
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.02


Financial Disclosures • Funding is provided for these studies via NIH Grants and Tonix Pharmaceuticals Inc.

Long - term rejection free renal allograft survival with Fc - modified anti - CD154 antibody monotherapy in nonhuman primates. Grace Lassiter, Takayuki Hirose, Ashley D’Attilio, Ryo Otsuka, Ahmad Karadagi, Toshihide Tomosugi, Tatsuo Kawai

Background • Current immunosuppressive regimens have significant side effects • Nephrotoxicity • Steroid induced diabetes • Cytopenia • Increased risk of infection • etc… • Belatacept is currently the only FDA approved costimulatory blockade alternative to calcineurin inhibitors. • Higher rate of acute cellular rejection compared to conventional immunosuppression
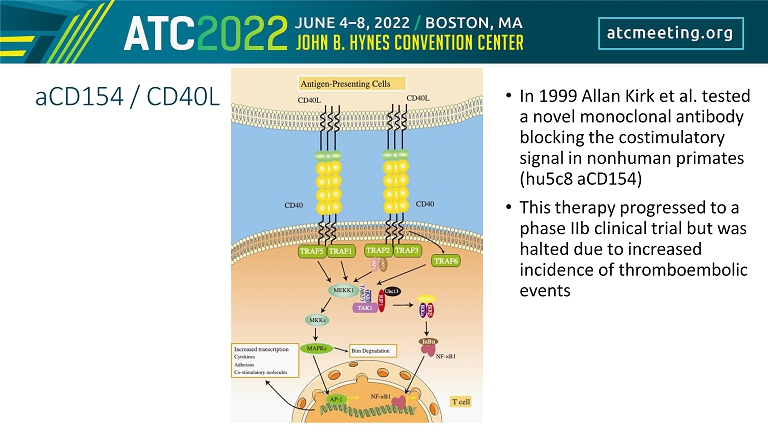
aCD154 / CD40L • In 1999 Allan Kirk et al. tested a novel monoclonal antibody blocking the costimulatory signal in nonhuman primates (hu5c8 aCD154) • This therapy progressed to a phase IIb clinical trial but was halted due to increased incidence of thromboembolic events
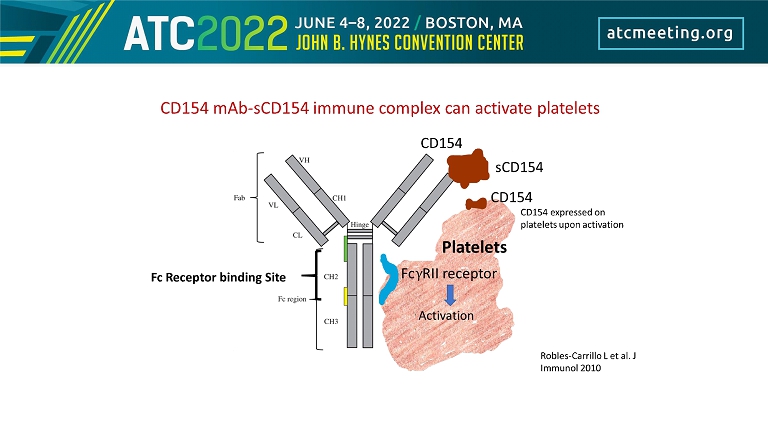
γ
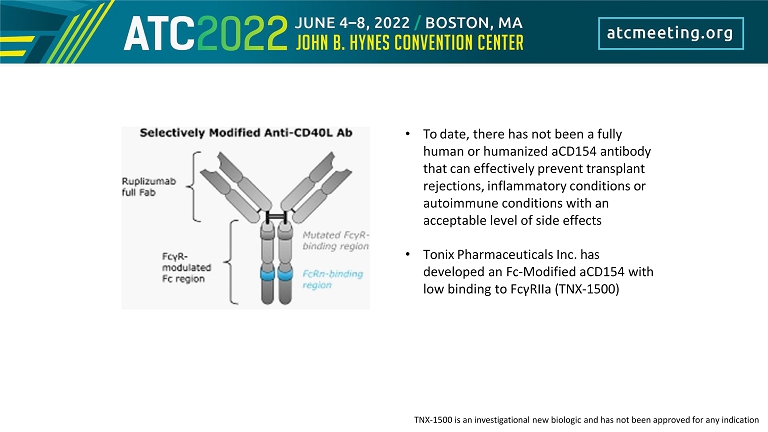
• To date, there has not been a fully human or humanized aCD154 antibody that can effectively prevent transplant rejections, inflammatory conditions or autoimmune conditions with an acceptable level of side effects • Tonix Pharmaceuticals Inc. has developed an Fc - Modified aCD154 with low binding to FcγRIIa (TNX - 1500) TNX - 1500 is an investigational new biologic and has not been approved for any indication
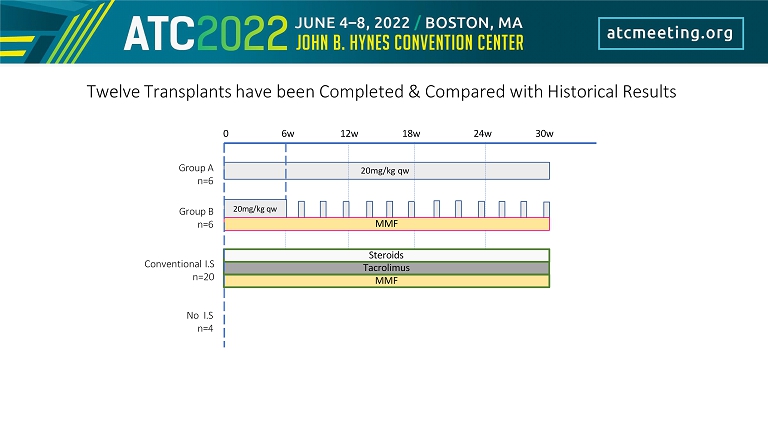
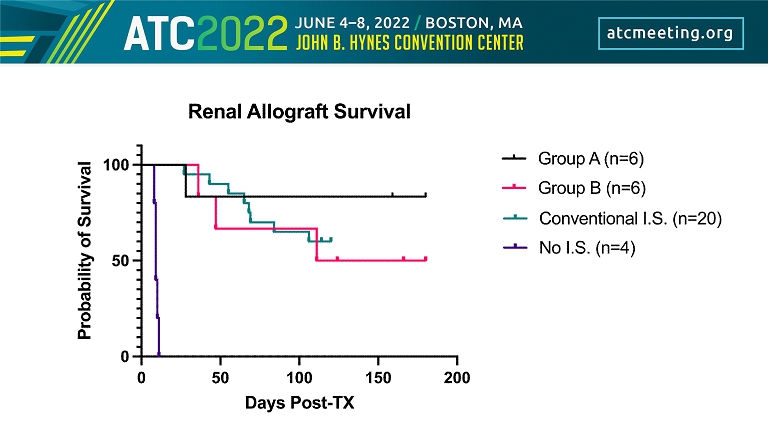
0 6w 12w 18w 24w 30w 20mg/kg qw 20mg/kg qw MMF Tacrolimus Group A n=6 Group B n=6 Conventional I.S n=20 Twelve Transplants have been Completed & Compared with Historical Results Steroids MMF No I.S n=4
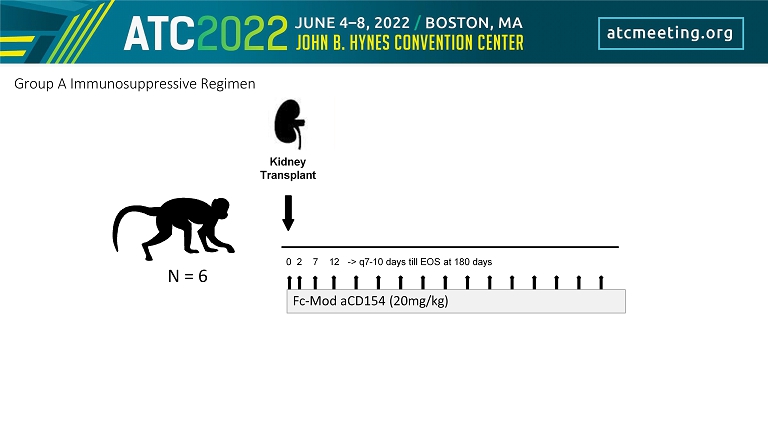
0 2 7 12 - > q7 - 10 days till EOS at 180 days Kidney Transplant Group A Immunosuppressive Regimen N = 6 Fc - Mod aCD154 (20mg/kg)
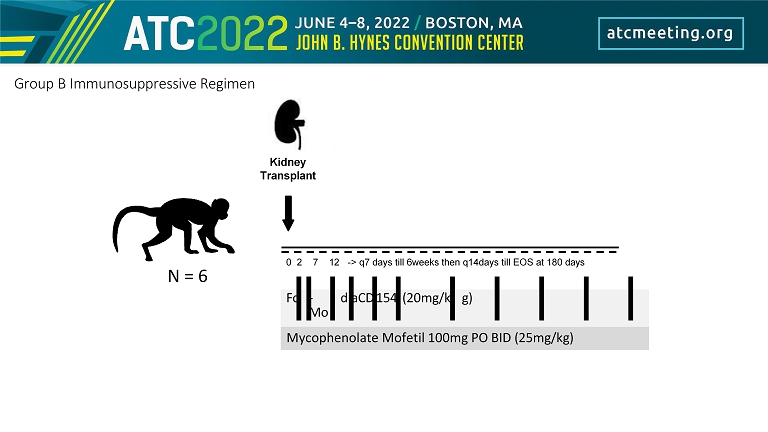
0 2 7 12 - > q7 days till 6weeks then q14days till EOS at 180 days Fc - Mo d aCD 154 (20mg/k g) Mycophenolate Mofetil 100mg PO BID (25mg/kg) Kidney Transplant N = 6 Group B Immunosuppressive Regimen
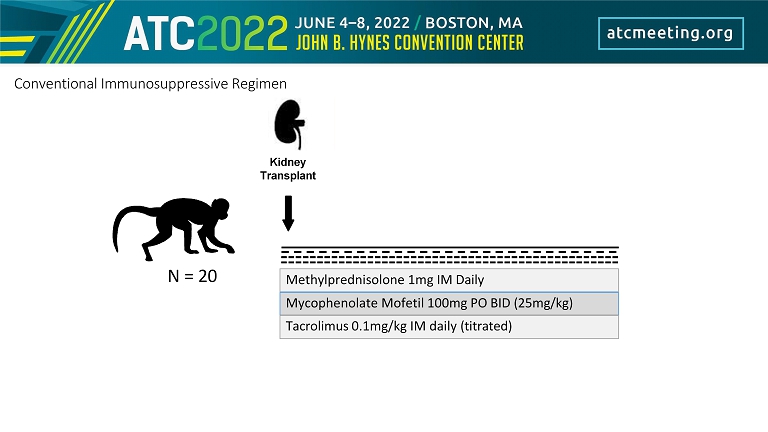
Methylprednisolone 1mg IM Daily Mycophenolate Mofetil 100mg PO BID (25mg/kg) Tacrolimus 0.1mg/kg IM daily (titrated) Kidney Transplant N = 20 Conventional Immunosuppressive Regimen
Kidney Transplant N = 4 No Immunosuppressive Regimen
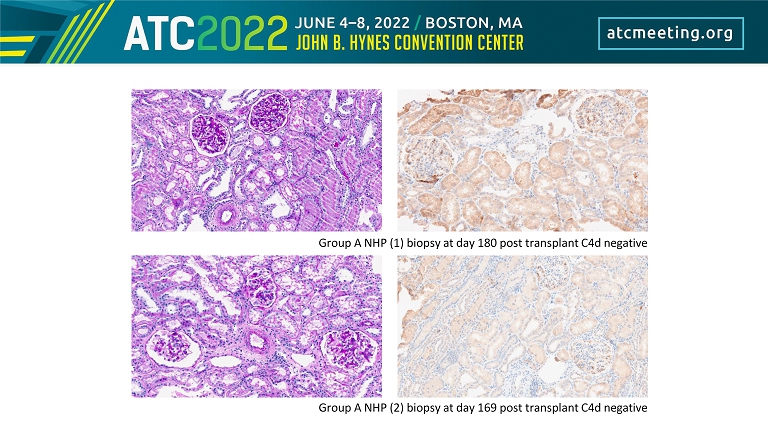
Group A NHP (1) biopsy at day 180 post transplant C4d negative Group A NHP (2) biopsy at day 169 post transplant C4d negative
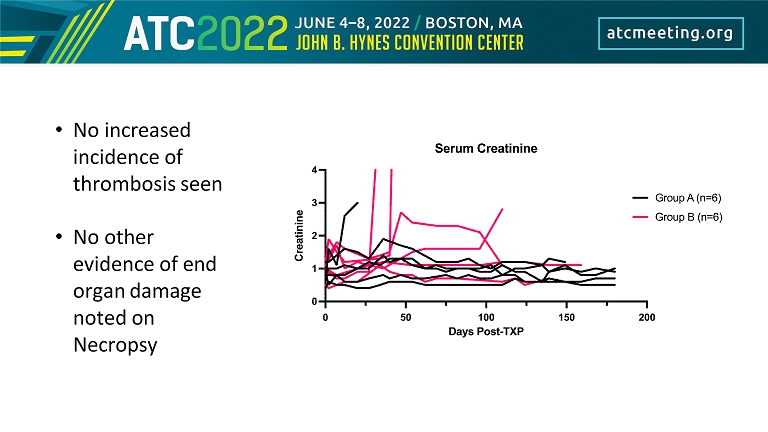
• No increased incidence of thrombosis seen • No other evidence of end organ damage noted on Necropsy
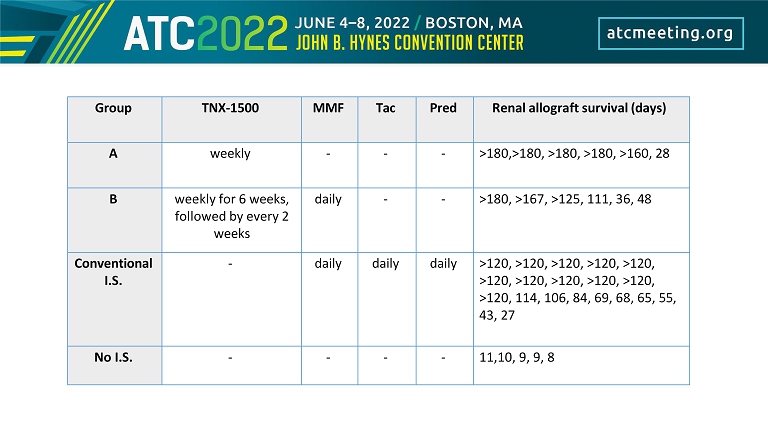
Group TNX - 1500 MMF Tac Pred Renal allograft survival (days) A weekly - - - >180,>180, >180, >180, >160, 28 B weekly for 6 weeks, followed by every 2 weeks daily - - >180, >167, >125, 111, 36, 48 Conventional I.S. - daily daily daily >120, >120, >120, >120, >120, >120, >120, >120, >120, >120, >120, 114, 106, 84, 69, 68, 65, 55, 43, 27 No I.S. - - - - 11,10, 9, 9, 8
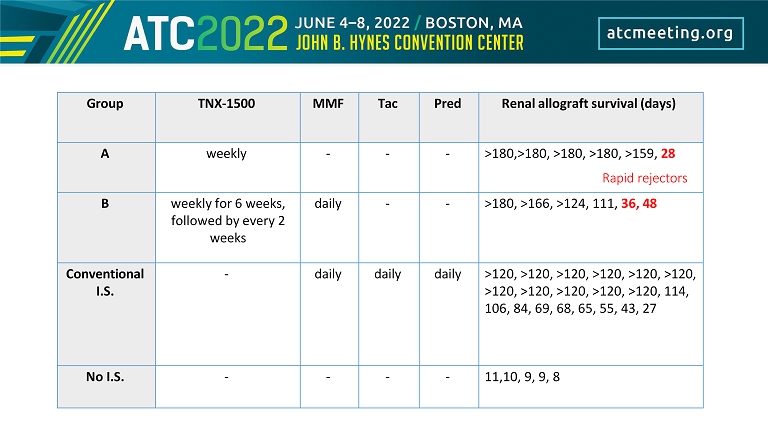
Group TNX - 1500 MMF Tac Pred Renal allograft survival (days) A weekly - - - >180,>180, >180, >180, >159, 28 Rapid rejectors B weekly for 6 weeks, followed by every 2 weeks daily - - >180, >166, >124, 111, 36, 48 Conventional I.S. - daily daily daily >120, >120, >120, >120, >120, >120, >120, >120, >120, >120, >120, 114, 106, 84, 69, 68, 65, 55, 43, 27 No I.S. - - - - 11,10, 9, 9, 8
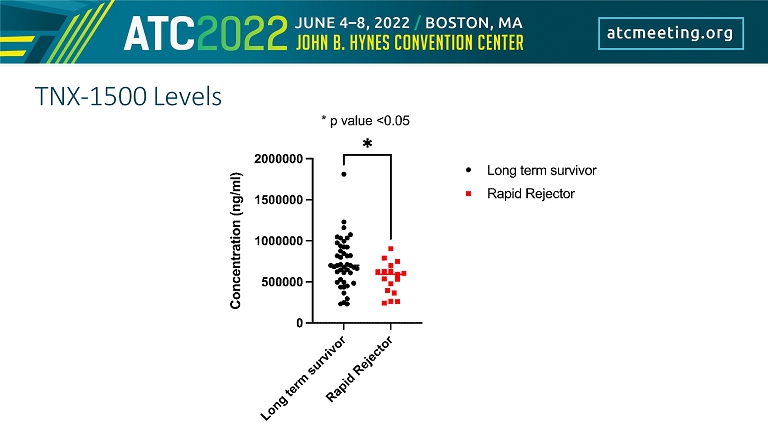
TNX - 1500 Levels

Conclusion • Fc - Modified aCD154 is well tolerated and can be an effective alternative to conventional immunosuppression therapy in nonhuman primates. ▪ TNX - 1500 in combination with MMF resulted in an increased rate of graft failure compared to monotherapy ▪ Optimal dosage remains to be defined

Takayuki Hirose Ryo Otsuka Ahmad Karadagi Toshi Tomosugi Kohei Kinoshita Abbas Dehnadi Cindy Miller Jane O Franzi Pollok R.N. Pierson, III A.Benedict Cosimi Tatsuo Kawai Knight Surgery Research Laboratory Jessica Burke Anet Calisir Nick Deluca Nelson Marquez Carvajal Eli Smith Michael Duggan MGH Pathology Ivy Rosales Robert Colvin Catherine Stevens Questions? Special Thanks to these wonderful people