
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2022 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION N oble C on 18 Investor Conference NASDAQ: TNXP Version P0348 April 12, 2022 (Doc 0993)

2 © 2022 Tonix Pharmaceuticals Holding Corp. CAUTIONARY NOTE ON FORWARD - LOOKING STATEMENTS Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
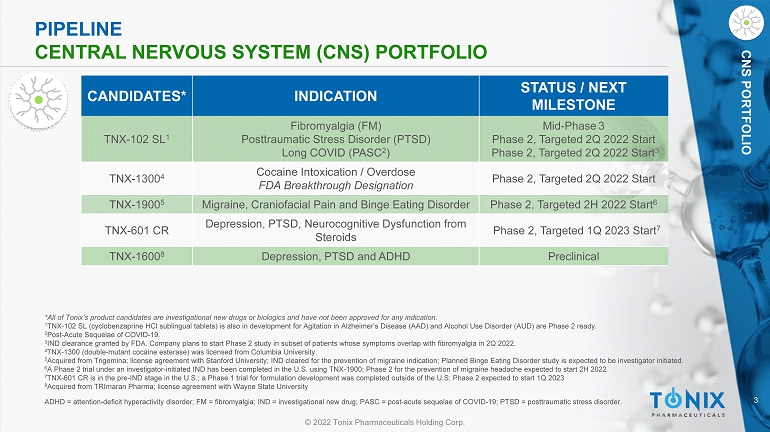
3 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO ADHD = attention - deficit hyperactivity disorder; FM = fibromyalgia; IND = investigational new drug; PASC = post - acute sequelae o f COVID - 19; PTSD = posttraumatic stress disorder. PIPELINE CENTRAL NERVOUS SYSTEM (CNS) PORTFOLIO CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 102 SL 1 Fibromyalgia (FM) Posttraumatic Stress Disorder (PTSD) Long COVID (PASC 2 ) Mid - Phase 3 Phase 2, Targeted 2Q 2022 Start Phase 2, Targeted 2Q 2022 Start 3 TNX - 1300 4 Cocaine Intoxication / Overdose FDA Breakthrough Designation Phase 2, Targeted 2Q 2022 Start TNX - 1900 5 Migraine, Craniofacial Pain and Binge Eating Disorder Phase 2, Targeted 2H 2022 Start 6 TNX - 601 CR Depression, PTSD, Neurocognitive Dysfunction from Steroids Phase 2, Targeted 1Q 2023 Start 7 TNX - 1600 8 Depression, PTSD and ADHD Preclinical *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is also in development for Agitation in A lzheimer’s Disease (AAD) and Alcohol Use D isorder (AUD) are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 IND clearance granted by FDA. Company plans to start Phase 2 study in subset of patients whose symptoms overlap with fibromya lgi a in 2Q 2022. 4 TNX - 1300 (double - mutant cocaine esterase) was licensed from Columbia University . 5 Acquired from Trigemina ; license agreement with Stanford University; IND cleared for the prevention of migraine indication; Planned Binge Eating Dis ord er study is expected to be investigator initiated. 6 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900; Phase 2 for the prevention of migraine headache expected to start 2H 2022 7 TNX - 601 CR is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was completed outside of the U.S; Ph ase 2 expected to start 1Q 2023 8 Acquired from TRImaran Pharma; license agreement with Wayne State University
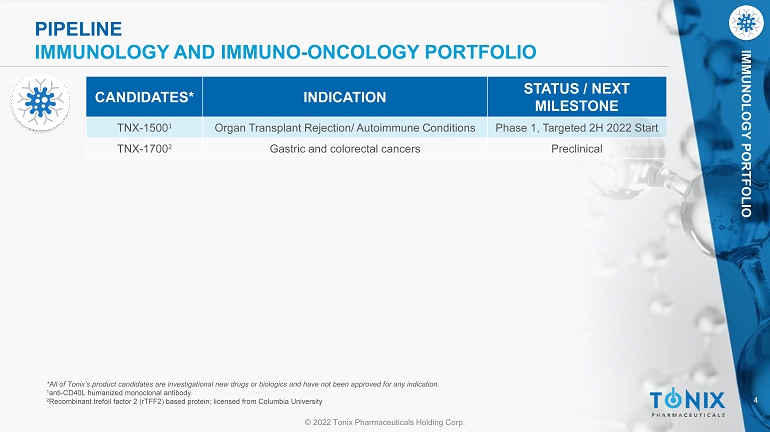
4 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 anti - CD40L humanized monoclonal antibody 2 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 1500 1 Organ Transplant Rejection/ Autoimmune Conditions Phase 1, Targeted 2H 2022 Start TNX - 1700 2 Gastric and colorectal cancers Preclinical PIPELINE IMMUNOLOGY AND IMMUNO - ONCOLOGY PORTFOLIO
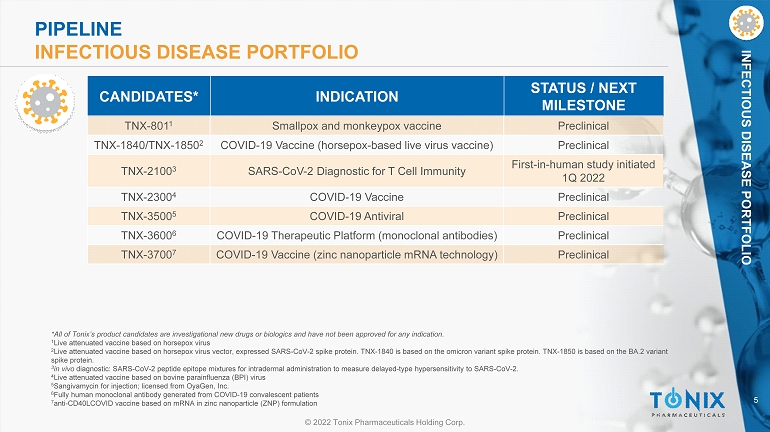
5 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Live attenuated vaccine based on horsepox virus 2 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. TNX - 1840 is based on the omicron var iant spike protein. TNX - 1850 is based on the BA.2 variant spike protein. 3 in vivo diagnostic: SARS - CoV - 2 peptide epitope mixtures for intradermal administration to measure delayed - type hypersensitivity to SARS - CoV - 2. 4 Live attenuated vaccine based on bovine parainfluenza (BPI) virus 5 Sangivamycin for injection; licensed from OyaGen , Inc. 6 Fully human monoclonal antibody generated from COVID - 19 convalescent patients 7 anti - CD40LCOVID vaccine based on mRNA in zinc nanoparticle (ZNP) formulation CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 801 1 Smallpox and monkeypox vaccine Preclinical TNX - 1840/TNX - 1850 2 COVID - 19 Vaccine (horsepox - based live virus vaccine) Preclinical TNX - 2100 3 SARS - CoV - 2 Diagnostic for T Cell Immunity First - in - human study initiated 1Q 2022 TNX - 2300 4 COVID - 19 Vaccine Preclinical TNX - 3500 5 COVID - 19 Antiviral Preclinical TNX - 3600 6 COVID - 19 Therapeutic Platform (monoclonal antibodies) Preclinical TNX - 3700 7 COVID - 19 Vaccine (zinc nanoparticle mRNA technology) Preclinical PIPELINE INFECTIOUS DISEASE PORTFOLIO
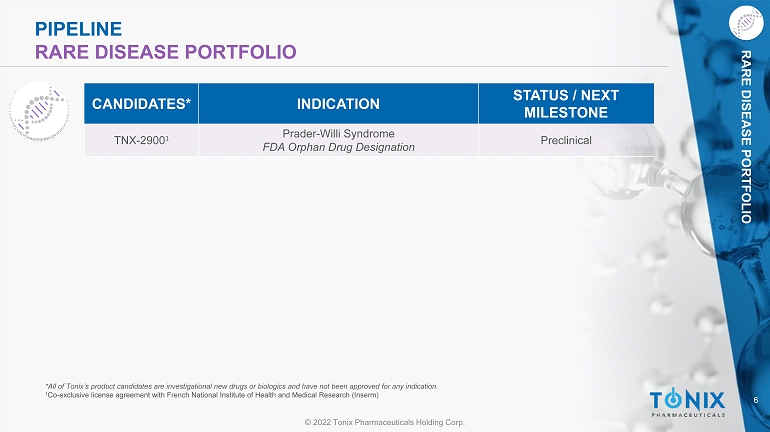
6 © 2022 Tonix Pharmaceuticals Holding Corp. RARE DISEASE PORTFOLIO PIPELINE RARE DISEASE PORTFOLIO TNX - 2900 1 Prader - Willi Syndrome FDA Orphan Drug Designation Preclinical CANDIDATE S * INDICATION STATUS / NEXT MILESTONE *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm )

© 2022 Tonix Pharmaceuticals Holding Corp. CNS: KEY CANDIDATES
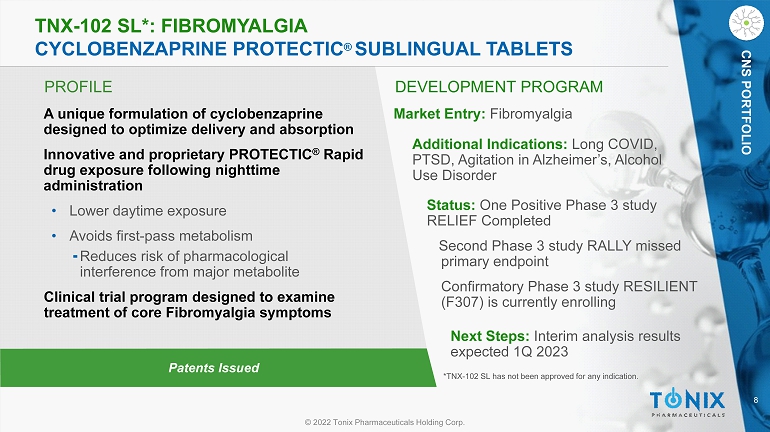
8 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: FIBROMYALGIA CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS CNS PORTFOLIO A unique formulation of cyclobenzaprine designed to optimize delivery and absorption Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration • Lower daytime exposure • Avoids first - pass metabolism ⁃ Reduces risk of pharmacological interference from major metabolite Clinical trial program designed to examine treatment of core Fibromyalgia symptoms Market Entry: Fibromyalgia Additional Indications: Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF Completed Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT (F307) is currently enrolling Next Steps: Interim analysis results expected 1Q 2023 *TNX - 102 SL has not been approved for any indication.

9 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: FIBROMYALGIA PROGRAM UPDATE CNS PORTFOLIO Phase 3 Study, RESILIENT (F307), will compare TNX - 102 SL 5.6 mg and placebo • First patient enrolled in April 2022 • Interim Analysis results expected 1Q 2023 • Parallel design, double - blind, randomized placebo - controlled study, all U.S. sites • Primary endpoint is pain at week 14 analyzed by MMRM with MI • Projecting adverse event related discontinuations to decrease towards rates in RELIEF and PTSD Studies Phase 3 Study, RALLY (F306), comparison of TNX - 102 SL 5.6 mg and placebo • As expected from interim results published July 2021, RALLY Study missed primary endpoint • Unexpected ~80% increase in adverse event - related discontinuations in both drug and placebo arms • Multiple imputation approach on 'Missing Data' attenuated statistical significance of efficacy endpoints’ • TNX - 102 SL was generally well tolerated with overall adverse event profile comparable to prior studies; no new safety signals observed
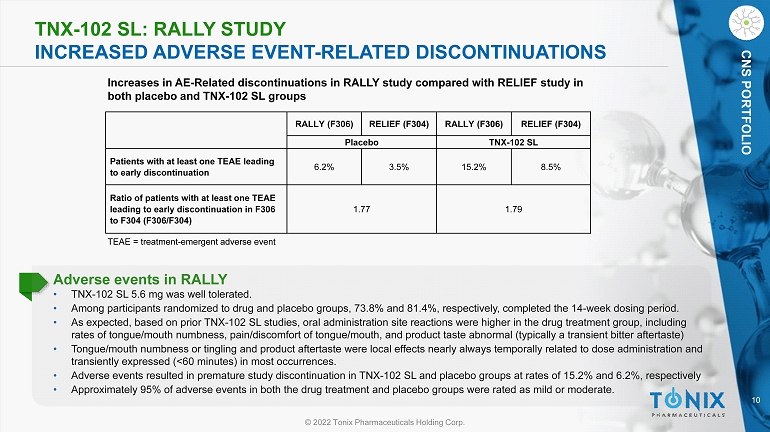
10 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: RALLY STUDY INCREASED ADVERSE EVENT - RELATED DISCONTINUATIONS CNS PORTFOLIO TEAE = treatment - emergent adverse event Increases in AE - Related discontinuations in RALLY study compared with RELIEF study in both placebo and TNX - 102 SL groups Adverse events in RALLY • TNX - 102 SL 5.6 mg was well tolerated. • Among participants randomized to drug and placebo groups, 73.8% and 81.4%, respectively, completed the 14 - week dosing period. • As expected, based on prior TNX - 102 SL studies, oral administration site reactions were higher in the drug treatment group, incl uding rates of tongue/mouth numbness, pain/discomfort of tongue/mouth, and product taste abnormal (typically a transient bitter aft ert aste) • Tongue/mouth numbness or tingling and product aftertaste were local effects nearly always temporally related to dose administ rat ion and transiently expressed (<60 minutes) in most occurrences. • Adverse events resulted in premature study discontinuation in TNX - 102 SL and placebo groups at rates of 15.2% and 6.2%, respecti vely • Approximately 95% of adverse events in both the drug treatment and placebo groups were rated as mild or moderate. RALLY (F306) RELIEF (F304) RALLY (F306) RELIEF (F304) Placebo TNX - 102 SL Patients with at least one TEAE leading to early discontinuation 6.2% 3.5% 15.2% 8.5% Ratio of patients with at least one TEAE leading to early discontinuation in F306 to F304 (F306/F304) 1.77 1.79
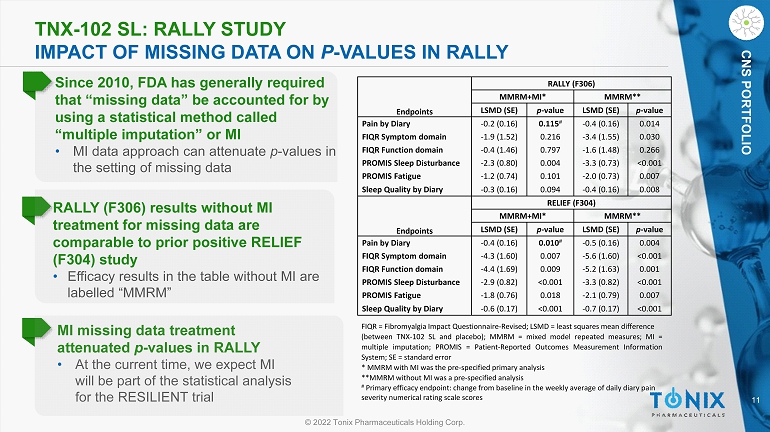
11 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: RALLY STUDY IMPACT OF MISSING DATA ON P - VALUES IN RALLY MI missing data treatment attenuated p - values in RALLY • At the current time, we expect MI will be part of the statistical analysis for the RESILIENT trial CNS PORTFOLIO FIQR = Fibromyalgia Impact Questionnaire - Revised ; LSMD = least squares mean difference (between TNX - 102 SL and placebo) ; MMRM = mixed model repeated measures ; MI = multiple imputation ; PROMIS = Patient - Reported Outcomes Measurement Information System ; SE = standard error * MMRM with MI was the pre - specified primary analysis **MMRM without MI was a pre - specified analysis # Primary efficacy endpoint: change from baseline in the weekly average of daily diary pain severity numerical rating scale scores Since 2010, FDA has generally required that “missing data” be accounted for by using a statistical method called “multiple imputation” or MI • MI data approach can attenuate p - values in the setting of missing data RALLY (F306) MMRM+MI* MMRM** Endpoints LSMD (SE) p - value LSMD (SE) p - value Pain by Diary - 0.2 (0.16) 0.115 # - 0.4 (0.16) 0.014 FIQR Symptom domain - 1.9 (1.52) 0.216 - 3.4 (1.55) 0.030 FIQR Function domain - 0.4 (1.46) 0.797 - 1.6 (1.48) 0.266 PROMIS Sleep Disturbance - 2.3 (0.80) 0.004 - 3.3 (0.73) <0.001 PROMIS Fatigue - 1.2 (0.74) 0.101 - 2.0 (0.73) 0.007 Sleep Quality by Diary - 0.3 (0.16) 0.094 - 0.4 (0.16) 0.008 RELIEF (F304) MMRM+MI* MMRM** Endpoints LSMD (SE) p - value LSMD (SE) p - value Pain by Diary - 0.4 (0.16) 0.010 # - 0.5 (0.16) 0.004 FIQR Symptom domain - 4.3 (1.60) 0.007 - 5.6 (1.60) <0.001 FIQR Function domain - 4.4 (1.69) 0.009 - 5.2 (1.63) 0.001 PROMIS Sleep Disturbance - 2.9 (0.82) <0.001 - 3.3 (0.82) <0.001 PROMIS Fatigue - 1.8 (0.76) 0.018 - 2.1 (0.79) 0.007 Sleep Quality by Diary - 0.6 (0.17) <0.001 - 0.7 (0.17) <0.001 RALLY (F306) results without MI treatment for missing data are comparable to prior positive RELIEF (F304) study • Efficacy results in the table without MI are labelled “MMRM”
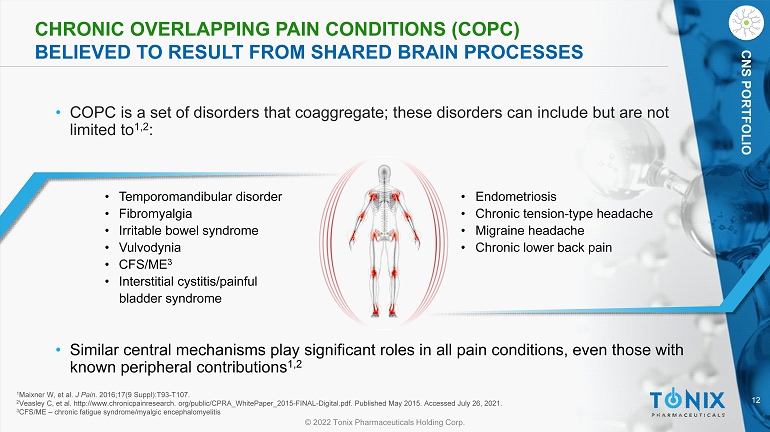
12 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CHRONIC OVERLAPPING PAIN CONDITIONS (COPC) BELIEVED TO RESULT FROM SHARED BRAIN PROCESSES • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2
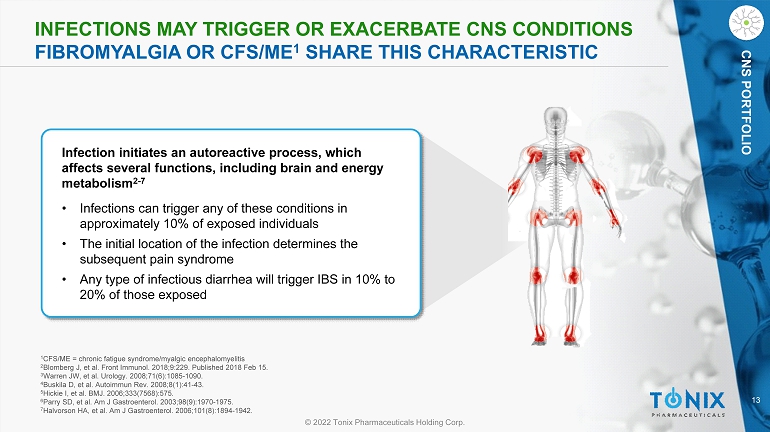
13 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO INFECTIONS MAY TRIGGER OR EXACERBATE CNS CONDITIONS FIBROMYALGIA OR CFS/ME 1 SHARE THIS CHARACTERISTIC Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger IBS in 10% to 20% of those exposed 1 CFS/ME = chronic fatigue syndrome/ myalgic encephalomyelitis 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942.
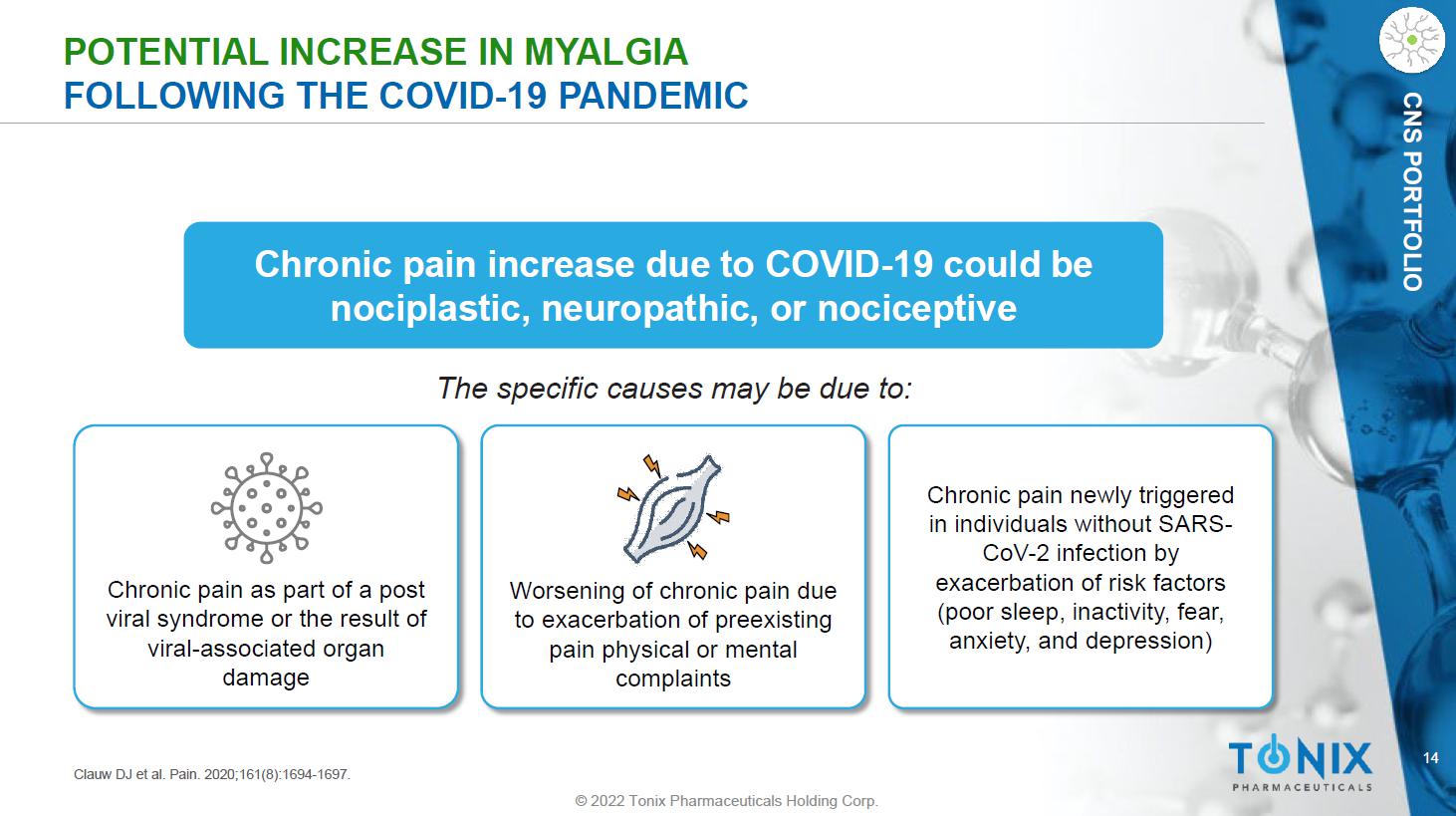
14 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO POTENTIAL INCREASE IN MYALGIA FOLLOWING THE COVID - 19 PANDEMIC The specific causes may be due to: Chronic pain increase due to COVID - 19 could be nociplastic , neuropathic, or nociceptive Chronic pain newly triggered in individuals without SARS - CoV - 2 infection by exacerbation of risk factors (poor sleep, inactivity, fear, anxiety, and depression) Chronic pain as part of a post viral syndrome or the result of viral - associated organ damage Worsening of chronic pain due to exacerbation of preexisting pain physical or mental complaints Clauw DJ et al. Pain. 2020;161(8):1694 - 1697.
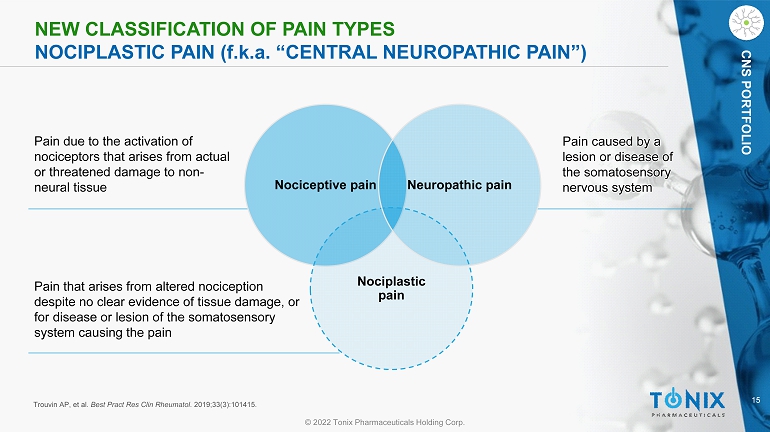
15 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO NEW CLASSIFICATION OF PAIN TYPES NOCIPLASTIC PAIN ( f.k.a . “CENTRAL NEUROPATHIC PAIN”) Nociceptive pain Nociplastic pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415.
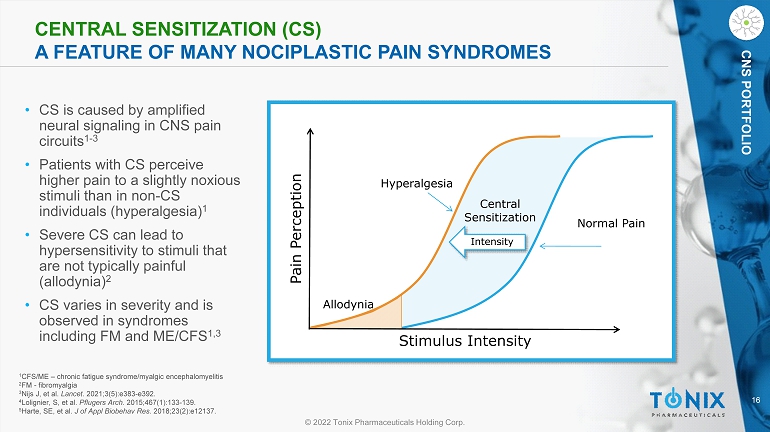
16 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CENTRAL SENSITIZATION (CS) A FEATURE OF MANY NOCIPLASTIC PAIN SYNDROMES • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 1 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis 2 FM - fibromyalgia 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392. 4 Lolignier, S, et al. Pflugers Arch. 2015;467(1):133 - 139. 5 Harte, SE, et al. J of Appl Biobehav Res. 2018;23(2):e12137.
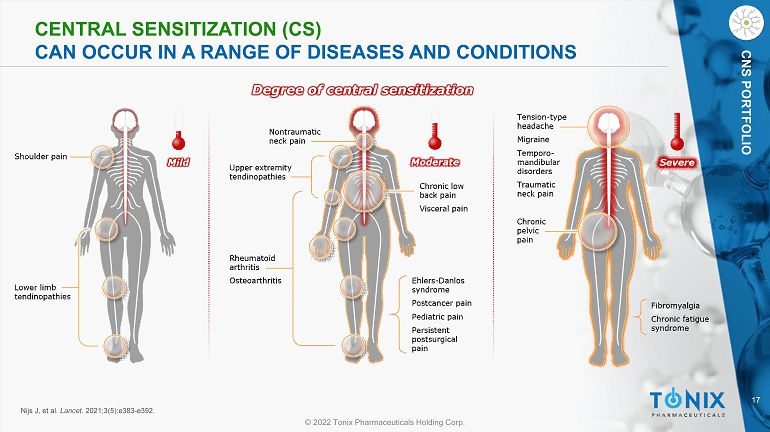
17 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CENTRAL SENSITIZATION (CS) CAN OCCUR IN A RANGE OF DISEASES AND CONDITIONS Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
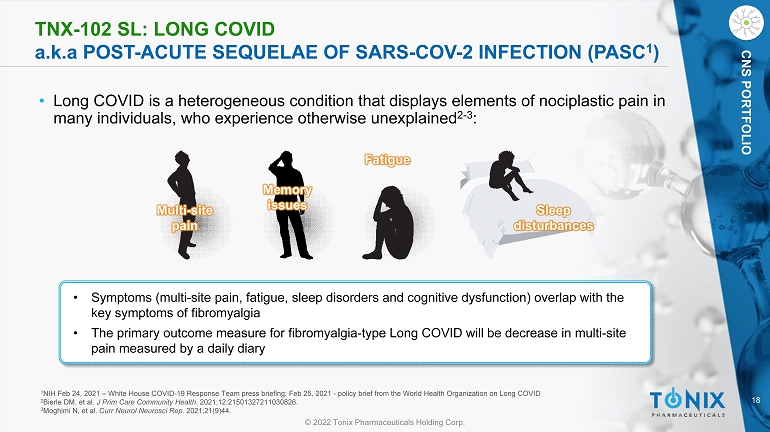
18 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: LONG COVID a.k.a POST - ACUTE SEQUELAE OF SARS - COV - 2 INFECTION (PASC 1 ) • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained 2 - 3 : • Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia • The primary outcome measure for fibromyalgia - type Long COVID will be decrease in multi - site pain measured by a daily diary Multi - site pain Memory issues Fatigue Sleep disturbances 1 NIH Feb 24, 2021 – White House COVID - 19 Response Team press briefing; Feb 25, 2021 - policy brief from the World Health Organiza tion on Long COVID 2 Bierle DM, et al. J Prim Care Community Health . 2021;12:21501327211030826. 3 Moghimi N, et al. C urr Neurol Neurosci Rep . 2021;21(9)44.
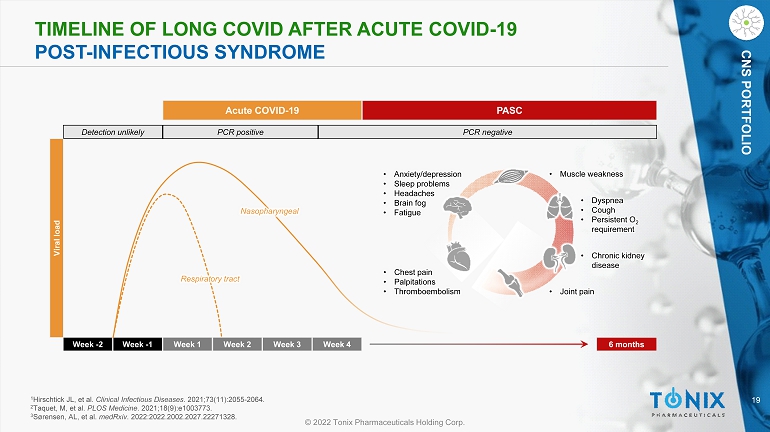
19 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TIMELINE OF L ONG COVID AFTER ACUTE COVID - 19 POST - INFECTIOUS SYNDROME Week - 2 Week - 1 Week 1 Week 2 Week 3 Week 4 6 months Detection unlikely PCR positive PCR negative Acute COVID - 19 PASC Viral load Nasopharyngeal Respiratory tract • Chest pain • Palpitations • Thromboembolism • Dyspnea • Cough • Persistent O 2 requirement • Anxiety/depression • Sleep problems • Headaches • Brain fog • Fatigue • Chronic kidney disease • Muscle weakness • Joint pain 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773. 3 Sørensen, AL, et al. medRxiv . 2022:2022.2002.2027.22271328.
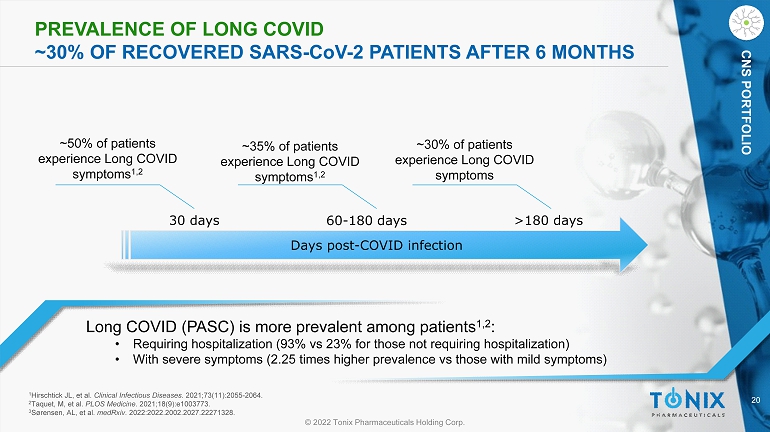
20 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PREVALENCE OF L ONG COVID ~30% OF RECOVERED SARS - C o V - 2 PATIENTS AFTER 6 MONTHS Long COVID (PASC) is more prevalent among patients 1,2 : • Requiring hospitalization (93% vs 23% for those not requiring hospitalization) • With severe symptoms (2.25 times higher prevalence vs those with mild symptoms) ~50% of patients experience L ong COVID symptoms 1,2 Days post - COVID infection 30 days 60 - 180 days >180 days ~35% of patients experience Long COVID symptoms 1,2 ~30% of patients experience Long COVID symptoms 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773. 3 Sørensen, AL, et al. medRxiv . 2022:2022.2002.2027.22271328.
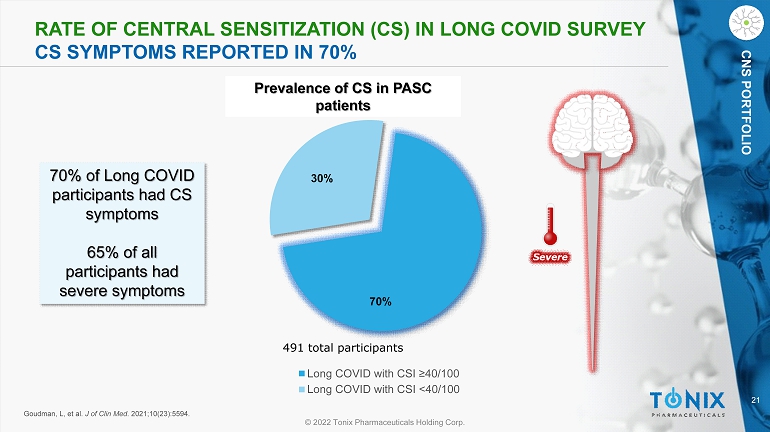
21 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO RATE OF CENTRAL SENSITIZATION (CS) IN L ONG COVID SURVEY CS SYMPTOMS REPORTED IN 70% 70% 30% Long COVID with CSI ≥40/100 Long COVID with CSI <40/100 491 total participants Goudman , L, et al. J of Clin Med . 2021;10(23):5594. 70% of Long COVID participants had CS symptoms 65% of all participants had severe symptoms Prevalence of CS in PASC patients
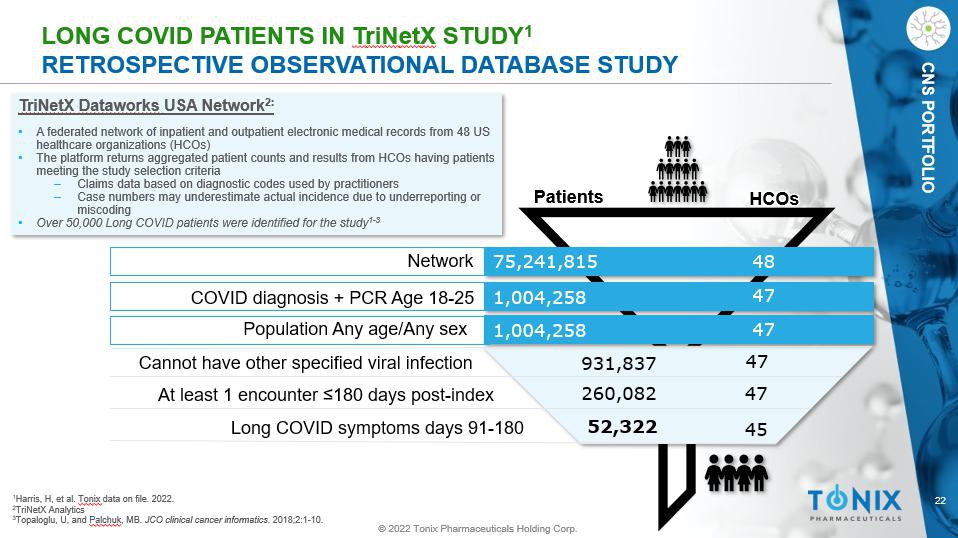
22 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO L ONG COVID PATIENTS IN T ri N et X STUDY 1 RETROSPECTIVE OBSERVATIONAL DATABASE STUDY 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 3 Topaloglu, U, and Palchuk , MB. JCO clinical cancer informatics . 2018;2:1 - 10. TriNetX Dataworks USA Network 2: • A federated network of inpatient and outpatient electronic medical records from 48 US healthcare organizations (HCOs) • The platform returns aggregated patient counts and results from HCOs having patients meeting the study selection criteria ‒ Claims data based on diagnostic codes used by practitioners ‒ Case numbers may underestimate actual incidence due to underreporting or miscoding • Over 50,000 Long COVID patients were identified for the study 1 - 3 52,322 260,082 931,837 47 47 45 48 47 47 75,241,815 1,004,258 1,004,258 HCOs Patients Network COVID diagnosis + PCR Age 18 - 25 Population Any age/Any sex Cannot have other specified viral infection At least 1 encounter ≥180 days post - index Long COVID symptoms days 91 - 180
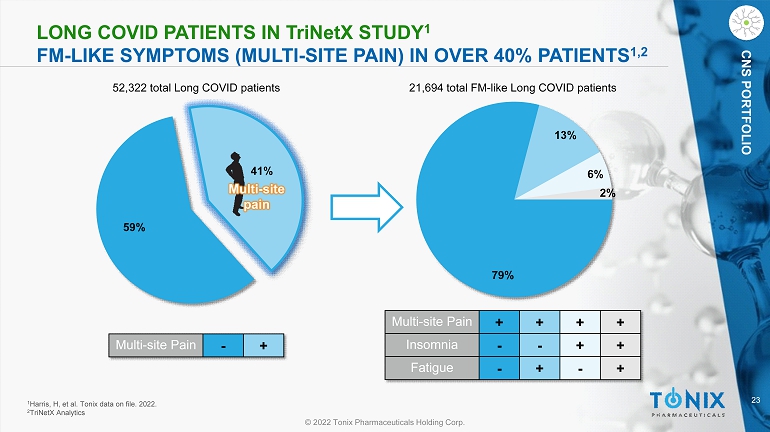
23 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO L ONG COVID PATIENTS IN T ri N et X STUDY 1 FM - LIKE SYMPTOMS (MULTI - SITE PAIN) IN OVER 40% PATIENTS 1,2 79% 13% 6% 2% 52,322 total Long COVID patients 21,694 total FM - like Long COVID patients 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 59% 41% Multi - site pain Multi - site Pain + + + + Insomnia - - + + Fatigue - + - + Multi - site Pain - +
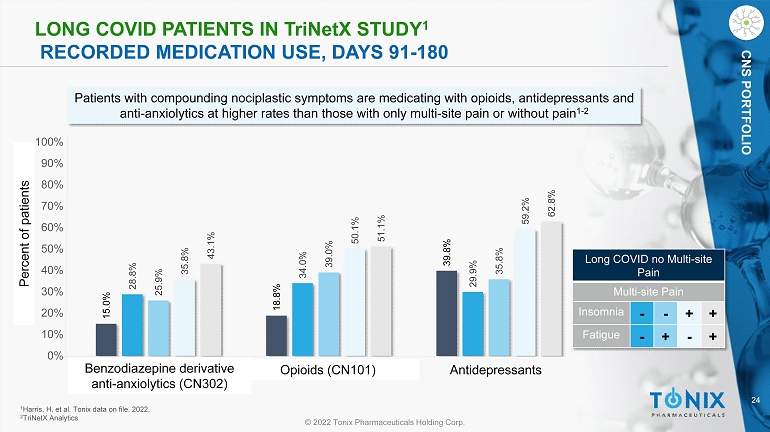
24 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO L ONG COVID PATIENTS IN T ri N et X STUDY 1 RECORDED MEDICATION USE, DAYS 91 - 180 15.0% 18.8% 39.8% 28.8% 34.0% 29.9% 25.9% 39.0% 35.8% 35.8% 50.1% 59.2% 43.1% 51.1% 62.8% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Patients with compounding nociplastic symptoms are medicating with opioids, antidepressants and anti - anxiolytics at higher rates than those with only multi - site pain or without pain 1 - 2 1 Harris, H, et al. Tonix dat a on file . 2022. 2 TriNetX Analytics Percent of patients Benzodiazepine derivative anti - anxiolytics (CN302) Opioids (CN101) Antidepressants Long COVID no Multi - site Pain Multi - site Pain Insomnia - - + + Fatigue - + - +
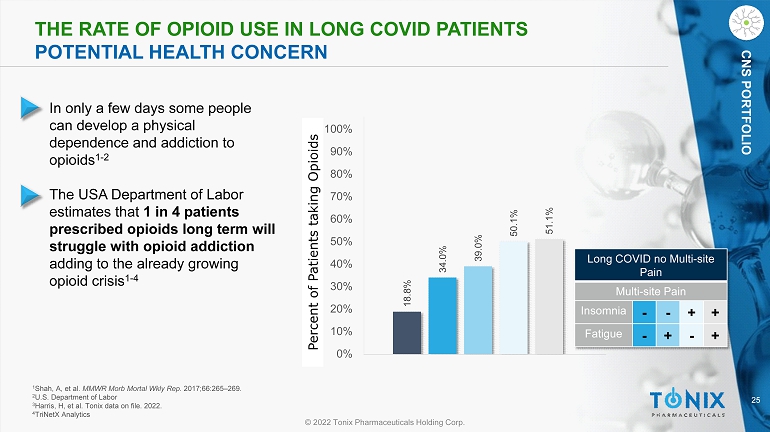
25 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO 18.8% 34.0% 39.0% 50.1% 51.1% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Percent of Patients taking Opioids THE RATE OF OPIOID USE IN L ONG COVID PATIENTS POTENTIAL HEALTH CONCERN • In only a few days some people can develop a physical dependence and addiction to opioids 1 - 2 • The USA Department of Labor estimates that 1 in 4 patients prescribed opioids long term will struggle with opioid addiction adding to the already growing opioid crisis 1 - 4 1 Shah, A, et al. MMWR Morb Mortal Wkly Rep. 2017;66:265 – 269. 2 U.S. Department of Labor 3 Harris, H, et al. Tonix data on file. 2022. 4 TriNetX Analytics Long COVID no Multi - site Pain Multi - site Pain Insomnia - - + + Fatigue - + - +
26 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO SIGNIFICANT FINANCIAL IMPACT OF L ONG COVID FOR HOUSEHOLDS AND ECONOMIES 25% of Long COVID patients are unable to return to work 1 Over 250,000 Quality Adjusted Life - Years (QUALYS) will be lost due to Long COVID in the UK 2 $23.3 billion is estimated to be paid by the UK government to avoid QUALY losses due to Long COVID 2 1 Davis, HE, et al. eClinicalMedicine . 2021;38. 2 Martin, C, et al. PloS one . 2021;16(12):e0260843 - e0260843.
27 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO L ONG COVID PRESIDENTIAL MEMORANDUM PRESIDENT BIDEN – APRIL 5, 2022 1 Policy • Commits to redoubling efforts to address the long - term effects of COVID - 19 Organizing Government Wide Response • Harnesses the full potential of the Federal Government, in coordination with public - and private - sector partners, to mount a full and effective response National Research Action Plane • Coordinates efforts across the public and private sectors • Orders establishment of the first - ever interagency national research agenda to, among other things, foster development of new treatments based on a better understanding of the pathophysiological mechanisms of the SARS - CoV - 2 virus Previously, Congress awarded NIH $1.15 billion to study Long COVID. 2 • Funded among other things the RECOVER Initiative implemented by the National Institutes of Health. 1 April 5, 2022 President Biden. “Memorandum on Addressing the Long - Term Effects of COVID - 19 - www.whitehouse.gov/briefing - room/pr esidential - actions/2022/04/05/memorandum - on - addressing - the - long - term - effects - of - covid - 19/ 2 The NIH provision of Title III Health and Human Services, Division M -- Coronavirus Response and Relief Supplemental Appropriation s Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260.

28 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: L ONG COVID ( PASC ) CYCLOBENZAPRINE PROTECTIC ® SUBLINGUAL TABLETS Long COVID or Post - acute Sequelae of COVID - 19 (PASC 1 ) • Symptoms can include fatigue, sleep disorders, pain, fevers, shortness of breath, cognitive impairment described as “brain fog”, gastrointestinal symptoms, anxiety, and depression 1 • Can persist for months and can range in severity from mild to incapacitating • Occurs in 30% of recovered COVID - 19 patients • Typically associated with moderate or severe COVID - 19, Long COVID can occur after mild COVID - 19 or even after asymptomatic SARS - CoV - 2 infection Market Entry : Long COVID (PASC) Status: Clinical – IND clearance granted Next Steps: Start Phase 2 study for treating subset of Long COVID patients whose symptoms overlap with fibromyalgia in 2Q 2022 1 Nalbandian, Ani, et al. "Post - acute COVID - 19 syndrome." Nature Medicine (2021): 1 - 15. *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO
29 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1300*: COCAINE INTOXICATION COCAINE ESTERASE ( CocE ) Cocaine is the main cause for drug - related ED visits 1 Cocaine use can cause irreversible structural damage to the heart and accelerate cardiovascular disease 2 • In one survey of 94 long - term cocaine users, 71% had some form of cardiovascular disease 3 CocE is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes Market Entry: Cocaine Intoxication Additional Indications: Cocaine Overdose Status: Phase 2 Open Label Next Steps: 2Q 2022 Initiate Trial 1 Havakuk O et al. J Am Coll Cardiol . 2017;70:101 - 113. 2 Phillips K et al. Am J Cardiovasc Drugs . 2009;9:177 - 196. 3 Maceira AM et al. J Cardiovasc Magn Reson . 2014;16:26. ED = emergency department. FDA Breakthrough Therapy Designation *TNX - 1300 has not been approved for any indication. CNS PORTFOLIO
30 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: MIGRAINE INTRANASAL POTENTIATED OXYTOCIN (OT) WITH MAGNESIUM CNS PORTFOLIO Intranasal OT has potential utility in treating migraine 1 • Intranasal OT reaches the trigeminal ganglion • Preclinical evidence of OT blocking CGRP release and suppressing pain • Association of low OT levels during and preceding migraine episodes • Novel non - CGRP antagonist approach to treatment Magnesium is known to potentiate the binding of OT to its receptor 2,3 One billion individuals worldwide suffer from migraines Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Clinical – IND cleared for prevention of migraine headache 4 Next Steps: 2H 2022 Initiate Phase 2 Trial and Investigator Initiated Phase 2 Trial in Binge Eating Disorder 1 Tzabazis A, et al. Oxytocin and Migraine Headache. Headache. 2017 May;57 Suppl 2:64 - 75. doi : 10.1111/head.13082. PMID: 28485846. 2 Antoni FA, Chadio SE. Essential role of magnesium in oxytocin - receptor affinity and ligand specificity. Biochem J. 1989 Jan 15;257(2):611 - 4. doi : 10.1042/bj2570611. PMID: 2539090; PMCID: PMC1135623. 3 Meyerowitz , J.G., et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol (2022). ( https://doi.org/10.1038/s41594 - 022 - 00728 - 4) 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide.
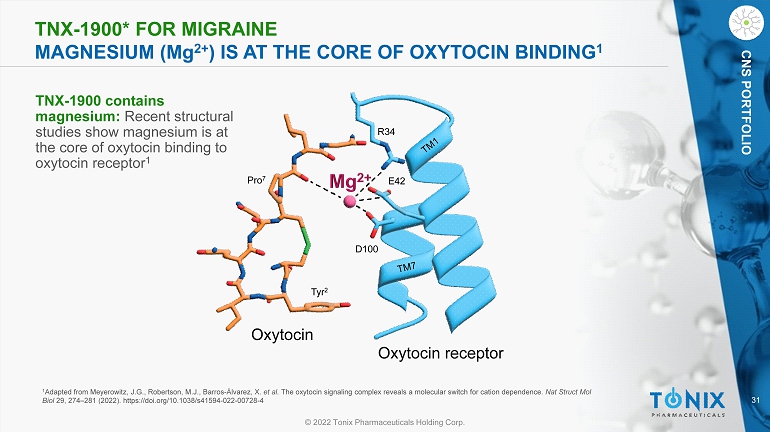
31 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900* FOR MIGRAINE MAGNESIUM (M g 2+ ) IS AT THE CORE OF OXYTOCIN BINDING 1 1 Adapted from Meyerowitz, J.G., Robertson, M.J., Barros - Álvarez, X. et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol 29, 274 – 281 (2022). https://doi.org/10.1038/s41594 - 022 - 00728 - 4 R34 E42 D100 Tyr 2 Pro 7 Oxytocin receptor Oxytocin TNX - 1900 contains magnesium: Recent structural studies show magnesium is at the core of oxytocin binding to oxytocin receptor 1
32 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 601 CR*: DEPRESSION TIANEPTINE OXALATE AND NALOXONE CNS PORTFOLIO A novel, oral, controlled release once - daily tablet Mechanistically different from traditional monoaminergic treatments for depression Indirectly modulates the glutamatergic system • No direct binding to NMDA, AMPA, or kainate receptors Naloxone added to deter parenteral abuse Treatment effect of tianeptine in depression is well - established Market Entry: Major Depressive Disorder Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids Status: pre - IND Next Steps: 1Q 2023 Initiate Phase 2 Trial AMPA= α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid; MAOI=monoamine oxidase inhibitors; NMDA=N - methyl - D - aspartate. *TNX - 601 CR is in the pre - IND stage of development and has not been approved for any indication.

© 2022 Tonix Pharmaceuticals Holding Corp. FUTURE OUTLOOK
34 © 2022 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND COLORECTAL CANCERS TNX - 3600: MONOCLONAL ANTIBODIES FOR COVID - 19 TREATMENT KEY DEVELOPMENT PARTNERS TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 801: SMALLPOX AND MONKEYPOX VACCINE TNX - 1840 and TNX - 1850: COVID - 19 VACCINES TNX - 2900: PRADER - WILLI SYNDROME TNX - 3700 : COVID - 19 VACCINE (ZINC NANOPARTICLE mRNA TECHNOLOGY ) TNX - 2300 : BOVINE PARAINFLUEZNA VIRUS

35 © 2022 Tonix Pharmaceuticals Holding Corp. Expected Clinical Trial Initiations □ 2 nd Quarter 2022 Phase 2 OL safety study start of TNX - 1300 in ED setting for cocaine intoxication □ 2 nd Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of PTSD in Kenya □ 2 nd Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of Long COVID □ 2 nd Half 2022 Phase 2 study start of TNX - 1900 for the treatment of migraine □ 2 nd Half 2022 Phase 1 study start of TNX - 1500 for prevention of allograft rejection □ 1 st Quarter 2023 Phase 2 study start of TNX - 601 CR for the treatment of major depressive disorder MILESTONES: RECENTLY COMPLETED AND UPCOMING* * We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones. □ 1 st Quarter 2021 Non - human primate positive efficacy data from TNX - 1800 in COVID - 19 models reported □ 1 st Quarter 2022 First - in - human study of TNX - 2100 initiated for skin test to detect T cell immunity to SARS - CoV - 2 □ 1 st Quarter 2022 Topline data from Phase 3 F306/RALLY study of TNX - 102 SL for the management of fibromyalgia □ 2 nd Quarter 2022 Phase 3 F307/RESILIENT study start of TNX - 102 SL for the management of fibromyalgia x x x x Expected Data □ 1 st Quarter 2023 Interim analysis results of Phase 3 F307/RESILIENT study of TNX - 102 SL in fibromyalgia

36 © 2022 Tonix Pharmaceuticals Holding Corp. MANAGEMENT TEAM Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

© 2022 Tonix Pharmaceuticals Holding Corp. THANK YOU