
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2022 Tonix Pharmaceuticals Holding Corp. Platform for Generating Fully Human anti - SARS - CoV - 2 Spike Therapeutic Monoclonal Antibodies Collaboration with Columbia University Version 1121 November 30 , 2022 (Doc 0391)

2 © 2022 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
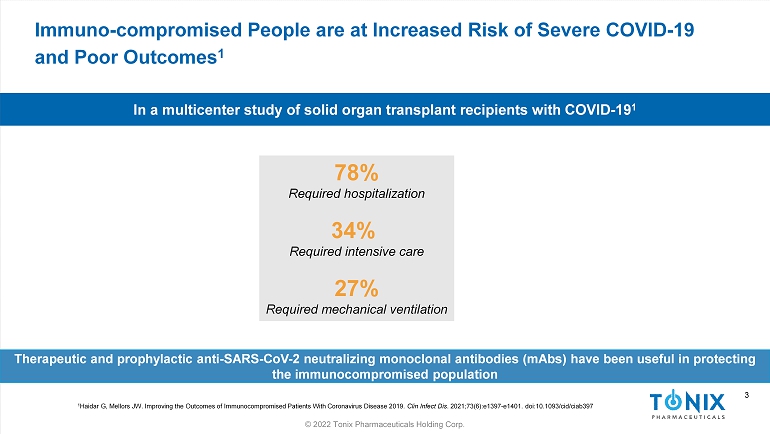
3 © 2022 Tonix Pharmaceuticals Holding Corp. 3 Immuno - compromised People are at Increased Risk of Severe COVID - 19 and Poor Outcomes 1 78% Required hospitalization 34% Required intensive care 27% Required mechanical ventilation In a multicenter study of solid organ transplant recipients with COVID - 19 1 1 Haidar G, Mellors JW. Improving the Outcomes of Immunocompromised Patients With Coronavirus Disease 2019. Clin Infect Dis . 2021;73(6):e1397 - e1401. doi:10.1093/ cid /ciab397 Therapeutic and prophylactic anti - SARS - CoV - 2 neutralizing monoclonal antibodies ( mAbs ) have been useful in protecting the immunocompromised population
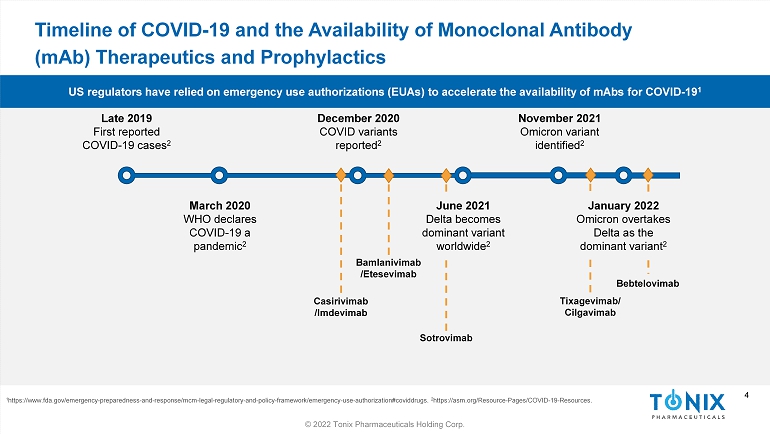
4 © 2022 Tonix Pharmaceuticals Holding Corp. 4 Timeline of COVID - 19 and the Availability of Monoclonal Antibody ( mAb ) Therapeutics and Prophylactics 1 https://www.fda.gov/emergency - preparedness - and - response/mcm - legal - regulatory - and - policy - framework/emergency - use - authorization#co viddrugs. 2 https://asm.org/Resource - Pages/COVID - 19 - Resources. Late 2019 First reported COVID - 19 cases 2 December 2020 COVID variants reported 2 June 2021 Delta becomes dominant variant worldwide 2 November 2021 Omicron variant identified 2 March 2020 WHO declares COVID - 19 a pandemic 2 Casirivimab /Imdevimab Bamlanivimab / Etesevimab Tixagevimab / Cilgavimab Bebtelovimab Sotrovimab January 2022 Omicron overtakes Delta as the dominant variant 2 US regulators have relied on emergency use authorizations (EUAs) to accelerate the availability of mAbs for COVID - 19 1

5 © 2022 Tonix Pharmaceuticals Holding Corp. 5 However, the Available anti - SARS - CoV - 2 Monoclonal Antibodies are Losing Their Activity as SARS - CoV - 2 Mutates and Evasive Variants Arise The efficacy of any mAb treatment varies as the dominant circulating variant changes 1,2 1 https://www.covid19treatmentguidelines.nih.gov/therapies/anti - sars - cov - 2 - antibody - products/anti - sars - cov - 2 - monoclonal - antibodies / 2 Wu, K.J. October 29, 2022. The Atlantic. “ The End of Evusheld : If you’re immunocompromised, this … isn’t great. www.theatlantic.com/health/archive/2022/10/covid - variants - antibody - treatments - immunocompromised/671929/ 3 Indicated for individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease 4 ”FDA Updates on Bebtelovimab ” – “ This information shows that bebtelovimab is not expected to neutralize Omicron subvariants BQ.1 and BQ.1.1. ” - www.fda.gov/drugs/drug - safety - and - availability/fda - updates - bebtelovimab - Accessed Nov 4, 2022 5 Vir isolated sotrovimab from the blood of a SARS - CoV - 1 patent 6 Regeneron used both convalescent patient cells and a humanized mouse platform: Hansen J et al. Science . 2020 Aug 21;369(6506):1010 - 1014. doi : 10.1126/science.abd0827 Most therapeutic and prophylactic mAbs have originated from COVID - convalescent patient blood 5,6 Monoclonal antibodies ( mAbs ) – two with active US Emergency Use Authorization (EUA) endorsed by NIH Guidelines Panel 1 ‒ AbCellera /NIAID - VRC/Eli Lilly - bebtelovimab – EUA for treatment of mild or moderate COVID 3 ▪ Nov 4 – FDA warns of reduced effect on omicron subvariants BQ.1 and BQ.1.1 4 ‒ AstraZeneca/Vanderbilt – Evusheld ® ( Tixagevimab / cilgavimab ) – EUA for long term prophylaxis Concerns about efficacy of mAbs against new variants ‒ Regeneron/Genentech - REGEN - COV® Casirivimab/imdevimab ▪ EUA revised Jan ‘22 to susceptible variants – unlikely to be effective against omicron 1 ‒ Eli Lilly/ AbCellera /NIAID/ Junshi - China Academy of Sciences – B amlanivimab /etesevimab 1 ▪ EUA revised Jan ‘22 to susceptible variants – unlikely to be effective against omicron 1 ‒ Vir /GSK – XEVURDY® (sotrovimab) 1 – active against omicron, but NIH COVID Guidelines panel recommends against use because less activity against omicron BA.2, BA.4 and BA.5 subvariants 1

6 © 2022 Tonix Pharmaceuticals Holding Corp. 6 Need for a Strategy to Frequently Update Monoclonal Antibodies Current and prior mAb therapeutics were developed in collaborations A platform to quickly develop and test novel SARS - CoV - 2 neutralizing mAbs may represent a significant advancement in the ability to update the pool of mAb treatments available to protect the immunocompromised population Dr. Luciana Borio is former National Security Council director for medical and biodefense preparedness and current senior fellow for global health at the think tank Council on Foreign Relations. a venture partner at ARCH.
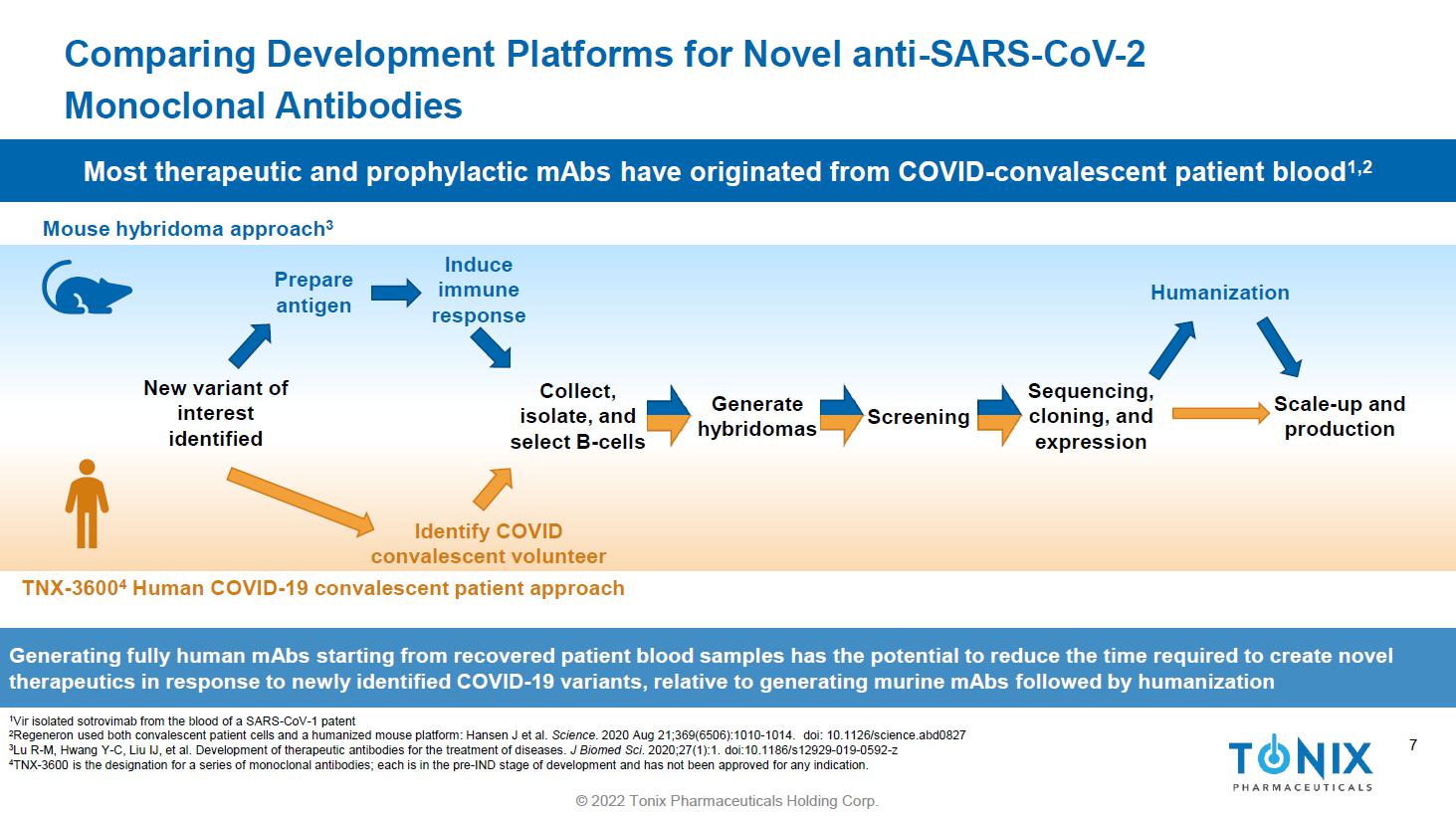
7 © 2022 Tonix Pharmaceuticals Holding Corp. 7 Comparing Development Platforms for Novel anti - SARS - CoV - 2 Monoclonal Antibodies 1 Vir isolated sotrovimab from the blood of a SARS - CoV - 1 patent 2 Regeneron used both convalescent patient cells and a humanized mouse platform: Hansen J et al. Science . 2020 Aug 21;369(6506):1010 - 1014. doi : 10.1126/science.abd0827 3 Lu R - M, Hwang Y - C, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci . 2020;27(1):1. doi:10.1186/s12929 - 019 - 0592 - z 4 TNX - 3600 is the designation for a series of monoclonal antibodies; each is in the pre - IND stage of development and has not been approved for any indication. Generating fully human mAbs starting from recovered patient blood samples has the potential to reduce the time required to create novel therapeutics in response to newly identified COVID - 19 variants, relative to generating murine mAbs followed by humanization Mouse hybridoma approach 3 TNX - 3600 4 Human COVID - 19 convalescent patient approach Collect, isolate, and select B - cells Generate hybridomas Screening Sequencing, cloning, and expression Scale - up and production Prepare antigen Induce immune response Humanization Identify COVID convalescent volunteer New variant of interest identified Most therapeutic and prophylactic mAbs have originated from COVID - convalescent patient blood 1,2
© 2022 Tonix Pharmaceuticals Holding Corp. 8 Fully Human anti - SARS - CoV - 2 Monoclonal Antibody Platform TNX - 3600 1 : COVID - 1 9 Therapeutic and Preventive Agents 8 Collaboration with Columbia University Fully human mAbs generated from SARS - CoV - 2 + asymptomatic individuals or COVID - 19 convalescent patients 3 Potential monotherapies or preventives • Plan to seek indication similar to current EUA therapeutic mAbs for treating individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease Potential combination therapy with other mAbs as therapeutics or prophylactics • Combination therapies for other anti - SARS - CoV - 2 monoclonal antibodies are believed to have reduced the emergence of drug resistant viral strains 4 *TNX - 3600 is the designation for a series of monoclonal antibodies; each is in the pre - IND stage of development and has not been approved for any indication 1 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https://www.nature.com/articles/d41586 - 022 - 00199 - z 3 Volunteers participated in an IRB - approved research protocol 4 Baum, A. et al. Science . 2020 Aug 21;369(6506):1014 - 1018. doi : 10.1126/science.abd0831. Epub 2020 Jun 15. Given the unpredictable trajectory of the SARS - CoV - 2 virus and new variants 2 , we seek to contribute to a broad set of monoclonal antibodies from a variety of SARS - CoV - 2 + volunteers and convalescent patients, that can be scaled up quickly and potentially combined with other monoclonal antibodies
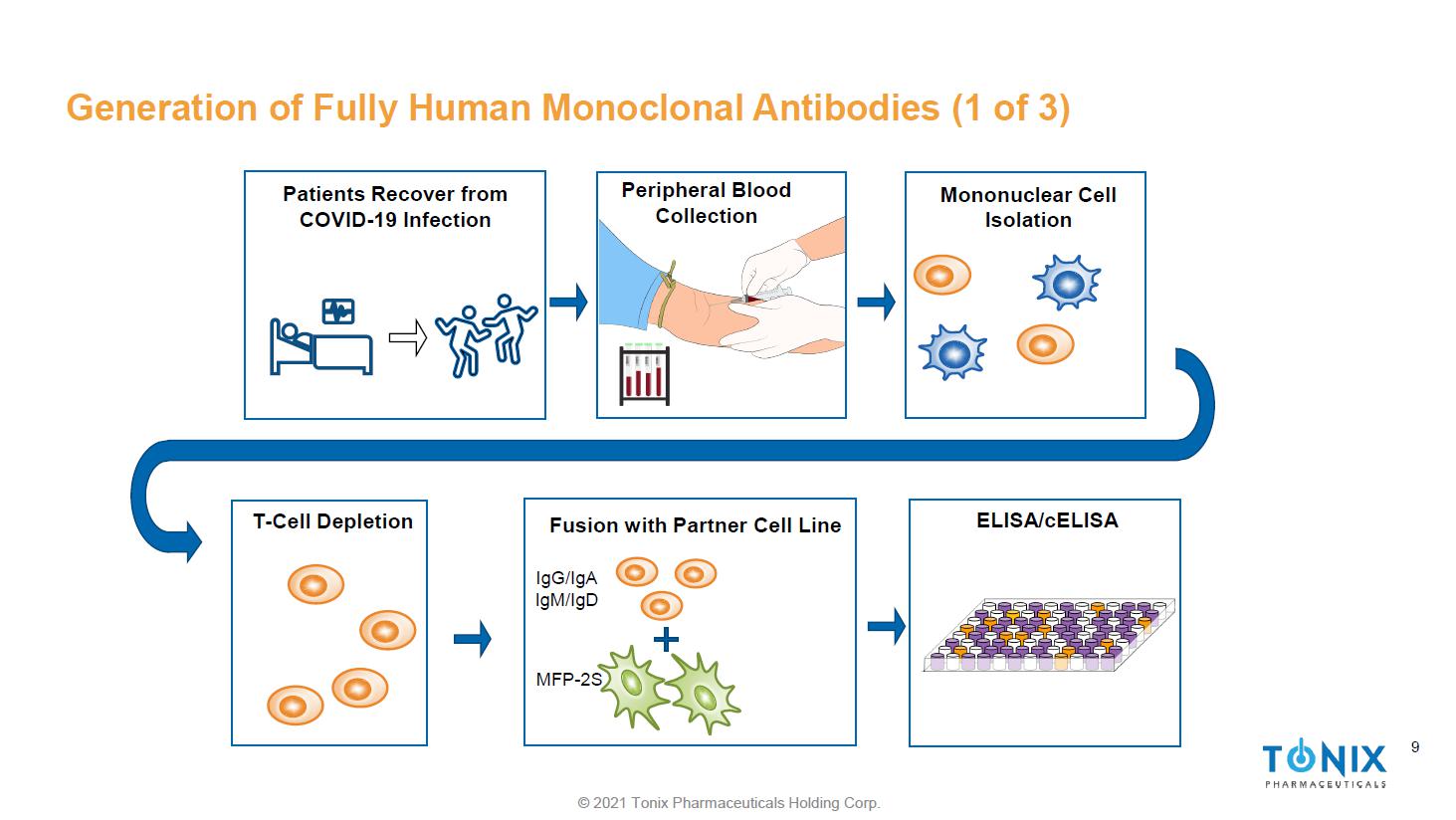
© 2021 Tonix Pharmaceuticals Holding Corp. 9 Generation of Fully Human Monoclonal Antibodies (1 of 3) Peripheral Blood Collection Mononuclear Cell Isolation ELISA/ cELISA T - Cell Depletion Fusion with Partner Cell Line IgG/IgA IgM/ IgD MFP - 2S Patients Recover from COVID - 19 Infection
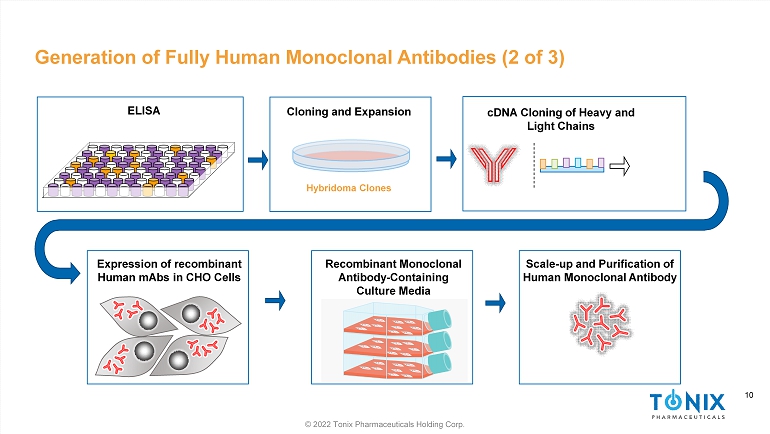
10 © 2022 Tonix Pharmaceuticals Holding Corp. 10 Generation of Fully Human Monoclonal Antibodies (2 of 3) ELISA Expression of recombinant Human mAbs in CHO Cells Cloning and Expansion Hybridoma Clones cDNA Cloning of Heavy and Light Chains Recombinant Monoclonal Antibody - Containing Culture Media Scale - up and Purification of H uman Monoclonal Antibody
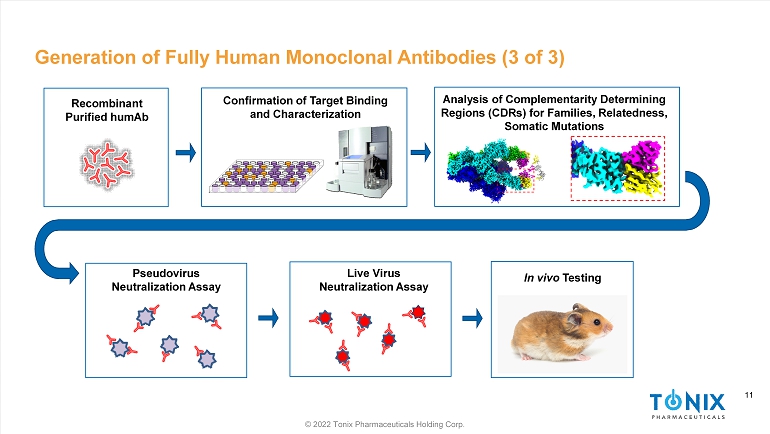
11 © 2022 Tonix Pharmaceuticals Holding Corp. 11 Generation of Fully Human Monoclonal Antibodies (3 of 3) Pseudovirus Neutralization Assay Recombinant Purified humAb Live Virus Neutralization Assay In vivo Testing Confirmation of Target Binding and Characterization Analysis of C omplementarity Determining Regions (CDRs) for Families, Relatedness, Somatic Mutations
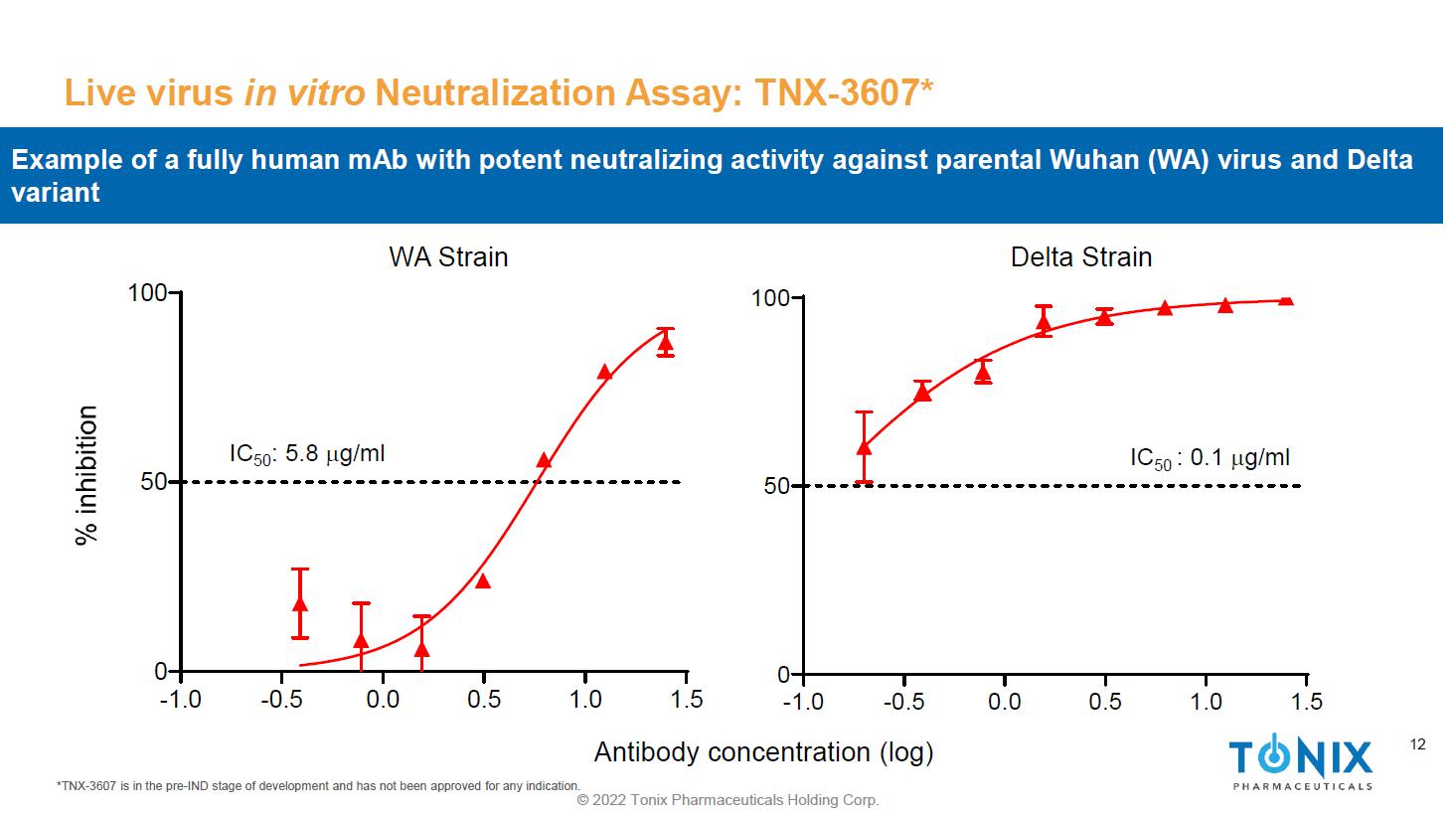
12 © 2022 Tonix Pharmaceuticals Holding Corp. 12 Live virus in vitro Neutralization Assay: TNX - 3607* *TNX - 3607 is in the pre - IND stage of development and has not been approved for any indication. -1.0 -0.5 0.0 0.5 1.0 1.5 0 50 100 IC 50 : 5.8 m g/ml -1.0 -0.5 0.0 0.5 1.0 1.5 0 50 100 IC 50 : 0.1 m g/ml WA Strain Delta Strain Antibody concentration (log) Example of a fully human mAb with potent neutralizing activity against parental Wuhan (WA) virus and Delta variant
13 © 2022 Tonix Pharmaceuticals Holding Corp. 13 Therapeutic Monoclonal Antibody Development for COVID - 19 has been Focused on a “Whack - a - Mole” 1x1 Monoclonal Antibody v. Variant Battle Delta Omicron 1 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https://www.nature.com/articles/d41586 - 022 - 00199 - z 2 https://www.fda.gov/emergency - preparedness - and - response/mcm - legal - regulatory - and - policy - framework/emergency - use - authorization#co viddrugs. • As new variants emerge, mAbs that were highly effective against older variants may quickly lose their place in the treatment landscape 1 • Antibodies receiving Emergency Use Authorizations (EUAs) may only have a lifespan of 1 - 2 years before shifts in the dominant circulating variant reduce their clinical utility 2
14 © 2022 Tonix Pharmaceuticals Holding Corp. 14 As the Circulating Mix of SARS - CoV - 2 Variants Changes, it Seems Prudent to Assemble a Diverse Inventory of Monoclonal Antibodies to Match It 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 Inventory of available anti - SARS - CoV - 2 mAbs Currently circulating “Variant Soup” SARS - CoV - 2 variants
15 © 2022 Tonix Pharmaceuticals Holding Corp. 15 The Platform is Designed to Develop and Maintain a Diverse Inventory of Monoclonal Antibodies to Keep Up with SARS - CoV - 2 “Variant Soup” 1 Currently circulating “Variant Soup” New variants Biotech industry Inventory of available anti - SARS - CoV - 2 mAbs Diabolical SARS - CoV - 2 Generator of Diversity Therapeutic mAb cocktail 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6
16 © 2022 Tonix Pharmaceuticals Holding Corp. 16 Desired Inventory Current Situation As the Circulating Mix of SARS - CoV - 2 Variants Changes, it Seems Prudent to Assemble a Diverse Inventory of Monoclonal Antibodies to Match It 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 Currently circulating “Variant Soup” New variants Inventory of available anti - SARS - CoV - 2 mAbs
© 2022 Tonix Pharmaceuticals Holding Corp. 17 • Immune - evading SARS - CoV - 2 variants are arising by divergent and convergent evolutionary processes 1 ‒ Potentially speeded by recombination between variants • To protect immuno - compromised individuals from a changing “soup” of SARS - CoV - 2 variants, we need an extensive palate of mAbs ‒ Rapid evasion confounds the durability of individual mAb therapeutic products ‒ Both new products are needed and potentially new combinations of new with existing mAbs • For life - saving, but short - lived products, we expect FDA to regulate with commensurate speed ‒ With respect to EUA product Bebtelovimab , the NIH Guidelines group wrote, “…there are no clinical efficacy data on the treatment of patients who are at high risk of progressing to severe COVID - 19” 2 ‒ For “updated” mRNA booster vaccines encoding omicron spike antigen, FDA approvals were granted without human efficacy data consistent with a “cartridge” approach Future of COVID - 19 mAb Therapeutics and Prophylactics 17 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 2 https://www.covid19treatmentguidelines.nih.gov/therapies/anti - sars - cov - 2 - antibody - products/anti - sars - cov - 2 - monoclonal - antibodies / - accessed Nov 3, 2022

© 2022 Tonix Pharmaceuticals Holding Corp. 18 • Tonix ‒ Seth Lederman ‒ Bruce Daugherty ‒ Herb Harris ‒ Candace Flint • Columbia ‒ Ilya Trakht ‒ Gavreel Kalantarov ‒ Sergei Rudchenko ‒ Milan Stojanovic Investigators and Collaborators 18 • Texas BioMed ‒ Viraj Kulkarni ‒ Marco Argonza • Chicago BioSolutions ‒ L ijun Rong • Curia ‒ Brian Zabel

19 © 2022 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Internal Development & Manufacturing Capabilities Infectious Disease R&D Center (RDC) – Frederick, MD • Function: Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description: ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 • Status: Operational Advanced Development Center (ADC) – North Dartmouth, MA • Function: Development and clinical scale manufacturing of biologics • Description: ~45,000 square feet, BSL - 2 • Status: Operational Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of biologics • Description: ~44 - acre green field site, planned BSL - 2 • Status: Planning for site enabling work in 2022 Architectural Rendering

© 2022 Tonix Pharmaceuticals Holding Corp. THANK YOU