
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.04

Long - term rejection free renal allograft survival with Fc - modified anti - CD154 antibody monotherapy in nonhuman primates. Grace Lassiter, MD Background • Triple xeno-antigen knock out (TKO) pigsareexpected to be optimaldonorsfor humanxenotransplantation due to reduced antibody binding from humans to TKO cells. • However, old world monkeys (OWMs) have higher IgM antibody titersagainst TKO pig than humans, raising the question whether TKO pig organs can sustain long term survival in OWMs. Center for Transplantation Sciences Massachusetts General Hospital
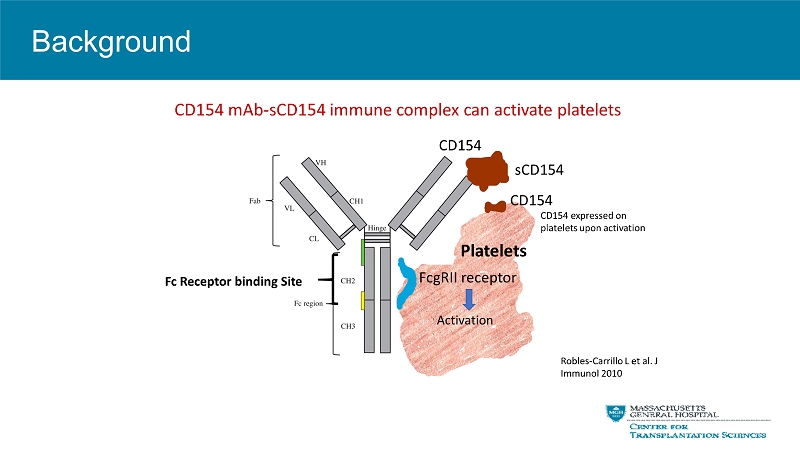
Background
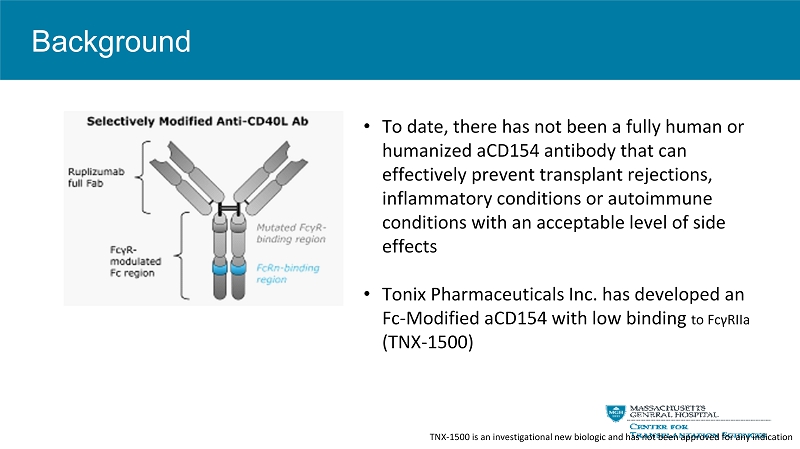
• To date, there has not been a fully human or humanized aCD154 antibody that can effectively prevent transplant rejections, inflammatory conditions or autoimmune conditions with an acceptable level of side effects • Tonix Pharmaceuticals Inc. has developed an Fc - Modified aCD154 with low binding to FcγRIIa (TNX - 1500) TNX - 1500 is an investigational new biologic and has not been approved for any indication Background
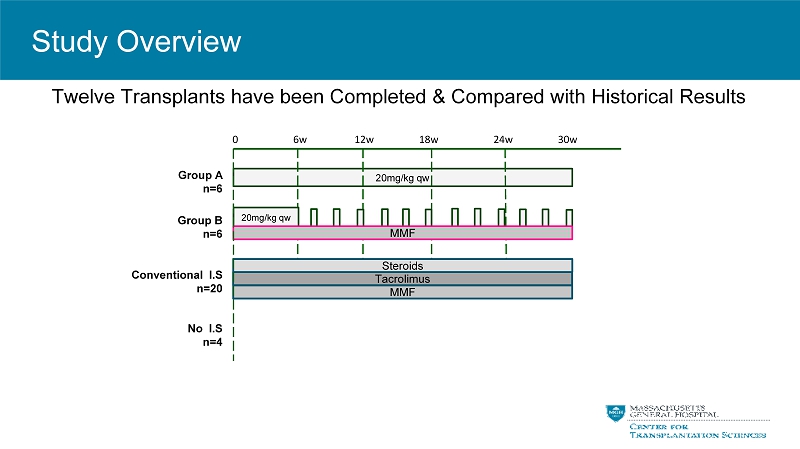
0 6w 12w 18w 24w 30w 20mg/kg qw 20mg/kg qw MMF Tacrolimus Group A n=6 Group B n=6 Conventional I.S n=20 Twelve Transplants have been Completed & Compared with Historical Results Steroids MMF No I.S n=4 Study Overview
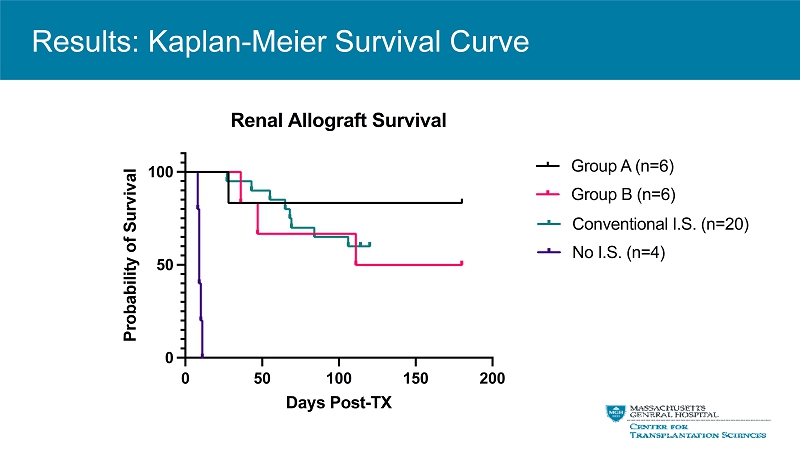
0 50 100 150 200 0 50 100 Renal Allograft Survival Days Post-TX P r o b a b i l i t y o f S u r v i v a l Group A (n=6) Group B (n=6) Conventional I.S. (n=20) No I.S. (n=4) Results: Kaplan - Meier Survival Curve
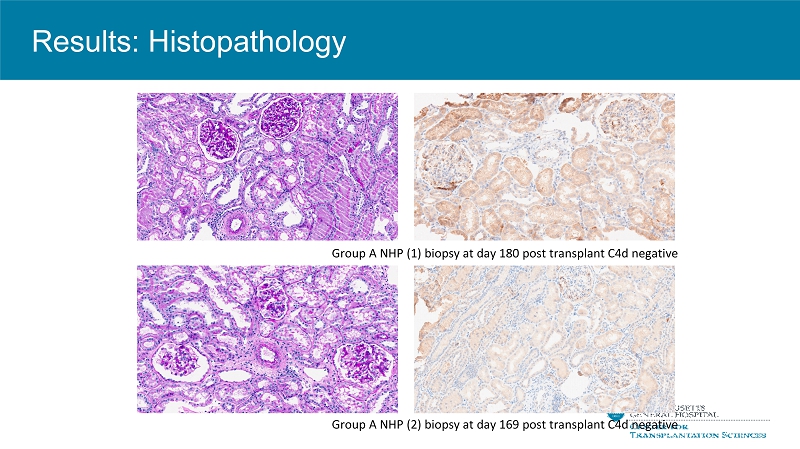
Group A NHP (1) biopsy at day 180 post transplant C4d negative Group A NHP (2) biopsy at day 169 post transplant C4d negative Results: Histopathology
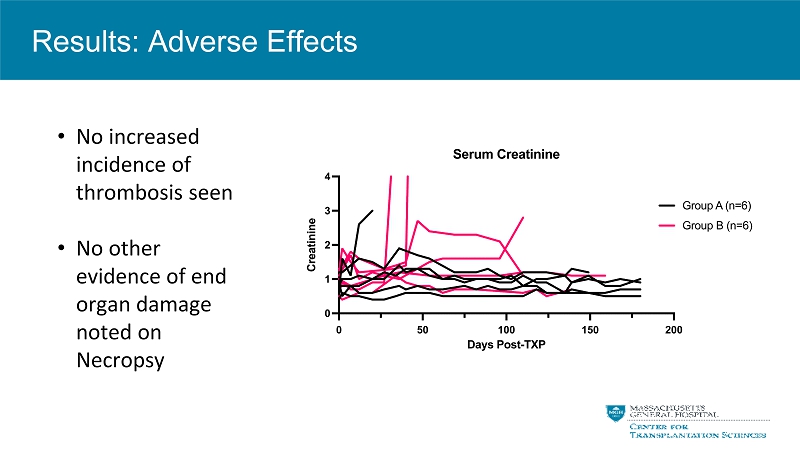
• No increased incidence of thrombosis seen • No other evidence of end organ damage noted on Necropsy Results: Adverse Effects
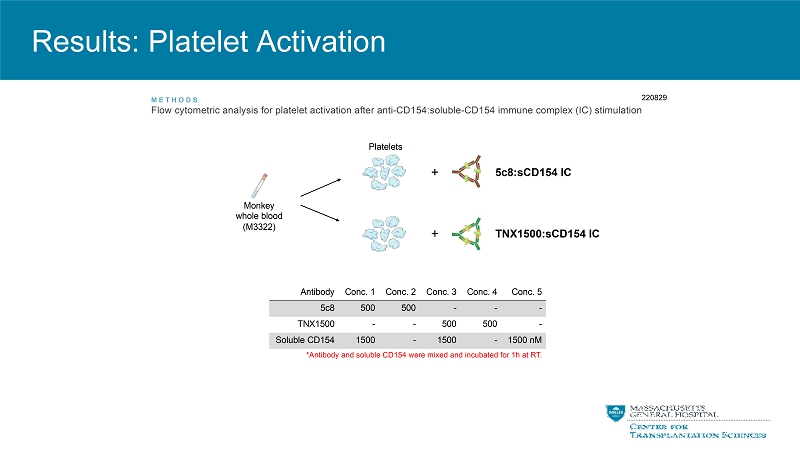
Results: Platelet Activation Antibody Conc. 1 Conc. 2 Conc. 3 Conc. 4 Conc. 5 5c8 500 500 - - - TNX1500 - - 500 500 - Soluble CD154 1500 - 1500 - 1500 nM ME THODS Flow cytometric analysis for platelet activation after anti-CD154:soluble-CD154 immune complex (IC) stimulation Monkey whole blood (M3322) + + Platelets 5c8:sCD154 IC TNX1500:sCD154 IC 220829 *Antibody and soluble CD154 were mixed and incubated for 1h at RT.
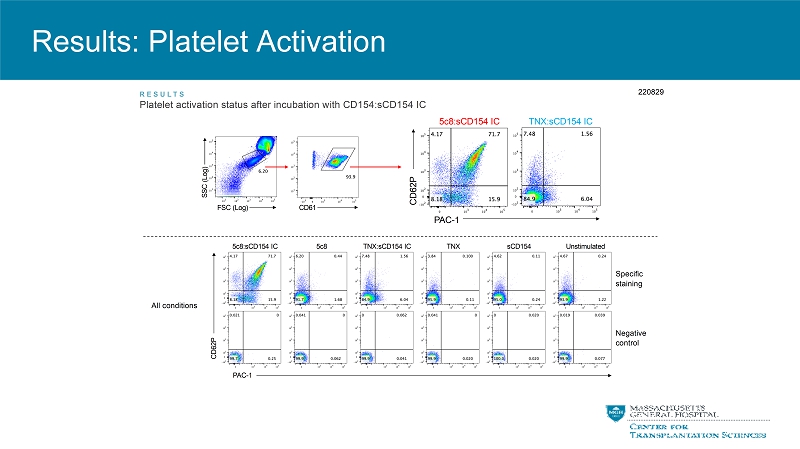
Results: Platelet Activation PAC-1 C D 6 2 P All conditions 5c8:sCD154 IC 5c8 TNX:sCD154 IC TNX sCD154 Specific staining Negative control Unstimulated FSC (Log) S S C ( L o g ) CD61 C D 6 2 P 5c8:sCD154 IC TNX:sCD154 IC RE SUL TS Platelet activation status after incubation with CD154:sCD154 IC 220829 PAC-1
Conclusion • Fc - Modified aCD154 is well tolerated and can be an effective alternative to conventional immunosuppression therapy in nonhuman primates. ▪ TNX - 1500 in combination with MMF resulted in an increased rate of graft failure compared to monotherapy ▪ Optimal dosage remains to be defined
Takayuki Hirose Ryo Otsuka Ahmad Karadagi Toshi Tomosugi Kohei Kinoshita Abbas Dehnadi Cindy Miller Jane O Franzi Pollok R.N. Pierson, III A.Benedict Cosimi Tatsuo Kawai Knight Surgery Research Laboratory Jessica Burke Anet Calisir Nick Deluca Nelson Marquez Carvajal Eli Smith Michael Duggan MGH Pathology Ivy Rosales Robert Colvin Catherine Stevens Special Thanks to these wonderful people Questions?