
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2022 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P0364 July 25, 2022 (Doc 1075)

2 © 2022 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2022 Tonix Pharmaceuticals Holding Corp. ADVANCING THE SCIENCE AND UNDERSTANDING OF DISEASES by developing innovative therapies that improve population health by focusing on unmet needs in patient care What we do Using our integrated development engine, we advance innovative programs across multiple therapeutic areas into the clinic while maximizing asset potential OUR MISSION OUR STRATEGY

4 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CNS PORTFOLIO ADHD = attention - deficit hyperactivity disorder; FM = fibromyalgia; IND = investigational new drug; PASC = post - acute sequelae o f COVID - 19; PTSD = posttraumatic stress disorder. Pipeline: Central Nervous System (CNS) Portfolio CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 102 SL 1 Fibromyalgia (FM) Posttraumatic Stress Disorder (PTSD) Long COVID (PASC 2 ) Mid - Phase 3 Phase 2, Targeted 3Q 2022 Start Phase 2, Targeted 3Q 2022 Start 3 TNX - 1300 4 Cocaine Intoxication / Overdose FDA Breakthrough Designation Mid - Phase 2 TNX - 1900 5 Migraine, Craniofacial Pain and Binge Eating Disorder Phase 2, Targeted 2H 2022 Start 6 TNX - 601 ER Depression, PTSD, Neurocognitive Dysfunction from Steroids Phase 2, Targeted 1Q 2023 Start 7 TNX - 1600 8 Depression, PTSD and ADHD Preclinical *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is also in development for Agitation in A lzheimer’s Disease (AAD) and Alcohol Use D isorder (AUD). Both indications are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 IND clearance granted by FDA. Company plans to start Phase 2 study in subset of patients whose symptoms overlap with fibromya lgi a in 3Q 2022. 4 TNX - 1300 (double - mutant cocaine esterase) was licensed from Columbia University . 5 Acquired from Trigemina ; license agreement with Stanford University; IND cleared for the prevention of migraine indication; Planned Binge Eating Dis ord er study is expected to be investigator initiated. 6 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900; Phase 2 for the prevention of migraine headache expected to start 2H 2022 7 TNX - 601 ER is in the pre - IND stage in the U.S.; a Phase 1 trial for formulation development was completed outside of the U.S; Ph ase 2 expected to start 1Q 2023 8 Acquired from TRImaran Pharma; license agreement with Wayne State University

5 © 2022 Tonix Pharmaceuticals Holding Corp. RARE DISEASE & IMMUNOLOGY PORTFOLIOS Pipeline Rare Disease Portfolio TNX - 2900 1 Prader - Willi Syndrome FDA Orphan Drug Designation Preclinical CANDIDATE S * INDICATION STATUS / NEXT MILESTONE *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 1500 1 Organ Transplant Rejection/ Autoimmune Conditions Phase 1, Targeted 1H 2023 Start TNX - 1700 2 Gastric and colorectal cancers Preclinical Pipeline Immunology and Immuno - Oncology portfolio *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 anti - CD40L humanized monoclonal antibody 2 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University
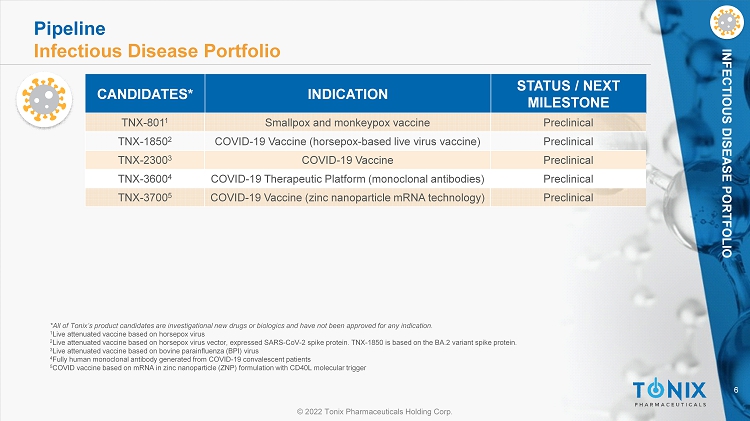
6 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 Live attenuated vaccine based on horsepox virus 2 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. TNX - 1850 is based on the BA.2 varian t spike protein. 3 Live attenuated vaccine based on bovine parainfluenza (BPI) virus 4 Fully human monoclonal antibody generated from COVID - 19 convalescent patients 5 COVID vaccine based on mRNA in zinc nanoparticle (ZNP) formulation with CD40L molecular trigger CANDIDATES* INDICATION STATUS / NEXT MILESTONE TNX - 801 1 Smallpox and monkeypox vaccine Preclinical TNX - 1850 2 COVID - 19 Vaccine (horsepox - based live virus vaccine) Preclinical TNX - 2300 3 COVID - 19 Vaccine Preclinical TNX - 3600 4 COVID - 19 Therapeutic Platform (monoclonal antibodies) Preclinical TNX - 3700 5 COVID - 19 Vaccine (zinc nanoparticle mRNA technology) Preclinical Pipeline Infectious Disease Portfolio

© 2022 Tonix Pharmaceuticals Holding Corp. CNS: KEY CANDIDATES
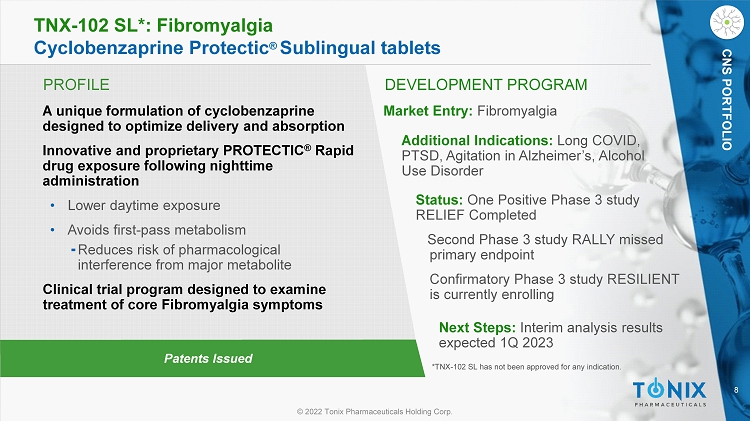
8 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual tablets CNS PORTFOLIO A unique formulation of cyclobenzaprine designed to optimize delivery and absorption Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration • Lower daytime exposure • Avoids first - pass metabolism ⁃ Reduces risk of pharmacological interference from major metabolite Clinical trial program designed to examine treatment of core Fibromyalgia symptoms Market Entry: Fibromyalgia Additional Indications: Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF Completed Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT is currently enrolling Next Steps: Interim analysis results expected 1Q 2023 *TNX - 102 SL has not been approved for any indication.

9 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Fibromyalgia Program Update CNS PORTFOLIO Phase 3 Study, RESILIENT (F307), will compare TNX - 102 SL 5.6 mg and placebo • First patient enrolled in April 2022 • Interim Analysis results expected 1Q 2023 • Parallel design, double - blind, randomized placebo - controlled study, all U.S. sites • Primary endpoint is pain at Week 14 analyzed by MMRM with MI • Projecting adverse event - related discontinuations to decrease towards rates in RELIEF and PTSD Studies Phase 3 Study, RALLY (F306), comparison of TNX - 102 SL 5.6 mg and placebo • As expected from interim analysis results published in July 2021, RALLY Study missed primary endpoint • Unexpected ~80% increase in adverse event - related discontinuations in both drug and placebo arms • Multiple imputation approach on 'Missing Data' attenuated statistical significance of efficacy endpoints’ • TNX - 102 SL was generally well tolerated with overall adverse event profile comparable to prior studies; no new safety signals observed

10 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets Long COVID or Post - acute Sequelae of COVID - 19 (PASC 1 ) • Symptoms can include fatigue, sleep disorders, pain, fevers, shortness of breath, cognitive impairment described as “brain fog”, gastrointestinal symptoms, anxiety, and depression 2 • Can persist for months and can range in severity from mild to incapacitating • Occurs in 30% of recovered COVID - 19 patients • Typically associated with moderate or severe COVID - 19, Long COVID can occur after mild COVID - 19 or even after asymptomatic SARS - CoV - 2 infection To address the urgent need for PASC therapies, Congress awarded the National Institutes of Health $1.15 billion to study Long COVID. 3 Market Entry : Long COVID (PASC) Status: Clinical – IND clearance granted Next Steps: Start Phase 2 study for treating subset of Long COVID patients whose symptoms overlap with fibromyalgia in 3Q 2022 1 Feb. 24, 2021 - White House COVID - 19 Response Team press briefing; Feb 25, 2021 - policy brief from the World Health Organizatio n on long COVID 2 Nalbandian, Ani, et al. "Post - acute COVID - 19 syndrome." Nature Medicine (2021): 1 - 15. 3 The NIH provision of Title III Health and Human Services, Division M -- Coronavirus Response and Relief Supplemental Appropriation s Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260. *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO Additional Indications: Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder

11 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Long COVID a.k.a Post - Acute Sequelae of SARS - CoV - 2 Infection (PASC) • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained 1 - 2 : • Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia • The primary outcome measure for fibromyalgia - type Long COVID will be decrease in multi - site pain measured by a daily diary Multisite pain Memory issues Fatigue Sleep disturbances 1 Bierle DM, et al. Central Sensitization Phenotypes in Post Acute Sequelae of SARS - CoV - 2 Infection (PASC): Defining the Post COVI D Syndrome. J Prim Care Community Health 2021 ;12:21501327211030826. doi : 10.1177/21501327211030826. 2 Moghimi, N. et al. The Neurological Manifestations of Post - Acute Sequelae of SARS - CoV - 2 infection Curr Neurol Neurosci Rep. 2021;21(9):44. doi : 10.1007/s11910 - 02101130 - 1.
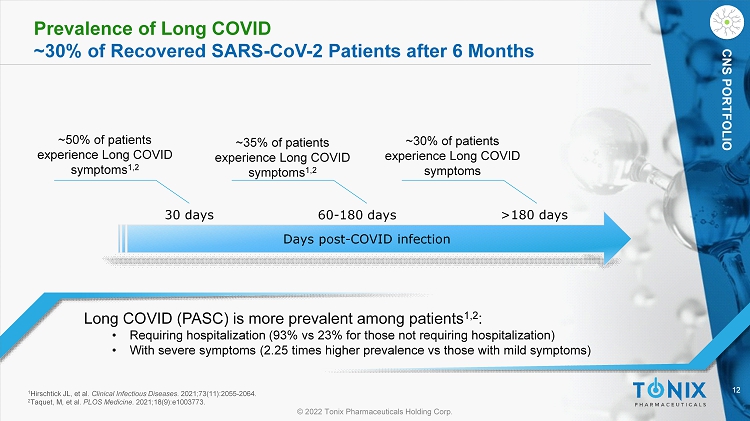
12 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Prevalence of Long COVID ~30% of Recovered SARS - CoV - 2 Patients after 6 Months Long COVID (PASC) is more prevalent among patients 1,2 : • Requiring hospitalization (93% vs 23% for those not requiring hospitalization) • With severe symptoms (2.25 times higher prevalence vs those with mild symptoms) ~50% of patients experience L ong COVID symptoms 1,2 Days post - COVID infection 30 days 60 - 180 days >180 days ~35% of patients experience Long COVID symptoms 1,2 ~30% of patients experience Long COVID symptoms 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773.

13 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO 18.8% 34.0% 39.0% 50.1% 51.1% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Percent of Patients taking Opioids Rate of Opioid Use in Long COVID Patients Potential Health Concern • In only a few days some people can develop a physical dependence and addiction to opioids 1 - 2 • The USA Department of Labor estimates that 1 in 4 patients prescribed opioids long term will struggle with opioid addiction adding to the already growing opioid crisis 1 - 2 1 Shah, A, et al. MMWR Morb Mortal Wkly Rep. 2017;66:265 – 269. 2 U.S. Department of Labor Source: Harris, H, et al. Tonix data on file. 2022.; TriNetX Analytics Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia

14 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX 102 SL*: Posttraumatic Stress disorder (PTSD) Cyclobenzaprine Protectic ® Sublingual Tablets PTSD is a serious chronic psychiatric illness • Defined as maladaptive prolonged stress response which occurs after experiencing severely injurious traumatic event(s) Affects approximately 12 million Americans adults 1,2 Large unmet clinical need and limited effective therapies available • Advances in pharmacological treatments beyond the currently approved SSRIs (e.g., Zoloft® (sertraline), Paxil® (paroxetine)) are needed 3 Market Entry: PTSD Additional Indications: Fibromyalgia, Long COVID, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Phase 2 study ( AtEase ) completed Two Phase 3 studies (HONOR, RECOVERY) conducted Next Steps: 3 Q 2022 Initiate Phase 2 Trial in Kenya 1 Goldstein RB, et al. The epidemiology of DSM - 5 posttraumatic stress disorder in the United States: results from the National Epi demiologic Survey on Alcohol and Related Conditions - III. Soc Psychiatry Psychiatr Epidemiol. 2016;51(8):1137 - 1148. 2 Pietrzak RH, et al. Prevalence and Axis I comorbidity of full and partial posttraumatic stress disorder in the United States: re sults from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Anxiety Disord . 2011;25(3):456 - 465. *TNX - 102 SL has not been approved for any indication. 3 Cain, C. K., et al. Targeting memory processes with drugs to prevent or cure PTSD. Expert Opin Investig Drugs. 2012; 21(9), 1323 - 1350
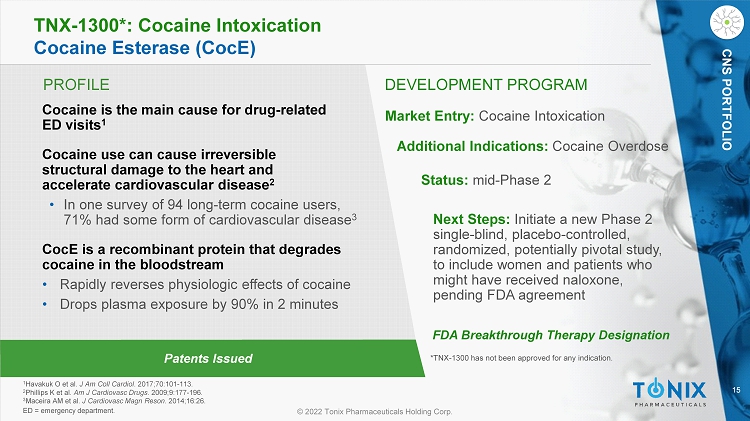
15 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1300*: Cocaine Intoxication Cocaine Esterase ( CocE ) Cocaine is the main cause for drug - related ED visits 1 Cocaine use can cause irreversible structural damage to the heart and accelerate cardiovascular disease 2 • In one survey of 94 long - term cocaine users, 71% had some form of cardiovascular disease 3 CocE is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes Market Entry: Cocaine Intoxication Additional Indications: Cocaine Overdose Status: mid - Phase 2 Next Steps: Initiate a new Phase 2 single - blind, placebo - controlled, randomized, potentially pivotal study, to include women and patients who might have received naloxone, pending FDA agreement 1 Havakuk O et al. J Am Coll Cardiol . 2017;70:101 - 113. 2 Phillips K et al. Am J Cardiovasc Drugs . 2009;9:177 - 196. 3 Maceira AM et al. J Cardiovasc Magn Reson . 2014;16:26. ED = emergency department. FDA Breakthrough Therapy Designation *TNX - 1300 has not been approved for any indication. CNS PORTFOLIO
16 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 601 E R*: Depression Tianeptine Hemioxalate Extended - Release Tablets CNS PORTFOLIO A novel, oral, extended - release once - daily tablet Mechanistically different from traditional monoaminergic treatments for depression Indirectly modulates the glutamatergic system • No direct binding to NMDA, AMPA, or kainate receptors Treatment effect of tianeptine in depression is well - established Market Entry: Major Depressive Disorder Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids Status: pre - IND Next Steps: 1Q 2023 Initiate Phase 2 Trial AMPA= α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid; MAOI=monoamine oxidase inhibitors; NMDA=N - methyl - D - aspartate. *TNX - 601 ER is in the pre - IND stage of development and has not been approved for any indication.

17 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium CNS PORTFOLIO Intranasal OT has potential utility in treating migraine 1 • Intranasal OT reaches the trigeminal ganglion • Preclinical evidence of OT blocking CGRP release and suppressing pain • Association of low OT levels during and preceding migraine episodes • Novel non - CGRP antagonist approach to treatment Magnesium is known to potentiate the binding of OT to its receptor 2,3 One billion individuals worldwide suffer from migraines Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Clinical – IND cleared for prevention of migraine headache 4 Next Steps: 2H 2022 Initiate Phase 2 Trial and Investigator Initiated Phase 2 Trial in Binge Eating Disorder 1 Tzabazis A, et al. Oxytocin and Migraine Headache. Headache. 2017 May;57 Suppl 2:64 - 75. doi : 10.1111/head.13082. PMID: 28485846. 2 Antoni FA, Chadio SE. Essential role of magnesium in oxytocin - receptor affinity and ligand specificity. Biochem J. 1989 Jan 15;257(2):611 - 4. doi : 10.1042/bj2570611. PMID: 2539090; PMCID: PMC1135623. 3 Meyerowitz , J.G., et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol (2022). ( https://doi.org/10.1038/s41594 - 022 - 00728 - 4) 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide.

18 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for Migraine Magnesium (Mg 2+ ) is at the Core of Oxytocin Binding 1 1 Adapted from Meyerowitz, J.G., Robertson, M.J., Barros - Álvarez, X. et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol 29, 274 – 281 (2022). https://doi.org/10.1038/s41594 - 022 - 00728 - 4 R34 E42 D100 Tyr 2 Pro 7 Oxytocin receptor Oxytocin TNX - 1900 contains magnesium: Recent structural studies show magnesium is at the core of oxytocin binding to oxytocin receptor 1

19 © 2022 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 1900 for Migraine Addition of Mg 2+ Expands Useful Dose Range of Oxytocin Bharadwaj VN, et al. Pharmaceutics . 2022;14(5):1105. • A nonlinear dose response has been demonstrated in the use of intranasal oxytocin • This decreases efficacy at higher doses • An “inverted U” dose response • Addition of Mg 2+ rescues the efficacy of oxytocin at high doses in preclinical study 1 3 10 30 100 300 1000 0 5 10 15 20 Oxytocin only Oxytocin with 1.75 nM Mg 2+ * * * * Oxytocin dose ( nM ) Hyperpolarization (mV) In vitro whole - cell voltage - clamp recordings of rat trigeminal nerves exposed to oxytocin solution with and without additional magnesium ions * P <0.05

© 2022 Tonix Pharmaceuticals Holding Corp. RARE DISEASE: KEY CANDIDATES

21 © 2022 Tonix Pharmaceuticals Holding Corp. RARE DISEASE PORTFOLIO Patents Issued PROFILE DEVELOPMENT PROGRAM TNX - 2900*: Prader - Willi Syndrome Intranasal Potentiated Oxytocin (OT) with Magnesium Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Rare disease occurring in 1 in 10 ,000 to 1 in 30,000 births Symptoms include lack of suckling as infants, poor muscle strength, and constant hunger (hyperphagia) • In animal models, OT has improved suckling and suppressed hunger ‒ Tonix’s patented potentiated OT formulation is believed to increase specificity for OT receptors relative to off - target vasopressin receptors Market Entry: Prader - Willi Syndrome Additional Indications: Rare Hyperphagia Conditions Status: Preclinical, granted orphan drug designation by FDA Next Steps: pre - IND Meeting to seek agreement on development plans *TNX - 2900 is in the pre - IND stage of development and has not been approved for any indication.

© 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY: KEY CANDIDATES
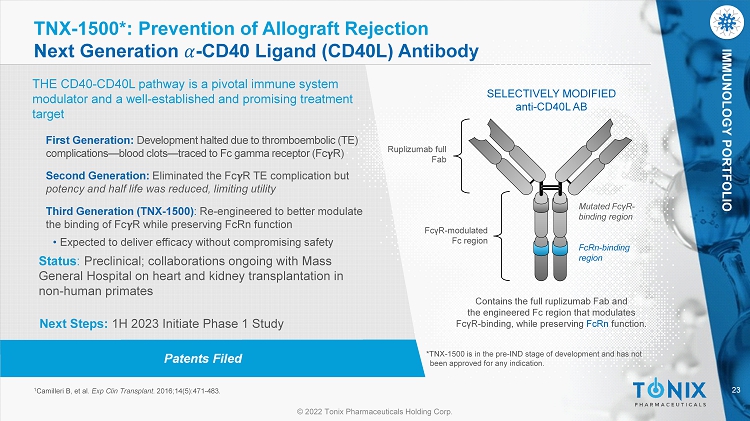
23 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Patents Filed TNX - 1500*: Prevention of Allograft Rejection Next Generation ߙ - CD40 Ligand (CD40L) Antibody THE CD40 - CD40L pathway is a pivotal immune system modulator and a well - established and promising treatment target 1 Camilleri B, et al. Exp Clin Transplant. 2016;14(5):471 - 483. SELECTIVELY MODIFIED anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500) : Re - engineered to better modulate the binding of Fc R while preserving FcRn function • Expected to deliver efficacy without compromising safety Status : Preclinical; collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates Next Steps: 1H 2023 Initiate Phase 1 Study *TNX - 1500 is in the pre - IND stage of development and has not been approved for any indication.
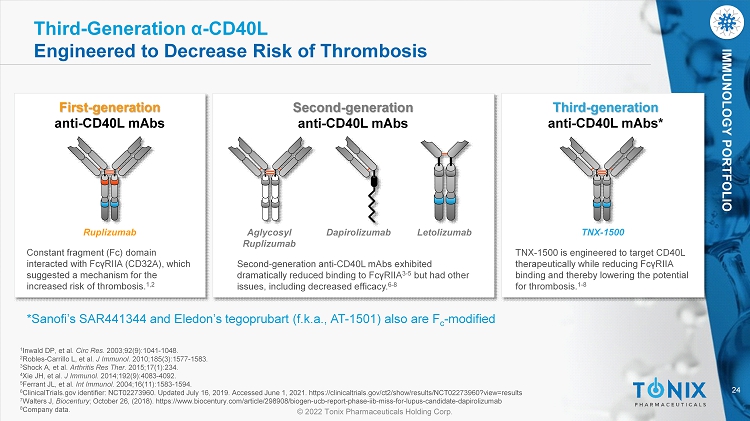
24 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Third - Generation α - CD40L Engineered to Decrease Risk of Thrombosis First - generation anti - CD40L mAbs Constant fragment (Fc) domain interacted with FcγRIIA (CD32A), which suggested a mechanism for the increased risk of thrombosis. 1,2 Ruplizumab Second - generation anti - CD40L mAbs Second - generation anti - CD40L mAbs exhibited dramatically reduced binding to FcγRIIA 3 - 5 but had other issues, including decreased efficacy. 6 - 8 Dapirolizumab Letolizumab Aglycosyl Ruplizumab Third - generation anti - CD40L mAbs * TNX - 1500 is engineered to target CD40L therapeutically while reducing FcγRIIA binding and thereby lowering the potential for thrombosis. 1 - 8 TNX - 1500 *Sanofi’s SAR441344 and Eledon’s tegoprubart ( f.k.a ., AT - 1501) also are F c - modified 1 Inwald DP, et al. Circ Res . 2003;92(9):1041 - 1048. 2 Robles - Carrillo L, et al. J Immunol . 2010;185(3):1577 - 1583. 3 Shock A, et al. Arthritis Res Ther . 2015;17(1):234. 4 Xie JH, et al. J Immunol . 2014;192(9):4083 - 4092. 5 Ferrant JL, et al. Int Immunol . 2004;16(11):1583 - 1594. 6 ClinicalTrials.gov identifier: NCT02273960. Updated July 16, 2019. Accessed June 1, 2021. https://clinicaltrials.gov/ct2/show /re sults/NCT02273960?view=results 7 Walters J, Biocentury ; October 26, (2018). https://www.biocentury.com/article/298908/biogen - ucb - report - phase - iib - miss - for - lupus - candidate - dapirolizum ab 8 Company data.

25 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Development and Regulatory Strategy • 1 st Indication – Kidney allotransplantation (human to human) ‒ Replacement for nephrotoxic CNI’s (calcineurin inhibitors, e.g. Prograf ® (tacrolimus) 1 , Neoral ® (cyclosporin) 2 ‒ Similar development path to the successful development of BMS’s Nulojix ® ( belatacept ) 3 , CTLA - 4/Ig biologic ‒ Clinical development may combine with Nulojix or Rapamune ® (rapamycin/sirolimus) 4 • 2 nd Indication – Heart or kidney xenotransplant (pig to human) ‒ CD40L:CD40 blockade considered essential ‒ The engineered pig organ is also considered a biologic • 3 rd Indication – Lou Gehrig's Disease, or ALS 5 ‒ Animal models show strong activity; competitor Eledon (ELDN) is pursuing ALS as primary indication • 4 th Indication (and beyond) – Autoimmune disease (e.g., Systemic Lupus Erythematosus, Rheumatoid Arthritis, Progressive Systemic Sclerosis) ‒ These indications require large studies; SLE and RA would represent very large target markets 1 http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/050708s027,050709s021lbl.pdf 2 http://www.novartis.us/sites/www.novartis.us/files/neoral.pdf 3 https://packageinserts.bms.com/pi/pi_nulojix.pdf 4 https://labeling.pfizer.com/showlabeling.aspx?id=139 5 Amyotrophic Lateral Sclerosis

26 © 2022 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Patents Filed TNX - 1700*: Gastric and Colorectal cancers Stabilized Recombinant Trefoil Factor 2 (rTFF2) POTENTIAL NEW CANCER TREATMENT • TNX - 1700 (rTFF2) has effects on cancer by altering the tumor micro - environment • Mechanism of action: suppresses myeloid - derived suppressor cells and activates anti - cancer CD8+ T cells • Potential synergy with anti - PD - 1 or anti - PD - L1 monoclonal antibodies ( mAbs ) PRECLINICAL EVIDENCE FOR INHIBITING GROWHT OF CANCER CELLS • Data showed that TFF2 - CTP augmented the efficacy of mAb anti - PD - 1 therapy. Anti - PD - 1 in combination with TFF2 - CTP showed greater anti - tumor activity in PD - L1 - overexpressing mice. LICENSED FROM COLUMBIA UNIVERSITY • Developing in partnership under sponsored research agreement DEVELOPMENT PROGRAM Market Entry: Gastric and colorectal cancers Status: Preclinical Next Steps: Animal studies ongoing *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication.

© 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: KEY CANDIDATES

28 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 801: Smallpox and Monkeypox Vaccine Live Virus Platform Development Program APPLICATION OF LIVE VIRUS PLATFORM • TNX - 801 is a cloned version of horsepox 1 (without any insert) purified from cell culture • In addition to being a potential addition to the U.S. Strategic National Stockpile, TNX - 801 will support recognition of the RPV/horsepox platform ANIMAL TESTING OF TNX - 801 WITH SOUTHERN RESEARCH INSTITUTE • Non - human primate monkeypox challenge testing: positive data reported in 1Q 2020 2 DEVELOPED IN COLLABORATION WITH UNIVERSITY OF ALBERTA • Proprietary synthetic biology approach and vector system DEVELOPMENT PROGRAM Market Entry: Smallpox and Monkeypox Vaccine Status: Preclinical, Pre - IND Next Steps: Developing GMP manufacturing for TNX - 801; initiate Phase 1 Trial, 2H 2023 *TNX - 801 is in the pre - IND stage of development and has not been approved for any indication. 1 Noyce RS, et al. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One. 2018 Jan 19;13(1):e0188453. 2 Noyce, RS, et al. Synthetic Chimeric Horsepox Virus ( scHPXV ) Vaccination Protects Macaques from Monkeypox* Presented as a poster at the American Society of Microbiology BioThreats Conference - January 29, 2020, Arlington, VA . (https://content.equisolve.net/tonixpharma/media/10929ac27f4fb5f5204f5cf41d59a121.pdf)

29 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Vaccinia and Horsepox Induce a Skin Reaction Called a “Take” Described by Dr. Edward Jenner *Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine with intradermal deli very, indicating successful vaccination 1,2 5 mm Vaccine Intradermal vaccination 1 Take 2 • Biomarker of protection ‒ Smallpox was eradicated using this marker ‒ Revaccination indicated for recipients without “take” • Measure of T cell immunity ‒ No need for blood draws or complex laboratory studies ‒ No other functional T cell assay is approved or in clinical use for vaccination 1 Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2 Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=3276
30 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 1850*: COVID - 19 Vaccine Live Virus Platform Development Program APPLICATION OF LIVE VIRUS PLATFORM • First version TNX - 1800 encodes spike protein from SARS - CoV - 2, Wuhan strain • Planned new version TNX - 1850 encodes spike protein from SARS - CoV - 2 BA.2 strain 1 ANIMAL TESTING OF TNX - 1800 WITH SOUTHERN RESEARCH INSTITUTE • Non - human primate immune response: positive results reported in 4Q 2020 • Non - human primate CoV - 2 challenge testing: positive data reported in 1Q 2021 DEVELOPED IN COLLABORATION WITH UNIVERSITY OF ALBERTA • Proprietary synthetic biology approach and vector system DEVELOPMENT PROGRAM Market Entry: COVID - 19 Vaccine Additional Indications: Future Pandemic, Infectious Disease, Smallpox, Cancer Status: Preclinical Next Steps: Developing TNX - 1850 (BA.2) version; initiate Phase 1 Trial, 2H 2023 *TNX - 1850 is in the pre - IND stage of development and has not been approved for any indication. 1 Brennan, Z. Endpoints March 2, 2022 ( https://endpts.com/weaker - omicron - variant - is - great - news - for - the - world - but - bad - news - for - covid - related - clinical - trials/)
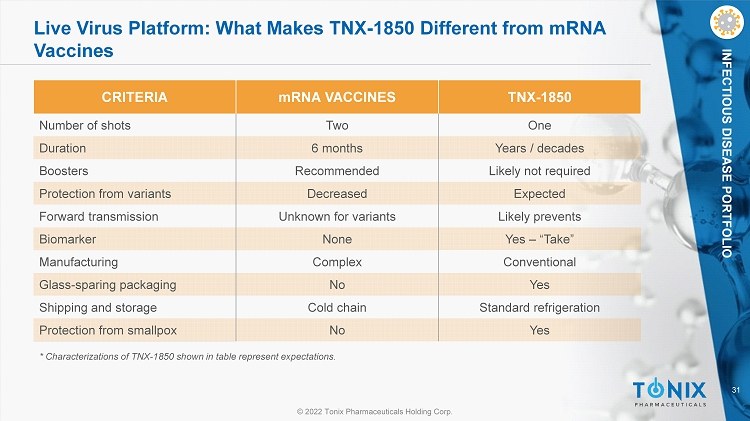
31 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Live Virus Platform: What Makes TNX - 1850 Different from mRNA Vaccines CRITERIA mRNA VACCINES TNX - 1850 Number of shots Two One Duration 6 months Years / decades Boosters Recommended Likely not required Protection from variants Decreased Expected Forward transmission Unknown for variants Likely prevents Biomarker None Yes – “Take” Manufacturing Complex Conventional Glass - sparing packaging No Yes Shipping and storage Cold chain Standard refrigeration Protection from smallpox No Yes * Characterizations of TNX - 1850 shown in table represent expectations.
32 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Patents Filed TNX - 2300*: COVID - 19 Vaccine Live Virus Vaccine Based on Bovine Parainfluenza (BPI) Virus LIVE VIRUS VACCINE 1 - 5 • Previously has been shown to be an effective antigen delivery vector in humans, notably well tolerated in infants and children • Vector is well suited for mucosal immunization using a nasal atomizer, but it can also be delivered parenterally ANIMAL TESTING OF TNX - 2300 ONGOING • Non - human primate immune response: positive results reported in 4Q 2020 • Non - human primate CoV - 2 challenge testing: positive data reported in 1Q 2021 DEVELOPED IN COLLABORATION WITH KANSAS STATE UNIVERSITY (KSU) • Uses a novel live attenuated vaccine vector platform, BPI, and the CD40 - ligand to stimulate T cell immunity DEVELOPMENT PROGRAM Market Entry: COVID - 19 Vaccine Additional Indications: Future Pandemic, Infectious Diseases Status: Preclinical Next Steps: Animal studies with KSU to test the effect of co - expression of the CD40 - ligand, also known as CD154 or 5c8 antigen, to stimulate T cell immunity. *TNX - 2300 is in the pre - IND stage of development and has not been approved for any indication. 1 Halle, AA et al. J Gen. Virology (2003) 84 :2153 – 2162; 2 Halle, AA et al. J Virology (2000) 74 (24): 11626 – 11635 ; 3 Karron RA et al. J Inf Dis (1995) 171: 1107 - 14; 4 Karron RA et al. Vaccine (2012) 30: 3975 – 3981; 5 Schmidt AC et al. J Virology (2001) 75(10): 4594 – 4603
33 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO PROFILE DEVELOPMENT PROGRAM TNX - 3600*: COVID - 1 9 Therapeutics Fully Human Monoclonal Antibody Platform Collaboration with Columbia University Human monoclonal antibodies ( mAbs ) generated from COVID - 19 convalescent patients Potential monotherapies • Plan to seek indication similar to current EUA therapeutic mAbs for treating individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease Potential combination therapy with other antibodies • Combination therapies for other anti - CoV - 2 monoclonal antibodies are believed to have reduced the emergence of drug resistant viral strains Market Entry: COVID - 19 Therapeutic Additional Indications: Symptomatic COVID in patients with risk factors for poor outcome Status: Preclinical Next Steps: Study inhibition of SARS CoV - 2 variants in tissue culture; 2Q 2022 Initiate Animal Studies *TNX - 3600 is in the pre - IND stage of development and has not been approved for any indication. 1 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https://www.nature.com/articles/d41586 - 022 - 00199 - z Given the unpredictable trajectory of the SARS - CoV - 2 virus and new variants 1 , we seek to contribute to a broad set of monoclonal antibodies from a variety of patients, that can be scaled up quickly and potentially combined with other monoclonal antibodies.

34 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Patents Filed PROFILE DEVELOPMENT PROGRAM TNX - 3700*: COVID - 19 Vaccine Zinc Nanoparticle (ZNP) Formulation for mRNA Vaccines Collaboration with Kansas State University ZNP technology is a potential replacement for the Lipid Nanoparticle (LNP) technology of current mRNA vaccines Potential improved stability • Plan to seek initial indications as booster, similar to the current EUA and FDA approved mRNA vaccines • Improved stability would facilitate shipping and storage Addresses limitations in current mRNA vaccines which require ultra - cold storage and shipping • Stability issues limit use in less developed countries Market Entry: Booster for COVID - 19 Vaccines Additional Indications: COVID - 19 vaccine for naïve individuals Status: Preclinical Next Steps: Research at K - State on CoV - 2 spike based vaccine in tissue culture and animals; 2Q 2022 Initiate Animal Studies *TNX - 3700 is in the pre - IND stage of development and has not been approved for any indication.

35 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Live Virus RPV P latform & COVID - 19 Vaccine Internal Development & Manufacturing Capabilities Infectious Disease R&D Center (RDC) – Frederick, MD • Function : Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description : ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 • Status : Operational; acquisition completed on October 1 st , 2021 Advanced Development Center (ADC) – North Dartmouth, MA • Function : Development and clinical scale manufacturing of live - virus vaccines • Description : ~45,000 square feet, BSL - 2 • Status : Partially operational as of 2Q 2022 Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of live - virus vaccines • Description : ~44 acre green field site, planned BSL - 2 • Status : Planning for site enabling work in 2022 Architectural Rendering
36 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO American Pandemic Preparedness Plan (AP3) • “Platforms” – Foundation of Pandemic Response ‒ Key element of AP3 from White House Office of Science and Technology Policy or OSTP 1,2 ▪ 100 days to human trials ▪ Technologies that do not require sterile injection • TNX - 801/TNX - 1850 (live virus RPV) platform addresses OSTP requirements 1,2 ‒ Our goal is to be able to test new live virus vaccines against novel pathogens within the 100 days of obtaining sequence ▪ RDC is equipped to make new vaccines ▪ ADC will be equipped to make clinical trial material ▪ CMC is planned to make commercial scale material 1 Sept 3, 2021 ( https://www.whitehouse.gov/wp - content/uploads/2021/09/American - Pandemic - Preparedness - Transforming - Our - Capabilities - Final - For - Web .pdf) 2 Sept 3, 2021 ( https://www.whitehouse.gov/briefing - room/statements - releases/2021/09/03/fact - sheet - biden - administration - to - transform - capabilitie s - for - pandemic - preparedness/)

© 2022 Tonix Pharmaceuticals Holding Corp. FUTURE OUTLOOK

38 © 2022 Tonix Pharmaceuticals Holding Corp. TNX - 1300: COCAINE INTOXICATION TNX - 1700: GASTRIC AND COLORECTAL CANCERS TNX - 3600: MONOCLONAL ANTIBODIES FOR COVID - 19 TREATMENT Key Development Partners TNX - 1500: ALLOGRAFT REJECTION TNX - 1900: MIGRAINE & OTHER INDICATIONS TNX - 801: SMALLPOX AND MONKEYPOX VACCINE TNX - 1850: COVID - 19 VACCINE TNX - 2900: PRADER - WILLI SYNDROME TNX - 3700 : COVID - 19 VACCINE (ZINC NANOPARTICLE mRNA TECHNOLOGY ) TNX - 2300 : BOVINE PARAINFLUEZNA VIRUS

39 © 2022 Tonix Pharmaceuticals Holding Corp. Expected Clinical Trial Initiations □ 3 rd Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of Long COVID □ 3 rd Quarter 2022 Phase 2 study start of TNX - 102 SL for the treatment of PTSD in Kenya □ 2 nd Half 2022 Phase 2 study start of TNX - 1900 for the treatment of migraine □ 1 st Quarter 2023 Phase 2 study start of TNX - 601 ER for the treatment of major depressive disorder □ 1 st Half 2023 Phase 1 study start of TNX - 1500 for prevention of allograft rejection Milestones: Recently Completed and Upcoming* * We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones. □ 1 st Quarter 2021 Non - human primate positive efficacy data from TNX - 1800 in COVID - 19 models reported □ 1 st Quarter 2022 Topline data from Phase 3 F306/RALLY study of TNX - 102 SL for the management of fibromyalgia □ 2 nd Quarter 2022 Phase 3 F307/RESILIENT study start of TNX - 102 SL for the management of fibromyalgia x x x Expected Data □ 1 st Quarter 2023 Interim analysis results of Phase 3 F307/RESILIENT study of TNX - 102 SL in fibromyalgia

40 © 2022 Tonix Pharmaceuticals Holding Corp. Management Team Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

© 2022 Tonix Pharmaceuticals Holding Corp. THANK YOU