
Tonix Pharmaceuticals Holding Corp. Form 8-K
Exhibit 99.2

© 2021 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P0332 December 14, 2021 (Doc 0945)

2 © 2021 Tonix Pharmaceuticals Holding Corp. CAUTIONARY NOTE ON FORWARD - LOOKING STATEMENTS Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2020, as filed with the Securities and Exchange Commission (the “SEC”) on March 15, 2021, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2021 Tonix Pharmaceuticals Holding Corp. ADVANCING THE SCIENCE AND UNDERSTANDING OF DISEASES by developing innovative therapies that improve population health by focusing on unmet needs in patient care WHAT WE DO Using our integrated development engine, we advance innovative programs across multiple therapeutic areas into the clinic while maximizing asset potential OUR MISSION OUR STRATEGY
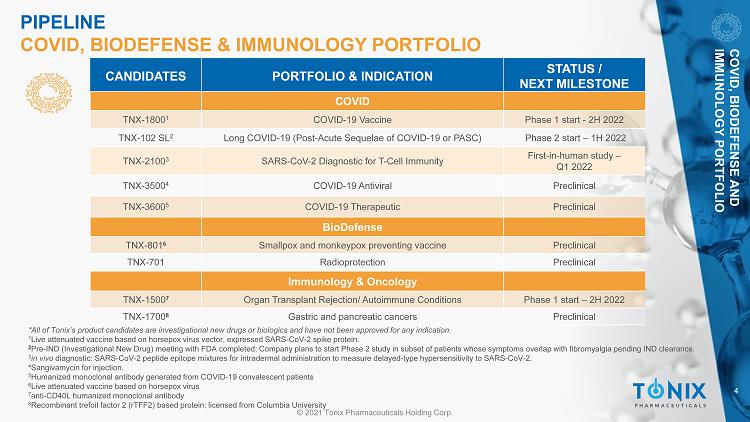
4 © 2021 Tonix Pharmaceuticals Holding Corp. *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. 2 Pre - IND (Investigational New Drug) meeting with FDA completed; Company plans to start Phase 2 study in subset of patients whose symptoms overlap with fibromyalgia pending IND clearance. 3 in vivo diagnostic: SARS - CoV - 2 peptide epitope mixtures for intradermal administration to measure delayed - type hypersensitivity to SARS - CoV - 2. 4 Sangivamycin for injection. 5 Humanized monoclonal antibody generated from COVID - 19 convalescent patients 6 Live attenuated vaccine based on horsepox virus 7 anti - CD40L humanized monoclonal antibody 8 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University CANDIDATES PORTFOLIO & INDICATION STATUS / NEXT MILESTONE BioDefense TNX - 801 6 Smallpox and monkeypox preventing vaccine Preclinical TNX - 701 Radioprotection Preclinical COVID TNX - 1800 1 COVID - 19 Vaccine Phase 1 start - 2H 2022 TNX - 102 SL 2 Long COVID - 19 (Post - Acute Sequelae of COVID - 19 or PASC) Phase 2 start – 1H 2022 TNX - 2100 3 SARS - CoV - 2 Diagnostic for T - Cell Immunity First - in - human study – Q1 2022 TNX - 3500 4 COVID - 19 Antiviral Preclinical TNX - 3600 5 COVID - 19 Therapeutic Preclinical COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO Immunology & Oncology TNX - 1500 7 Organ Transplant Rejection/ Autoimmune Conditions Phase 1 start – 2H 2022 TNX - 1700 8 Gastric and pancreatic cancers Preclinical PIPELINE COVID, BIODEFENSE & IMMUNOLOGY PORTFOLIO
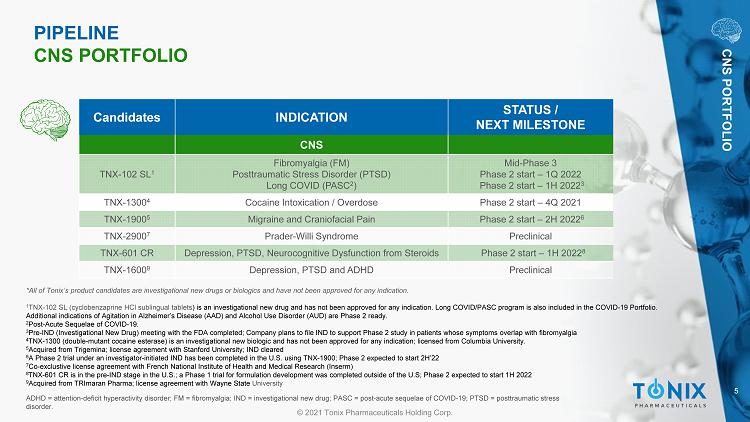
5 © 2021 Tonix Pharmaceuticals Holding Corp. ADHD = attention - deficit hyperactivity disorder; FM = fibromyalgia; IND = investigational new drug; PASC = post - acute sequelae o f COVID - 19; PTSD = posttraumatic stress disorder. Candidates INDICATION STATUS / NEXT MILESTONE CNS TNX - 102 SL 1 Fibromyalgia (FM) Posttraumatic Stress Disorder (PTSD) Long COVID (PASC 2 ) Mid - Phase 3 Phase 2 start – 1Q 2022 Phase 2 start – 1H 2022 3 TNX - 1300 4 Cocaine Intoxication / Overdose Phase 2 start – 4Q 2021 TNX - 1900 5 Migraine and Craniofacial Pain Phase 2 start – 2H 2022 6 TNX - 2900 7 Prader - Willi Syndrome Preclinical TNX - 601 CR Depression, PTSD, Neurocognitive Dysfunction from Steroids Phase 2 start – 1H 2022 8 TNX - 1600 9 Depression, PTSD and ADHD Preclinical PIPELINE CNS PORTFOLIO CNS PORTFOLIO *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets ) is an investigational new drug and has not been approved for any indication. Long COVID/PASC program is also included in th e C OVID - 19 Portfolio. Additional indications of Agitation in A lzheimer’s Disease (AAD) and Alcohol Use D isorder (AUD) are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 Pre - IND (Investigational New Drug) meeting with the FDA completed; Company plans to file IND to support Phase 2 study in patient s whose symptoms overlap with fibromyalgia 4 TNX - 1300 (double - mutant cocaine esterase) is an investigational new biologic and has not been approved for any indication; licensed from Columbia University . 5 Acquired from Trigemina ; license agreement with Stanford University; IND cleared 6 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900; Phase 2 expected to start 2H’ 22 7 Co - exclustive license agreement with French National Institute of Health and Medical Research ( Inserm ) 8 TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was completed outside of the U.S; Ph ase 2 expected to start 1H 2022 9 Acquired from TRImaran Pharma; license agreement with Wayne State University

© 2021 Tonix Pharmaceuticals Holding Corp. KEY CANDIDATES: COVID, BIODEFENSE & IMMUNOLOGY

7 © 2021 Tonix Pharmaceuticals Holding Corp. • Vaccines : early vaccines slowed pandemic, but are showing limitations ‒ Short term protection – requirement for boosters; uncertain protection against variants • Anti - viral drugs : only remdesivir is available ‒ Pfizer’s PAXLOVID™ 1 looks promising; Merck’s molnupiravir did not show benefit in 2 nd cohort 2 • Anti - SARS - CoV - 2 monoclonal antibodies : increasing adoption ‒ Concerns about whether monoclonals will work on new variants • Tests : measurement of functional T cell immunity is a new frontier • Long COVID : no approved treatment for ‘Long Covid’ COVID - 19: THE MISSING PIECES DELTA VARIANT IS SURGING IN THE US – OMICRON VARIANT IS POISED T O SPREAD 1 PAXLOVID™ (PF - 07321332; ritonavir) 2 Merck Says Its Covid Pill Is Less Effective in a Final Analysis - The New York Times (nytimes.com) COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO

8 © 2021 Tonix Pharmaceuticals Holding Corp. Durability of protection ‒ Vaccinated people lose protection, starting at 6 months 1 ‒ Increasing rates of “breakthrough” COVID ‒ White House advocating booster vaccinations with mRNA vaccines at 6 months Effect on forward transmission (spread of infection to others) ‒ Concerns about whether vaccinated people can be infectious to others Detecting vaccine failure ‒ Need a strategy for identifying individuals at risk after vaccination No recognized, clinical applicable biomarker of vaccine protection ‒ Best proxy is neutralizing antibodies, which are hard to measure Current and future variants (e.g., delta, omicron variants) ‒ Less protection from existing vaccines ‒ Unknown effectiveness for future variants COVID - 19 VACCINES: WHERE WE ARE TODAY 1 www.cdc.gov/media/releases/2021/s0818 - covid - 19 - booster - shots.html COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO

9 © 2021 Tonix Pharmaceuticals Holding Corp. mRNA vaccines have slowed pandemic, but may not be a long - term solution ‒ Vaccinated people lost protection, increasing rates of “breakthrough” COVID ‒ COVID is becoming endemic; vaccination of entire world every 6 months not practical Operation Warp Speed (OWS) identified 4 types of vaccines: 1. RNA/DNA – Pfizer is fully approved by the FDA 1 and Moderna has EUA 2. Subunit – NovaVax has good data, but manufacturing issues – not available 3. Non - replicating – J&J has EUA; AstraZeneca widely used ex - US 4. Live Virus Vaccines – none were ultimately adopted by OWS Live Virus Vaccines ‒ Merck was developing two programs: VSV and Measles, but they were not included in OWS and were abandoned in January 2021 2 COVID - 19 VACCINES: WHERE DO WE GO FROM HERE? 1 COMIRNATY is the brand name for the Pfizer - BioNTech COVID - 19 vaccine 2 https://www.merck.com/news/merck - discontinues - development - of - sars - cov - 2 - covid - 19 - vaccine - candidates - continues - development - of - two - investigational - therapeutic - candidates/ COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO

10 © 2021 Tonix Pharmaceuticals Holding Corp. • Control of smallpox, measles, mumps, rub ella, chickenpox and other viral conditions ‒ Prevent forward transmission • Effective in eliciting durable or long - term immunity • Economical to manufacture at scale ‒ Low dose because replication amplifies dose in vivo ‒ Single shot administration • Standard cold chain required for shipping and storage • Live virus vaccines are the oldest vaccine technology ‒ Starting with Edward Jenner’s smallpox vaccine, the first vaccine, eradicated smallpox LIVE VIRUS VACCINES: DEVELOPMENT RATIONALE COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
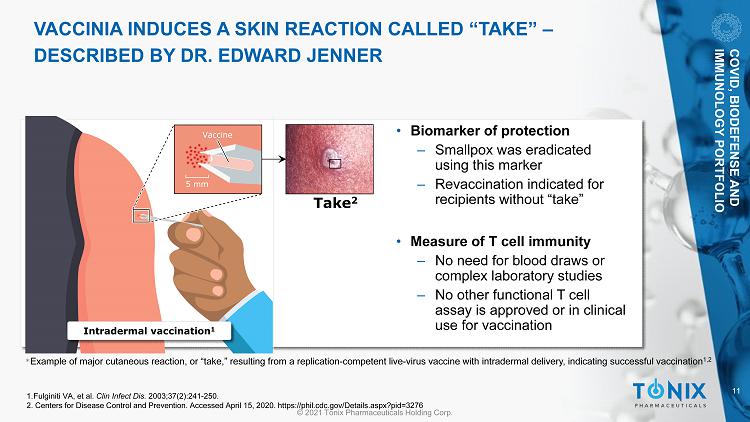
11 © 2021 Tonix Pharmaceuticals Holding Corp. VACCINIA INDUCES A SKIN REACTION CALLED “TAKE” – DESCRIBED BY DR. EDWARD JENNER * Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine with intradermal deliv ery, indicating successful vaccination 1,2 5 mm Vaccine Intradermal vaccination 1 Take 2 • Biomarker of protection ‒ Smallpox was eradicated using this marker ‒ Revaccination indicated for recipients without “take” • Measure of T cell immunity ‒ No need for blood draws or complex laboratory studies ‒ No other functional T cell assay is approved or in clinical use for vaccination 1. Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2. Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276 COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
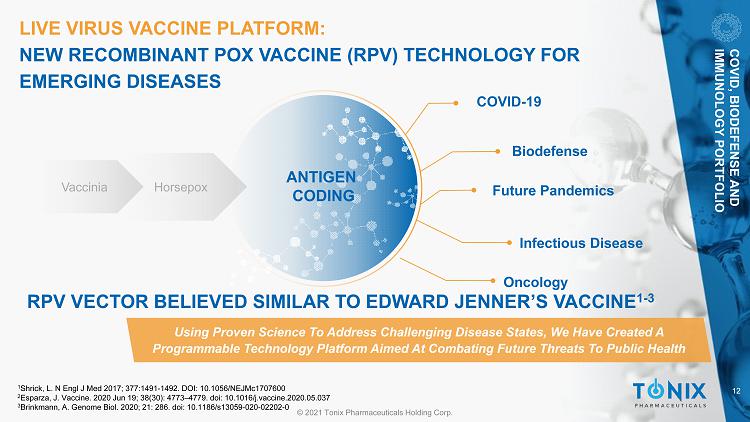
12 © 2021 Tonix Pharmaceuticals Holding Corp. LIVE VIRUS VACCINE PLATFORM: NEW RECOMBINANT POX VACCINE (RPV) TECHNOLOGY FOR EMERGING DISEASES COVID - 19 Future Pandemics Infectious Disease Biodefense Using Proven Science To Address Challenging Disease States, We Have Created A Programmable Technology Platform Aimed At Combating Future Threats To Public Health Vaccinia Horsepox ANTIGEN CODING COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO Oncology RPV VECTOR BELIEVED SIMILAR TO EDWARD JENNER’S VACCINE 1 - 3 1 Shrick, L. N Engl J Med 2017; 377:1491 - 1492. DOI: 10.1056/NEJMc1707600 2 Esparza, J. Vaccine. 2020 Jun 19; 38(30): 4773 – 4779. doi : 10.1016/j.vaccine.2020.05.037 3 Brinkmann, A. Genome Biol. 2020; 21: 286. doi : 10.1186/s13059 - 020 - 02202 - 0

13 © 2021 Tonix Pharmaceuticals Holding Corp. VIRUS - BASED SCIENCE IS WELL ESTABLISHED • Streamlined development • Ability to vertically integrate development and manufacturing • Multi - dose packaging, standard cold - chain products LIVE VIRUS RECOMBINANT POX VACCINE ( RPV ) PLATFORM PROFILE POTENTIALLY LONGER DURABILITY DUE TO POX - ENGINEERED ARCHITECTURE • Enables broad CD8+ T - cell response , resulting in strong immune response PROGRAMMABLE VECTOR DESIGN FOR USE IN DIFFERENT DISEASE MODELS • Responsive to new variants • Wide range of clinical applications: pandemic, biodefense, infectious disease, smallpox, oncology COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
14 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1800 COVID - 19 VACCINE LIVE VIRUS PLATFORM DEVELOPMENT PROGRAM ESTABLISHES LIVE VIRUS PLATFORM • Encodes a protein from SARS - CoV - 2, the cause of COVID - 19 • Provides a novel, variant - reflexive alternative to mRNA products ANIMAL TESTING WITH SOUTHERN RESEARCH INSTITUTE • Non - human primate immune response: positive results reported in Q4 2020 • Non - human primate CoV - 2 challenge testing: positive data reported in Q1 2021 DEVELOPED IN COLLABORATION WITH UNIVERSITY OF ALBERTA AND MANUFACTURING AGREEMENT WITH FUJIFILM DIOSYNTH • GMP clinical supply expected to be ready for human trials in 2H 2022 DEVELOPMENT PROGRAM Market Entry: COVID - 19 Vaccine Additional Indications: Future Pandemic, Infectious Disease, Smallpox, Cancer Status: Preclinical Next Steps: 2H 2022 Initiate Phase 1 Study COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO Patents Filed
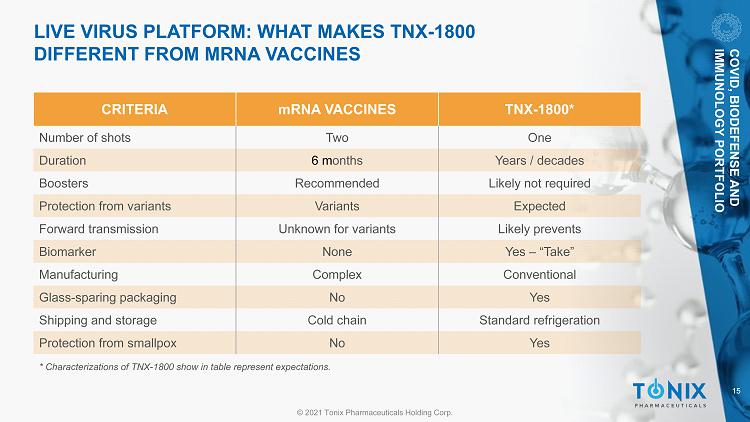
15 © 2021 Tonix Pharmaceuticals Holding Corp. LIVE VIRUS PLATFO RM: WHAT MAKES TNX - 1800 DIFFERENT FROM MRNA VACCINES CRITERIA mRNA VACCINES TNX - 1800* Number of shots Two One Duration 6 m onths Years / decades Boosters Recommended Likely not required Protection from variants Variants Expected Forward transmission Unknown for variants Likely prevents Biomarker None Yes – “Take” Manufacturing Complex Conventional Glass - sparing packaging No Yes Shipping and storage Cold chain Standard refrigeration Protection from smallpox No Yes COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO * Characterizations of TNX - 1800 show in table represent expectations.

16 © 2021 Tonix Pharmaceuticals Holding Corp. LIVE VIRUS RPV P LATFORM & COVID - 19 VACCINE INTERNAL DEVELOPMENT & MANUFACTURING CAPABILITIES Infectious Disease R&D Center (RDC) – Frederick, MD • Function : Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description : ~48,000 square feet; currently BSL - 2 but being converted to BSL - 3 • Status : Operational; acquisition completed on October 1 st , 2021 Advanced Development Center (ADC) – New Bedford, MA • Function : Development and clinical scale manufacturing of live - virus vaccines to support Phase 1 and Phase 2 trials • Description : ~45,000 square feet, under construction, planned BSL - 2 • Status : Expected to be operational in first half 2022 Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of live - virus vaccines • Description : ~44 acre green field site, planned BSL - 2 • Status : Planning for site enabling work in 2022 Architectural Rendering COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO

17 © 2021 Tonix Pharmaceuticals Holding Corp. • “Platforms” – Foundation of Pandemic Response ‒ Key element of AP3 from White House Office of Science and Technology Policy or OSTP 1,2 ▪ 100 days to human trials ▪ Technologies that do not require sterile injection • TNX - 801/ - 1800 (live virus RPV) platform addresses OSTP requirements 1,2 ‒ Our goal is to be able to test new live virus vaccines against novel pathogens within the 100 days of obtaining sequence ▪ RDC is equipped to make new vaccines ▪ ADC will be equipped to make clinical trial material ▪ CDC is planned to make commercial scale material AMERICAN PANDEMIC PREPAREDNESS PLAN (AP3) 1 Sept 3, 2021 https://www.whitehouse.gov/wp - content/uploads/2021/09/American - Pandemic - Preparedness - Transforming - Our - Capabilities - Final - For - Web .pdf 2 Sept 3, 2021 https://www.whitehouse.gov/briefing - room/statements - releases/2021/09/03/fact - sheet - biden - administration - to - transform - capabilitie s - for - pandemic - preparedness/ COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO

18 © 2021 Tonix Pharmaceuticals Holding Corp. IMPORTANCE OF TESTING PROTECTIVE IMMUNITY • Personalized approach to determine need for vaccine boosters • More cost effective • Reduces risk with unnecessary vaccination • One - size - fits - all booster strategy is expensive and likely unsustainable Testing protective immunity to assess personalized need for vaccine boosters is expected to be more cost effective and reduce risks with unnecessary vaccination US TRENDS IN COVID - 19 VACCINE BOOSTER DEVELOPMENT CURRENT US GOVERNMENT STANCE IS BOOSTERS RECOMMENDED POST - PFIZER MODERNA, AND J&J VACCINATIONS 1,2 • CDC, FDA, White House, COVID - 19 Response Team stated that immunity wanes and booster vaccines should be used in all adults and most children • FDA has authorized and CDC approved a single booster shot of the Pfizer - BioNTech and Moderna COVID - 19 vaccines for Americans age 18 and older, six months after second dose • FDA has authorized a single booster shot of the J&J vaccine for everyone who received the initial J&J vaccine two or more months ago COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO 1 www.cdc.gov/media/releases/2021/s0818 - covid - 19 - booster - shots.html 2 https://www.fda.gov/news - events/press - announcements/fda - authorizes - booster - dose - pfizer - biontech - covid - 19 - vaccine - certain - populat ions
19 © 2021 Tonix Pharmaceuticals Holding Corp. TWO TYPES OF IMMUNITY • Antibodies – can be measured in a blood test, but anti - SARS - CoV - 2 antibodies are not predictive of protection • T cell – can be measured in a blood test, but requires sophisticated lab, unknown if predictive ASSESSING ANTI - SARS - COV - 2 PROTECTIVE IMMUNITY FUNCTIONAL T CELL IMMUNITY • in vivo – classic skin test – correlation with protection under investigation 2,3 NEUTRALIZING ANTIBODIES – APPEAR TO CORRELATE WITH PROTECTION 1 • Not part of standard antibody tests • Requires culture of antibodies with live SARS - CoV - 2; possibly “pseudo - type” assays 1 Krammer, F. (2021) Nature Medicine. 27:1145 – 1153. https://www.nature.com/articles/s41591 - 021 - 01432 - 4.pdf 2 Barrios, Y et al. Clinical Immunol. (2021) 226:108730 3 Barrios, Y et al. Vaccines (2021) 9:575 COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
20 © 2021 Tonix Pharmaceuticals Holding Corp. MEASURES THE PRESENCE AND STRENGTH OF FUNCTIONAL IN VIVO T - CELL IMMUNITY • Designed to elicit delayed - type hypersensitivity in individuals who have been exposed to SARS - CoV - 2 or successfully vaccinated • SARS - CoV - 2 epitope peptide mixtures for intradermal administration (Skin Test) TNX - 2100*: SARS - COV - 2 DIAGNOSTIC TO MEASURE T - CELL IMMUNITY *TNX - 2100 is in the pre - IND stage of development and has not been approved for any indication. † Intracellular cytokine staining (ICS) measured by flow cytometry after in vitro stimulation of purified peripheral blood mono nuc lear cells. DEVELOPMENT PLANS • Q1 2022: Plan to initiate first - in - human clinical testing • Patents filed POTENTIALLY SCALABLE FOR WIDESPREAD USE • Many tests † for T - cell immunity to SARS - CoV - 2 require specialized laboratories and are not amendable to standardization • Adaptive Biotech’s T Detect™ COVID - 19 test received FDA EUA based on genetic analysis of T - cell receptors COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
21 © 2021 Tonix Pharmaceuticals Holding Corp. The only COVID - 19 antiviral that is FDA approved is Remdesivir/ Veklury ® ‒ Gilead – Intravenous ( i.v. ) medicine ‒ FDA approved for patients who are at least 12 years old and require hospitalization ‒ May shorten the time to recover from acute COVID - 19 ‒ World Health Organization has recommended against its use 1 ‒ Resistance reported 2 Anti - virals in Phase 3 development ‒ Pfizer – PAXLOVID™ (PF - 07321332; ritonavir) - oral protease C3L inhibitor ‒ Merck/Ridgeback – molnupiravir , oral, in Phase 3 development with US gov’t supply agreement 3 Concerns about anti - viral efficacy ‒ Remdesivir resistance reported 2 ‒ Molnupiravir efficacy was not repeated in second cohort of Phase 3 trial 4 SMALL MOLECULE COVID - 19 THERAPEUTICS 1 World Health Organization (2021). Therapeutics and COVID - 19: living guideline, 6 July 2021 (Report). hdl : 10665/342368 . Therapeutics and COVID - 19: living guideline, 6 July 2021 (who.int) WHO/2019 - nCoV/therapeutics/2021.2 2 https://yaledailynews.com/blog/2021/12/02/yale - scientists - identify - remdesivir - resistance - in - immunocompromised - covid - 19 - patient/ 3 www.merck.com/news/merck - announces - supply - agreement - with - u - s - government - for - molnupiravir - an - investigational - oral - antiviral - candi date - for - treatment - of - mild - to - moderate - covid - 19 4 www.merck.com/news/merck - announces - supply - agreement - with - u - s - government - for - molnupiravir - an - investigational - oral - antiviral - candi date - for - treatment - of - mild - to - moderate - covid - 19 COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO

22 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 3500*: COVID - 19 ANTIVIRAL TREATMENT SANGIVAMYCIN PROFILE New variants heighten need for therapeutics NIH Treatment Guidelines for COVID - 19 are mixed on use of remdesivir Potential monotherapy antiviral 1,2 • 65 times more potent than remdesivir in inhibiting SARS - CoV - 2 in cell culture infectivity studies (dose to achieve IC 90 ) Potential combination therapy with remdesivir 1,2 • TNX - 3500 antiviral effect is additive when combined with remdesivir and reduces the amount of each drug necessary for an IC 90 • Combination therapies for other viruses have reduced the emergence of drug resistant viral strains DEVELOPMENT PROGRAM Market Entry: COVID - 19 Antiviral Additional Indications: MERS, Ebola, Lassa, Oncology Status: Preclinical Next Steps: 1H 2022 Initiate Animal Studies Patents Filed *TNX - 3500 is in the pre - IND stage of development and has not been approved for any indication. . MERS = Middle East Respiratory Syndrome; NIH = National Institutes of Health; PK = pharmacokinetics. COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO 1. Bennett RP et al. Viruses . 2020;13(1):52. doi : 10.3390/v13010052 2. Bennett, RP et al. JCI Insight . 2021 in press preview 10.1172/jci.insight.153165

23 © 2021 Tonix Pharmaceuticals Holding Corp. Monoclonal antibodies ( mAbs ) (EUA) – 4 granted US Emergency Use Authorization 1 ‒ Regeneron/Genentech - REGEN - COV® Casirivimab /imdevimab 2 ‒ Vir /GSK – XEVURDY® ( sotrovimab ) 3 ‒ Eli Lilly – B amlanivimab /etesevimab 4 - US distribution halted 5 ‒ AstraZeneca – Evusheld ( Tixagevimab / cilgavimab ) – EUA for long term prophylaxis New mAbs under development 6 ‒ AstraZeneca – AZD7442 – EUA request submitted 7 ‒ Brii Biosciences – BRII - 196 and BRII - 198 8 ‒ Adagio Therapeutics – ADG20 9 ‒ Many other companies 10 Concerns about efficacy of mAbs against new variants ‒ Delta and Omicron variants have many changes in the spike protein, which is the target of current mAbs 11 ‒ Antibodies are being studies for activity against new variants MONOCLONAL ANTIBODY COVID - 19 THERAPEUTICS 1 Indicated for individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease 2 www.fda.gov/news - events/press - announcements/coronavirus - covid - 19 - update - fda - authorizes - monoclonal - antibodies - treatment - covid - 19 3 www.fda.gov/news - events/press - announcements/coronavirus - covid - 19 - update - fda - authorizes - additional - monoclonal - antibody - treatment - covid - 19 4 www.fda.gov/news - events/press - announcements/coronavirus - covid - 19 - update - fda - authorizes - monoclonal - antibodies - treatment - covid - 19 - 0 5 www.fiercepharma.com/pharma/lilly - s - covid - antibody - combo - halted - nationwide - dealing - huge - blow - to - blockbuster - program 6 Dolgin, E. Nature Biotechnology volume 39 , pages783 – 785 (2021) https://doi.org/10.1038/s41587 - 021 - 00980 - x 7 https://www.cnbc.com/2021/11/18/astrazeneca - antibody - drug - 83percent - effective - at - preventing - covid - trial.html 8 https:://endpts.com/brii - bio - gets - all - hands - on - deck - for - covid - 19 - antibody - hunt - leveraging - chinese - partners - work - with - recovered - p atients/ 9 https://endpts.com/qa - tillman - gerngross - explains - why - his - covid - mab - will - have - an - edge - over - an - already - crowded - field/ 10 e.g., Centivax , Corat Therapeutics, IDBiologics , Leyden Labs, Memo Therapeutics and SpikImm 11 Dec 7, 2021 Glaxo Says Its Covid - 19 Antibody Drug Works Against Omicron - WSJ COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
24 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 3600*: COVID - 1 9 THERAPEUTIC HUMANIZED MONOCLONAL ANTIBODY PROFILE Collaboration with Columbia University Human monoclonal antibodies ( mAbs ) generated from COVID - 19 convalescent patients Potential monotherapy • Plan to seek indication similar to current EUA therapeutic mAbs for treating individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease Potential combination therapy with other antibodies • Combination therapies for other anti - CoV - 2 monoclonal antibodies are believed to have reduced the emergence of drug resistant viral strains DEVELOPMENT PROGRAM Market Entry: COVID - 19 Therapeutic Additional Indications: Symptomatic COVID in patients with risk factors for poor outcome Status: Preclinical Next Steps: Study inhibition of SARS CoV - 2 variants in tissue culture; 1H 2022 Initiate Animal Studies *TNX - 3600 is in the pre - IND stage of development and has not been approved for any indication. COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
25 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: LONG COVID (PASC) CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS PROFILE Long COVID or Post - acute Sequelae of COVID - 19 (PASC 1 ) – What is it? • Symptoms can include fatigue, sleep disorders, pain, fevers, shortness of breath, cognitive impairment described as “brain fog”, gastrointestinal symptoms, anxiety, and depression 2 • Can persist for months and can range in severity from mild to incapacitating • Occurs in 30% of recovered COVID - 19 patients • Typically associated with moderate or severe COVID - 19, Long COVID can occur after mild COVID - 19 or even after asymptomatic SARS - CoV - 2 infection To address the urgent need for PASC therapies, Congress awarded the National Institutes of Health $1.15 billion to study Long COVID. 3 DEVELOPMENT PROGRAM Market Entry : Long COVID (PASC) Status: Clinical – pre - IND; FDA minutes from pre - IND meeting received in Q3 2021 Next Steps: Start Phase 2 study for treating subset of Long COVID patients whose symptoms overlap with fibromyalgia in 1H 2022 Patents Issued 1 Feb. 24, 2021 - White House COVID - 19 Response Team press briefing; Feb 25, 2021 - policy brief from the World Health Organizatio n on long COVID 2 Nalbandian, Ani, et al. "Post - acute COVID - 19 syndrome." Nature Medicine (2021): 1 - 15. 3 The NIH provision of Title III Health and Human Services, Division M -- Coronavirus Response and Relief Supplemental Appropriation s Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260. COVID, BIODEFENSE AND IMMUNOLOGY PORTFOLIO
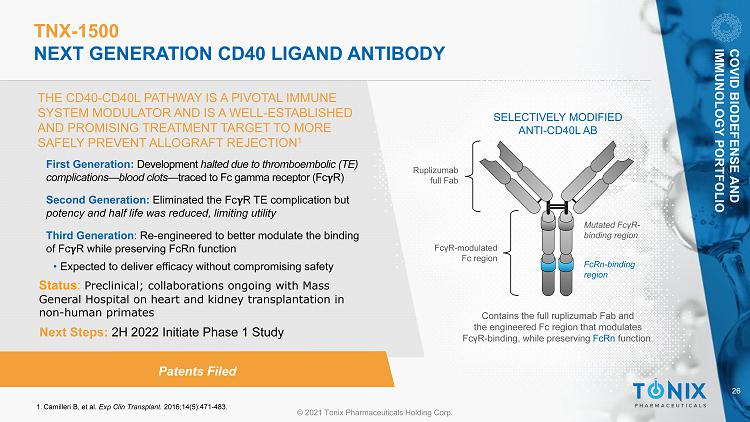
26 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1500 NEXT GENERATION CD40 LIGAND ANTIBODY THE CD40 - CD40L PATHWAY IS A PIVOTAL IMMUNE SYSTEM MODULATOR AND IS A WELL - ESTABLISHED AND PROMISING TREATMENT TARGET TO MORE SAFELY PREVENT ALLOGRAFT REJECTION 1 1. Camilleri B, et al. Exp Clin Transplant. 2016;14(5):471 - 483. SELECTIVELY MODIFIED ANTI - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region Patents Filed First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation : Re - engineered to better modulate the binding of Fc R while preserving FcRn function • Expected to deliver efficacy without compromising safety Status : Preclinical; collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates COVID BIODEFENSE AND IMMUNOLOGY PORTFOLIO Next Steps: 2H 2022 Initiate Phase 1 Study

© 2021 Tonix Pharmaceuticals Holding Corp. KEY CANDIDATES: CNS

28 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: FIBROMYALGIA CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS CNS PORTFOLIO A unique formulation of cyclobenzaprine designed to optimize delivery and absorption Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration • Lower daytime exposure • Avoids first - pass metabolism ⁃ Reduces risk of pharmacological interference from major metabolite Clinical trial program designed to examine treatment of core Fibromyalgia symptoms Market Entry: Fibromyalgia Additional Indications: PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder, Long COVID Status: One Positive Phase 3 study (RELIEF) Completed Next Steps: Second Phase 3 Study (RALLY/F306): clinical phase completed, and topline data expected 1Q 2022. Confirmatory Phase 3 planned for 1H 2022 PROFILE DEVELOPMENT PROGRAM Patents Issued

29 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL: FIBROMYALGIA CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS PROGRAM UPDATE Phase 3 Study, RALLY (F306) • July 2021: Tonix stopped enrollment in the RALLY study following an unblinded, pre planned interim analysis by the Independent Data Monitoring Committee (IDMC). • Based on interim analysis results of the first 50% (n=336) enrolled participants, the IDMC recommended stopping the trial as TNX - 102 SL is unlikely to demonstrate a statistically significant improvement in the primary endpoint. • Clinical phase of study completed, with 514 participants enrolled overall – 399 completers; topline results expected 1Q 2022 • Confirmatory Phase 3 study (F307) planned 1H 2022 Following analysis of F306 results, including pharmacogenetic comparison of F304 and F306, Tonix may modify F307 protocol TNX 102 - SL Development Beyond Fibromyalgia • Development efforts continue in PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder, Long COVID CNS PORTFOLIO
30 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 601 CR: PSYCHIATRY TIANEPTINE OXALATE AND NALOXONE CNS PORTFOLIO A novel, oral, controlled release once - daily tablet Mechanistically different from traditional monoaminergic treatments for depression Indirectly modulates the glutamatergic system • No direct binding to NMDA, AMPA, or kainate receptors Naloxone added to deter parenteral abuse Treatment effect of tianeptine in depression is well - established Market Entry: Major Depressive Disorder Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids Status: Clinical – pre - IND Next Steps: 1H 2022 Initiate Phase 2 Trial PROFILE DEVELOPMENT PROGRAM AMPA= α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid; MAOI=monoamine oxidase inhibitors; NMDA=N - methyl - D - aspartate. Patents Issued

31 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1900: MIGRAINE INTRANASAL POTENTIATED OXYTOCIN (OT) WITH MAGNESIUM CNS PORTFOLIO Intranasal OT has potential utility in treating migraine 1 • Intranasal OT reaches the trigeminal ganglion • Preclinical evidence of OT blocking CGRP release and suppressing pain • Association of low OT levels during and preceding migraine episodes • Novel non - CGRP antagonist approach to treatment Magnesium is known to potentiate the binding of OT to its receptor 2 One billion individuals worldwide suffer from migraines Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance Status: Clinical – IND cleared 3 Next Steps: 2H 2022 Initiate Phase 2 Trial Patents Issued PROFILE DEVELOPMENT PROGRAM 1. Tzabazis et al., 2017. 2. Antoni and Chadio, 1989. 3. A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 CGRP = calcitonin gene - related peptide.
32 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 2900: PRADER - WILLI SYNDROME INTRANASAL POTENTIATED OXYTOCIN (OT) WITH MAGNESIUM PROFILE CNS PORTFOLIO Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Orphan disease occurring in 1 in 15,000 births Symptoms include lack of suckling as infants, poor muscle strength, and constant hunger (hyperphagia) • In animal models, OT has improved suckling and suppressed hunger ‒ Tonix’s patented potentiated OT formulation is believed to increase specificity for OT receptors relative to off - target vasopressin receptors DEVELOPMENT PROGRAM Market Entry: Prader - Willi Syndrome Additional Indications: Rare, Orphan Hyperphagia Conditions Status: pre - IND; orphan drug designation application submitted to FDA Next Steps: Submit application to the FDA for Fast Track designation Patents Issued
33 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION COCAINE ESTERASE ( CoCe ) CNS PORTFOLIO PROFILE Cocaine is the main cause for drug - related ED visits 1 Cocaine use can cause irreversible structural damage to the heart and accelerate cardiovascular disease 2 • In one survey of 94 long - term cocaine users, 71% had some form of cardiovascular disease 3 CoCe is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes DEVELOPMENT PROGRAM Market Entry: Cocaine Intoxication Additional Indications: Cocaine Overdose Status: Phase 2 Open Label Next Steps: Q4 2021 Initiate Trial 1. Havakuk O et al. J Am Coll Cardiol . 2017;70:101 - 113. 2. Phillips K et al. Am J Cardiovasc Drugs . 2009;9:177 - 196. 3. Maceira AM et al. J Cardiovasc Magn Reson . 2014;16:26. ED = emergency department. FDA Breakthrough Therapy Designation Patents Issued

© 2021 Tonix Pharmaceuticals Holding Corp. FUTURE OUTLOOK

35 © 2021 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND PANCREATIC CANCERS TNX - 3600: MONOCLONAL ANTIBODIES FOR COVID - 19 TREATMENT KEY DEVELOPMENT PARTNERS TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 1800: COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME

36 © 2021 Tonix Pharmaceuticals Holding Corp. Data □ 1 st Quarter 2022 Topline data from TNX - 102 SL Phase 3 F306/RALLY study in fibromyalgia expected Expected Clinical Trial Initiations □ 4 th Quarter 2021 Phase 2 OL safety study start of TNX - 1300 in ED setting for cocaine intoxication □ 1 st Quarter 2022 First - in - human clinical study start of TNX - 2100 for SARS - CoV - 2 skin test □ 1 st Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of PTSD in Kenya □ 1 st Half 2022 Phase 3 study start of TNX - 102 SL for the management of fibromyalgia □ 1 st Half 2022 Phase 2 study start of TNX - 102 SL for the treatment of Long COVID □ 1 st Half 2022 Phase 2 study start of TNX - 601 CR for the treatment of major depressive disorder □ 2 nd Half 2022 Phase 2 study start of TNX - 1900 for the treatment of migraine □ 2 nd Half 2022 Phase 1 study start of TNX - 1800 for COVID - 19 □ 2 nd Half 2022 Phase 1 study start of TNX - 1500 for prevention of allograft rejection MILESTONES: RECENTLY COMPLETED AND UPCOMING* * We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones. □ 4 th Quarter 2020 Positive topline data from TNX - 102 SL Phase 3 F304/RELIEF study in fibromyalgia reported □ 1 st Quarter 2021 Non - human primate positive efficacy data from TNX - 1800 in COVID - 19 models reported x x

37 © 2021 Tonix Pharmaceuticals Holding Corp. MANAGEMENT TEAM Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

© 2021 Tonix Pharmaceuticals Holding Corp. THANK YOU