticals Holding Corp. KEY CANDIDATES: CNS
19 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: FIBROMYALGIA CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS CNS PORTFOLIO A unique formulation of cyclobenzaprine designed to optimize delivery and absorption Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration • Lower daytime exposure • Avoids first - pass metabolism ⁃ Reduces risk of pharmacological interference from major metabolite Clinical trial program designed to examine treatment of core Fibromyalgia symptoms Market Entry: Fibromyalgia Additional Indications: PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder, Long COVID Status: One Positive Phase 3 study (RELIEF) Completed Next Steps: Second Phase 3 Study: Clinical phase completed; topline data expected 1Q 2022; Confirmatory Phase 3 planned 1H 2022 PROFILE DEVELOPMENT PROGRAM Patents Issued

20 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: FIBROMYALGIA CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS PROGRAM UPDATE Phase 3 Study, RALLY (F306) • July 2021: Tonix stopped enrollment in the RALLY study following an unblinded, pre planned interim analysis by the Independent Data Monitoring Committee (IDMC). • Based on interim analysis results of the first 50% (n=336) enrolled participants, the IDMC recommended stopping the trial as TNX - 102 SL is unlikely to demonstrate a statistically significant improvement in the primary endpoint. • Clinical phase of study completed, with 514 participants enrolled overall – 399 completers; topline results expected 1Q 2022 • Confirmatory Phase 3 study (F307) planned 1H 2022 Following analysis of F306 results, including pharmacogenetic comparison of F304 and F306, Tonix may modify F307 protocol TNX 102 - SL Development Beyond Fibromyalgia • Development efforts continue in PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder, Long COVID CNS PORTFOLIO
21 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 601 CR: PSYCHIATRY TIANEPTINE OXALATE AND NALOXONE CNS PORTFOLIO A novel, oral, controlled release once - daily tablet Mechanistically different from traditional monoaminergic treatments for depression Indirectly modulates the glutamatergic system • No direct binding to NMDA, AMPA, or kainate receptors Naloxone added to deter parenteral abuse Treatment effect of tianeptine in depression is well - established Market Entry: Major Depressive Disorder Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids Status: Clinical – pre - IND Next Steps: 1H 2022 Initiate Phase 2 Trial PROFILE DEVELOPMENT PROGRAM AMPA= α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid; MAOI=monoamine oxidase inhibitors; NMDA=N - methyl - D - aspartate. Patents Issued

22 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1900: MIGRAINE INTRANASAL POTENTIATED OXYTOCIN (OT) WITH MAGNESIUM CNS PORTFOLIO Intranasal OT has potential utility in treating migraine 1 • Intranasal OT reaches the trigeminal ganglion • Preclinical evidence of OT blocking CGRP release and suppressing pain • Association of low OT levels during and preceding migraine episodes • Novel non - CGRP antagonist approach to treatment Magnesium is known to potentiate the binding of OT to its receptor 2 One billion individuals worldwide suffer from migraines Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance Status: Clinical – IND cleared 3 Next Steps: 2H 2022 Initiate Phase 2 Trial Patents Issued PROFILE DEVELOPMENT PROGRAM 1. Tzabazis et al., 2017. 2. Antoni and Chadio, 1989. 3. A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 CGRP = calcitonin gene - related peptide.
23 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 2900: PRADER - WILLI SYNDROME INTRANASAL POTENTIATED OXYTOCIN (OT) WITH MAGNESIUM PROFILE CNS PORTFOLIO Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Orphan disease occurring in 1 in 15,000 births Symptoms include lack of suckling as infants, poor muscle strength, and constant hunger (hyperphagia) • In animal models, OT has improved suckling and suppressed hunger ‒ Tonix’s patented potentiated OT formulation is believed to increase specificity for OT receptors relative to off - target vasopressin receptors DEVELOPMENT PROGRAM Market Entry: Prader - Willi Syndrome Additional Indications: Rare, Orphan Hyperphagia Conditions Status: pre - IND Next Steps: Submit applications to the FDA for Orphan Drug and Fast Track designations for TNX - 2900 Patents Issued
24 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION COCAINE ESTERASE ( CoCe ) CNS PORTFOLIO PROFILE Cocaine is the main cause for drug - related ED visits 1 Cocaine use can cause irreversible structural damage to the heart and accelerate cardiovascular disease 2 • In one survey of 94 long - term cocaine users, 71% had some form of cardiovascular disease 3 CoCe is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes DEVELOPMENT PROGRAM Market Entry: Cocaine Intoxication Additional Indications: Cocaine Overdose Status: Phase 2 Open Label Next Steps: Q4 2021 Initiate Trial 1. Havakuk O et al. J Am Coll Cardiol . 2017;70:101 - 113. 2. Phillips K et al. Am J Cardiovasc Drugs . 2009;9:177 - 196. 3. Maceira AM et al. J Cardiovasc Magn Reson . 2014;16:26. ED = emergency department. FDA Breakthrough Therapy Designation Patents Issued

© 2021 Tonix Pharmaceuticals Holding Corp. FUTURE OUTLOOK
26 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND PANCREATIC CANCERS KEY DEVELOPMENT PARTNERS TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 1800: COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME

27 © 2021 Tonix Pharmaceuticals Holding Corp. Data □ 1 st Quarter 2022 Topline data from TNX - 102 SL Phase 3 F306/RALLY study in fibromyalgia expected Expected Clinical Trial Initiations □ 4 th Quarter 2021 Phase 2 OL safety study start of TNX - 1300 in ED setting for cocaine intoxication □ 1 st Quarter 2022 First - in - human clinical study start of TNX - 2100 for SARS - CoV - 2 skin test □ 1 st Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of PTSD in Kenya □ 1 st Half 2022 Phase 3 study start of TNX - 102 SL for the management of fibromyalgia □ 1 st Half 2022 Phase 2 study start of TNX - 102 SL for the treatment of Long COVID □ 1 st Half 2022 Phase 2 study start of TNX - 601 CR for the treatment of major depressive disorder □ 2 nd Half 2022 Phase 2 study start of TNX - 1900 for the treatment of migraine □ 2 nd Half 2022 Phase 1 study start of TNX - 1800 for COVID - 19 □ 2 nd Half 2022 Phase 1 study start of TNX - 1500 for prevention of allograft rejection MILESTONES: RECENTLY COMPLETED AND UPCOMING* * We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones. □ 4 th Quarter 2020 Positive topline data from TNX - 102 SL Phase 3 F304/RELIEF study in fibromyalgia reported □ 1 st Quarter 2021 Non - human primate positive efficacy data from TNX - 1800 in COVID - 19 models reported x x

28 © 2021 Tonix Pharmaceuticals Holding Corp. MANAGEMENT TEAM Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

© 2021 Tonix Pharmaceuticals Holding Corp. THANK YOU