
TONIX PHARMACEUTICALS HOLDING CORP. 8-K
Exhibit 99.03

TNX - 1500, an Fc - modified Anti - CD154 Antibody, Prolongs Nonhuman Primate Cardiac Allograft Survival Shuhei Miura 1 , Zahra H. Abady 1 , Franziska Pollok 1 , Madelyn Ma 1 , Kohei Kinoshita 1 , Siobhan Fogarty 2 , Patrick Maguire 2 , Bruce Daugherty 2 , Seth Lederman 2 , Richard N. Pierson III 1 1 Center for Transplantation Sciences, Massachusetts General Hospital, Boston MA, 2 Tonix Pharmaceuticals, Chatham, NJ, USA

Shuhei Miura, MD, Research fellow Center for Transplantation Sciences, Massachusetts General Hospital, Boston MA I have no financial relationships with commercial interests to disclose AND My presentation does not include discussion of off - label or investigational use. SF, BD and SL are Tonix employees, and PM is a Tonix consultant This work was supported by Tonix through a Sponsored Research Agreement.
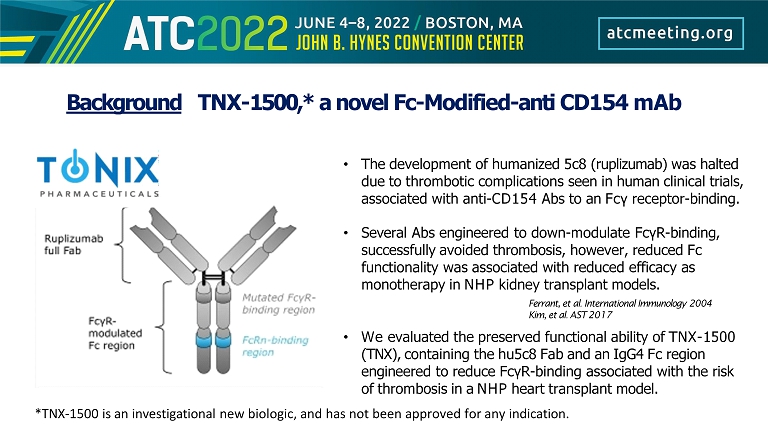
Background TNX - 1500,* a novel Fc - Modified - anti CD154 mAb • The development of humanized 5c8 (ruplizumab) was halted due to thrombotic complications seen in human clinical trials, associated with anti - CD154 Abs to an Fcγ receptor - binding. • Several Abs engineered to down - modulate FcγR - binding, successfully avoided thrombosis, however, reduced Fc functionality was associated with reduced efficacy as monotherapy in NHP kidney transplant models. Ferrant, et al. International Immunology 2004 Kim, et al. AST 2017 • We evaluated the preserved functional ability of TNX - 1500 (TNX), containing the hu5c8 Fab and an IgG4 Fc region engineered to reduce FcγR - binding associated with the risk of thrombosis in a NHP heart transplant model. *TNX - 1500 is an investigational new biologic, and has not been approved for any indication.
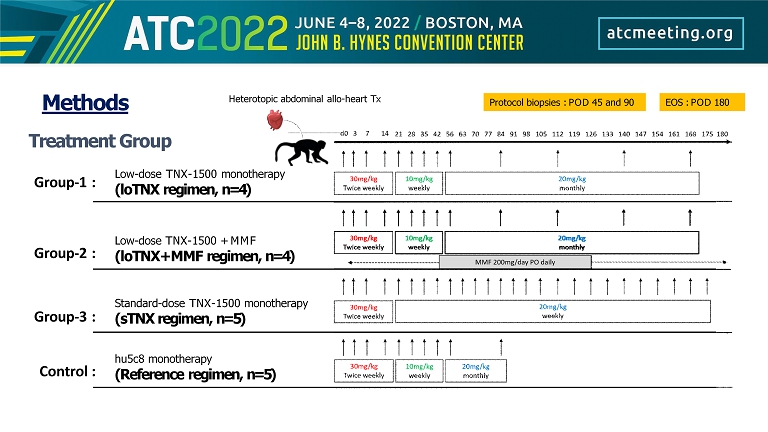
Treatment Group Low - dose TNX - 1500 monotherapy (loTNX regimen, n=4) Low - dose TNX - 1500 + MMF (loTNX+MMF regimen, n=4) Protocol biopsies : POD 45 and 90 Methods Heterotopic abdominal allo - heart Tx EOS : POD 180 Group - 1 : Group - 2 : Group - 3 : Control : hu5c8 monotherapy (Reference regimen, n=5) Standard - dose TNX - 1500 monotherapy (sTNX regimen, n=5)
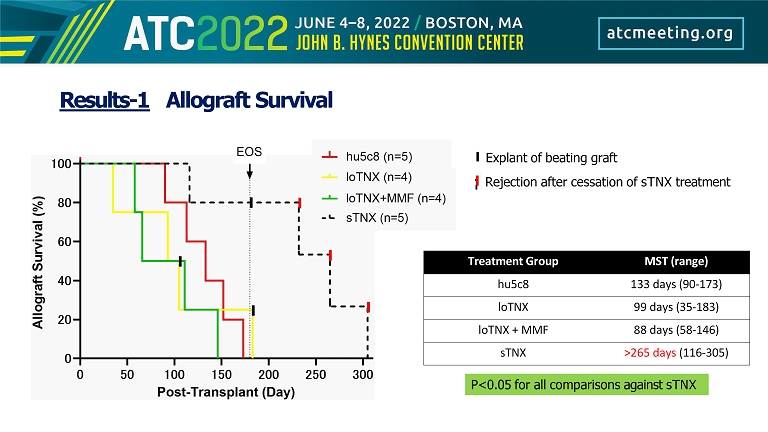
Results - 1 Allograft Survival Treatment Group MST (range) hu5c8 133 days (90 - 173) loTNX 99 days (35 - 183) loTNX + MMF 88 days (58 - 146) sTNX >265 days (116 - 305) Explant of beating graft Rejection after cessation of sTNX treatment P<0.05 for all comparisons against sTNX
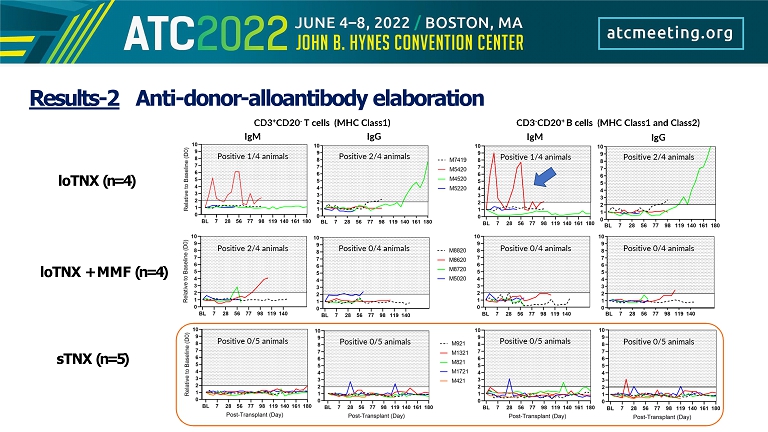
Results - 2 Anti - donor - alloantibody elaboration loTNX (n=4) loTNX + MMF (n=4) sTNX (n=5)
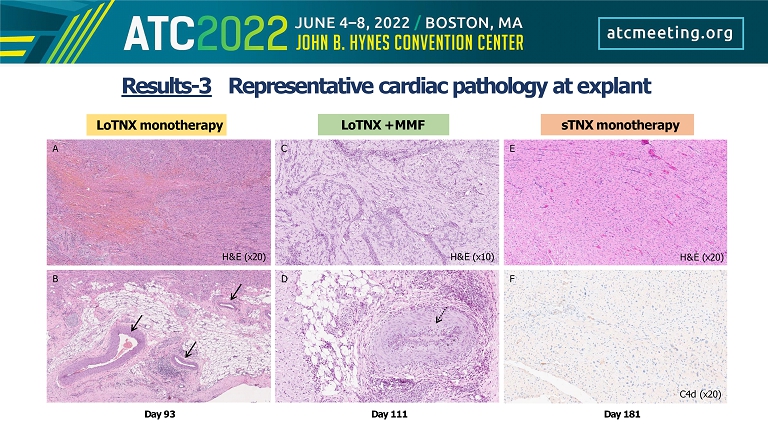
LoTNX monotherapy Day 111 Day 181 LoTNX + MMF sTNX monotherapy Day 93 A B C D E F H&E (x20) H&E (x10) H&E (x20) C4d (x20) Results - 3 Representative cardiac pathology at explant
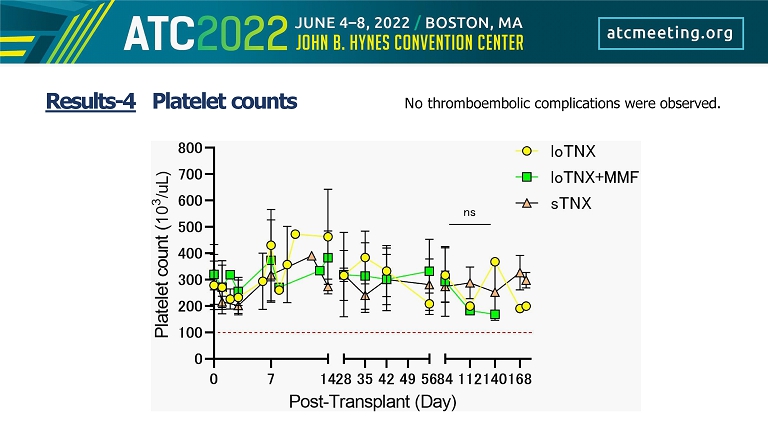
Results - 4 Platelet counts No thromboembolic complications were observed.
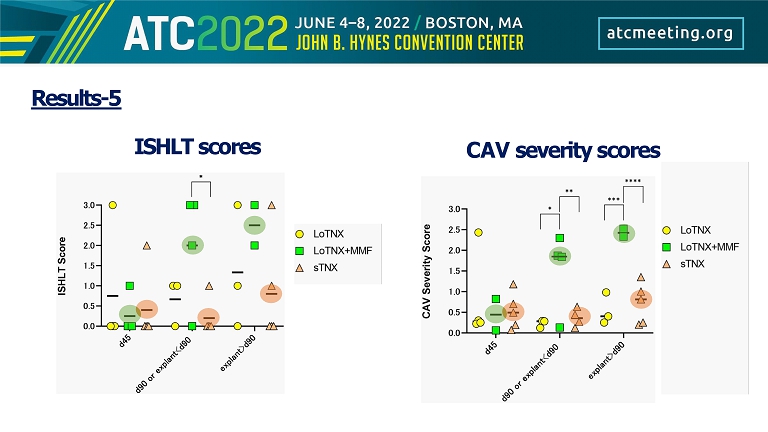
Results - 5 ISHLT scores CAV severity scores

Conclusion • Blockade of CD154 with TNX - 1500 monotherapy consistently and safely prevented pathologic alloimmunity in a NHP cardiac allograft model at least as effectively as hu5c8 monotherapy, without clinical thrombotic events. • "Standard - dose” TNX - 1500 regimen was associated with prolonged allograft survival relative to “low - dose” maintenance regimen, with or without MMF, as supported by prevention of antidonor alloAb elaboration, reduced ISHLT and CAV severity scores.