
TONIX PHARMACEUTICALS HOLDING CORP. FORM 8-K
Exhibit 99.01

© 2023 Tonix Pharmaceuticals Holding Corp. Biotech Showcase COVID antiviral agents: anti - SARS - CoV - 2 Spike Protein Monoclonal Antibodies for Treatment and Prevention of COVID - 19 Version 1145 January 7, 2023 (Doc 0402)

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (the “SEC”) on March 14, 2022, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2023 Tonix Pharmaceuticals Holding Corp. Who We Are Tonix Pharmaceuticals is committed to improving population health by inventing and developing innovative therapies and vaccines, through broad in - house capabilities and creative collaborations , to help address important unmet needs. OUR MISSION Tonix strives to be a leader in providing novel drug therapies and vaccines to improve population health around the world. OUR VISION

4 © 2023 Tonix Pharmaceuticals Holding Corp. Investment Highlights DIVERSE PIPELINE Tonix’s c ore focus is on central nervous system disorders , but we also target unmet needs across multiple therapeutic areas including immunology, infectious disease and rare disease. STRATEGIC PARTNERSHIPS Partnering strategically with other biotech companies , world - class academic and non - profit research organizations to bring innovative therapeutics to market faster. IN - HOUSE CAPABILITIES Investment in domestic , in - house, R&D and manufacturing to accelerate development timelines and improve the ability to respond to pandemics. FINANCIAL POSITION Tonix had $140 M of cash as of 9/30/22. Tonix has no debt .
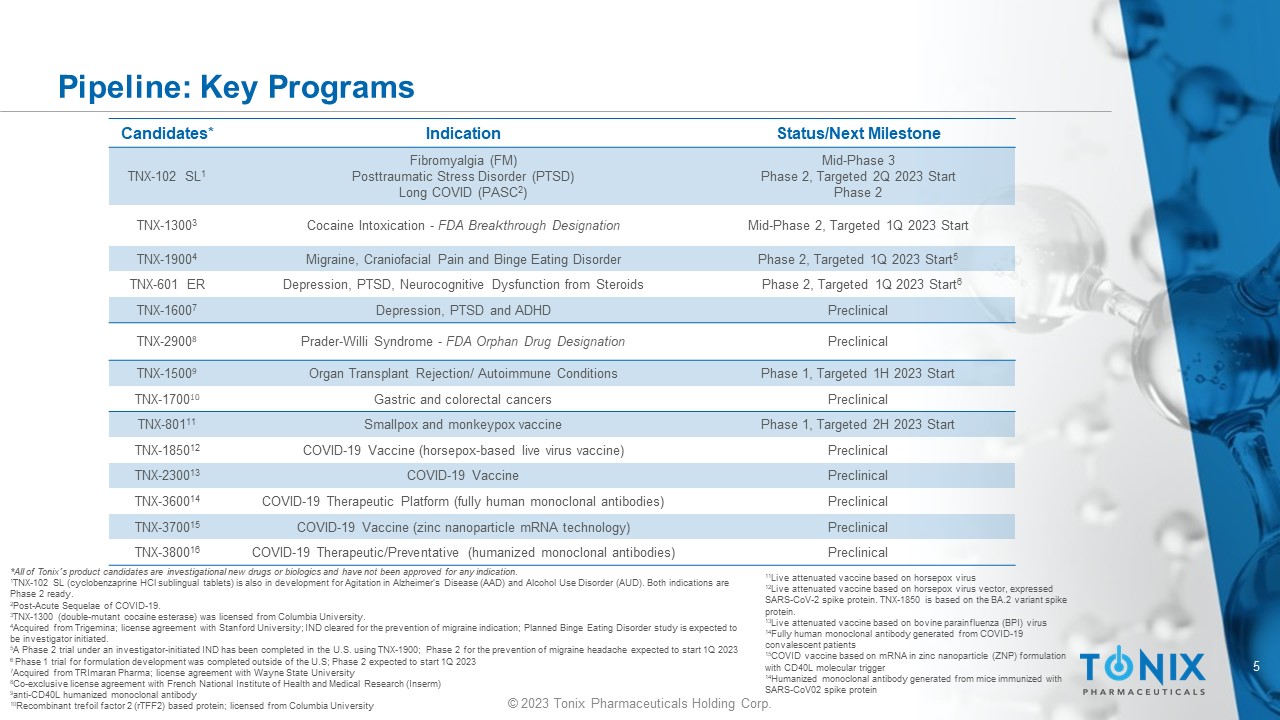
5 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Programs Candidates* Indication Status/Next Milestone TNX - 102 SL 1 Fibromyalgia (FM) Posttraumatic Stress Disorder (PTSD) Long COVID (PASC 2 ) Mid - Phase 3 Phase 2, Targeted 2Q 2023 Start Phase 2 TNX - 1300 3 Cocaine Intoxication - FDA Breakthrough Designation Mid - Phase 2, Targeted 1Q 2023 Start TNX - 1900 4 Migraine, Craniofacial Pain and Binge Eating Disorder Phase 2, Targeted 1Q 2023 Start 5 TNX - 601 ER Depression, PTSD, Neurocognitive Dysfunction from Steroids Phase 2, Targeted 1Q 2023 Start 6 TNX - 1600 7 Depression, PTSD and ADHD Preclinical TNX - 2900 8 Prader - Willi Syndrome - FDA Orphan Drug Designation Preclinical TNX - 1500 9 Organ Transplant Rejection/ Autoimmune Conditions Phase 1, Targeted 1H 2023 Start TNX - 1700 10 Gastric and colorectal cancers Preclinical TNX - 801 11 Smallpox and monkeypox vaccine Phase 1, Targeted 2H 2023 Start TNX - 1850 12 COVID - 19 Vaccine (horsepox - based live virus vaccine) Preclinical TNX - 2300 13 COVID - 19 Vaccine Preclinical TNX - 3600 14 COVID - 19 Therapeutic Platform (fully human monoclonal antibodies) Preclinical TNX - 3700 15 COVID - 19 Vaccine (zinc nanoparticle mRNA technology) Preclinical TNX - 3800 16 COVID - 19 Therapeutic/Preventative (humanized monoclonal antibodies) Preclinical *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is also in development for Agitation in A lzheimer’s Disease (AAD) and Alcohol Use D isorder (AUD). Both indications are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 TNX - 1300 (double - mutant cocaine esterase) was licensed from Columbia University . 4 Acquired from Trigemina ; license agreement with Stanford University; IND cleared for the prevention of migraine indication; Planned Binge Eating Dis ord er study is expected to be investigator initiated. 5 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900; Phase 2 for the prevention of migraine headache expected to start 1Q 2023 6 Phase 1 trial for formulation development was completed outside of the U.S; Phase 2 expected to start 1Q 2023 7 Acquired from TRImaran Pharma; license agreement with Wayne State University 8 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) 9 anti - CD40L humanized monoclonal antibody 10 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University 11 Live attenuated vaccine based on horsepox virus 12 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. TNX - 1850 is based on the BA.2 variant spike protein. 13 Live attenuated vaccine based on bovine parainfluenza (BPI) virus 14 Fully human monoclonal antibody generated from COVID - 19 convalescent patients 15 COVID vaccine based on mRNA in zinc nanoparticle (ZNP) formulation with CD40L molecular trigger 14 Humanized monoclonal antibody generated from mice immunized with SARS - CoV02 spike protein
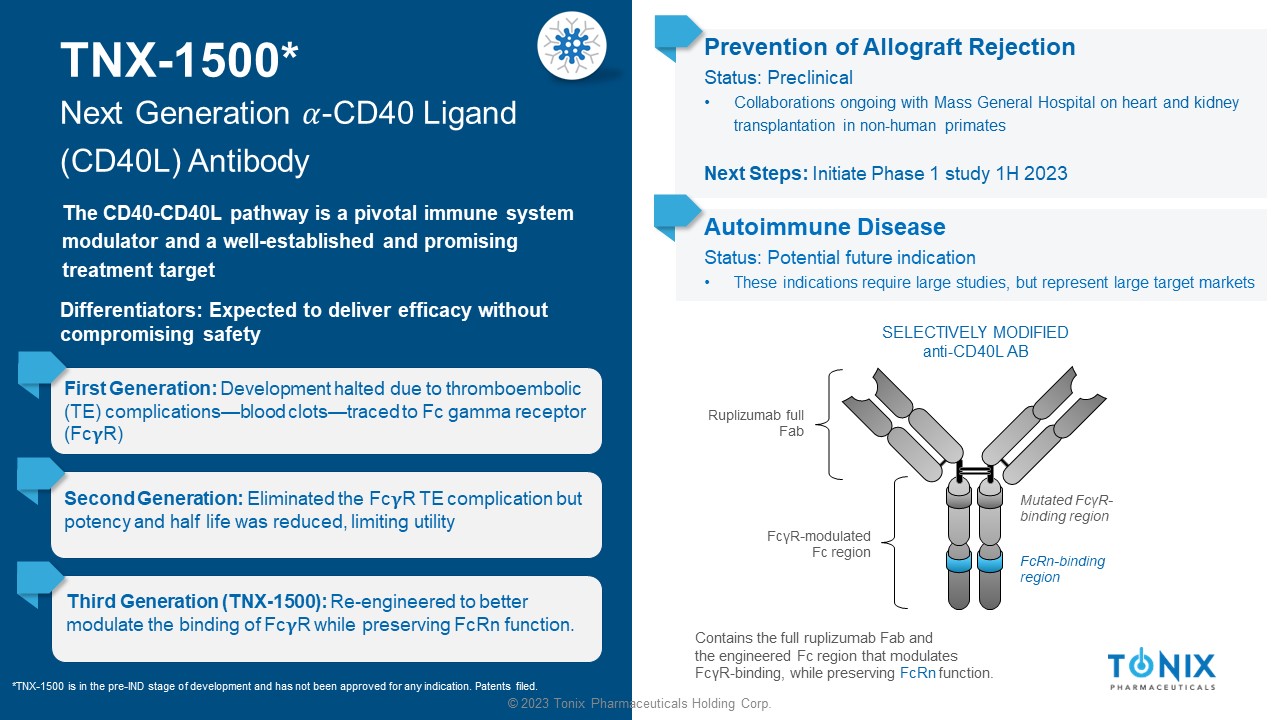
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 1500* Next Generation ߙ - CD40 Ligand (CD40L) Antibody The CD40 - CD40L pathway is a pivotal immune system modulator and a well - established and promising treatment target First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Prevention of Allograft Rejection Status: Preclinical • Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates Next Steps: Initiate Phase 1 study 1H 2023 SELECTIVELY MODIFIED anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500): Re - engineered to better modulate the binding of Fc R while preserving FcRn function. *TNX - 1500 is in the pre - IND stage of development and has not been approved for any indication. Patents filed. Differentiators: Expected to deliver efficacy without compromising safety Autoimmune Disease Status: Potential future indication • These indications require large studies, but represent large target markets

7 © 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Internal Development & Manufacturing Capabilities Infectious Disease R&D Center (RDC) – Frederick, MD • Function: Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases • Description: ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 • Status: Operational Advanced Development Center (ADC) – North Dartmouth, MA • Function: Development and clinical scale manufacturing of biologics • Description: ~45,000 square feet, BSL - 2 • Status: Operational Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of biologics • Description: ~44 - acre green field site, planned BSL - 2 • Status: Planning for site enabling work in 2023 Architectural Rendering

© 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: Anti - SARS - C o V - 2 MONOCLONAL ANTIBODIES
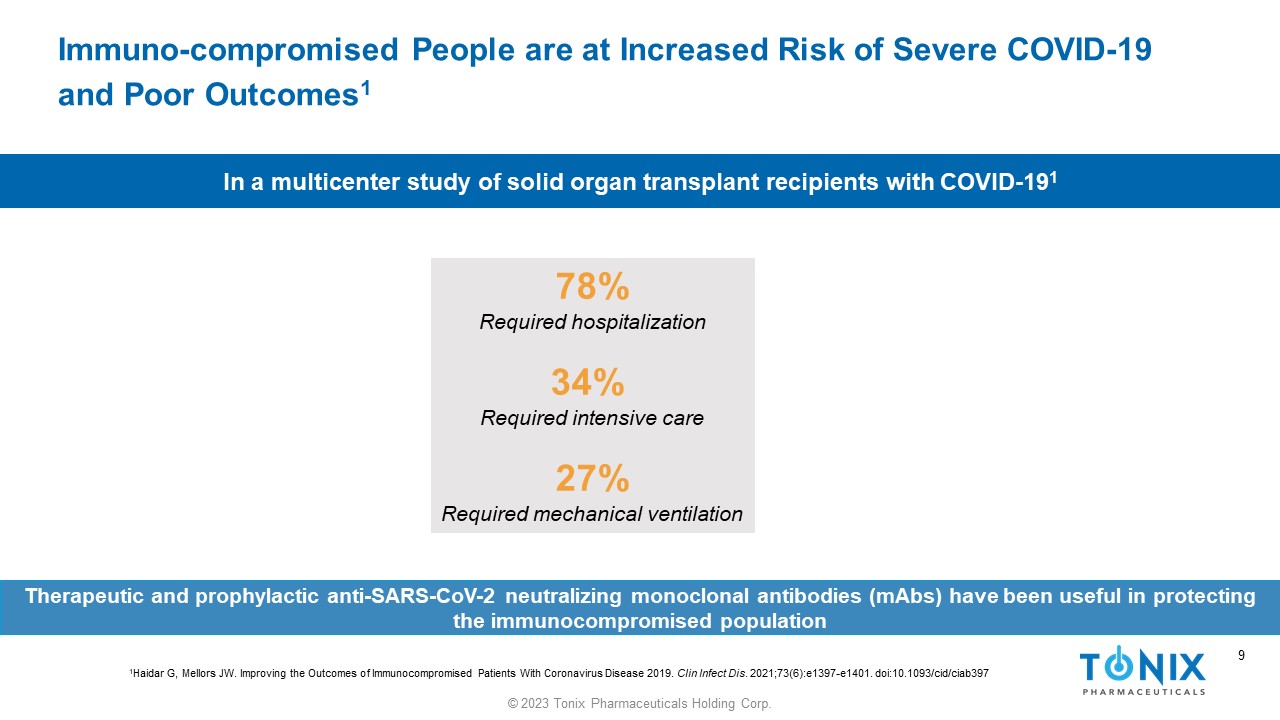
9 © 2023 Tonix Pharmaceuticals Holding Corp. 9 Immuno - compromised People are at Increased Risk of Severe COVID - 19 and Poor Outcomes 1 78% Required hospitalization 34% Required intensive care 27% Required mechanical ventilation In a multicenter study of solid organ transplant recipients with COVID - 19 1 1 Haidar G, Mellors JW. Improving the Outcomes of Immunocompromised Patients With Coronavirus Disease 2019. Clin Infect Dis . 2021;73(6):e1397 - e1401. doi:10.1093/ cid /ciab397 Therapeutic and prophylactic anti - SARS - CoV - 2 neutralizing monoclonal antibodies ( mAbs ) have been useful in protecting the immunocompromised population
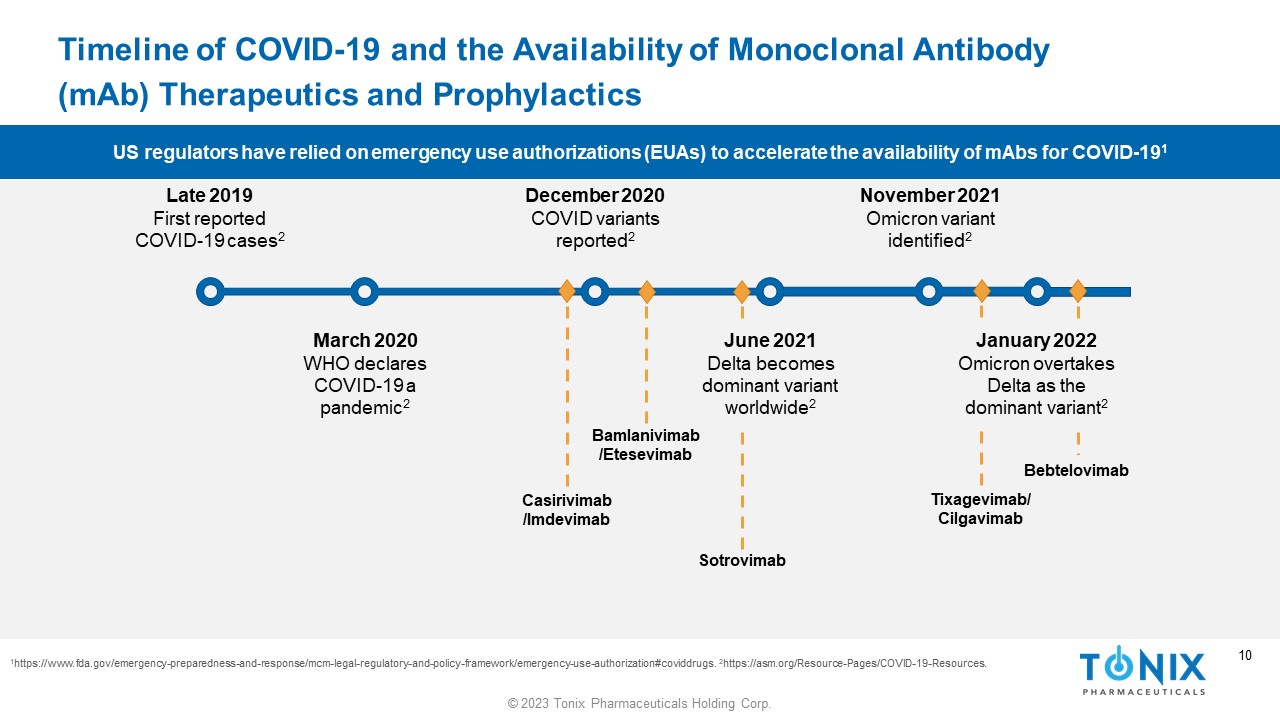
10 © 2023 Tonix Pharmaceuticals Holding Corp. 10 Timeline of COVID - 19 and the Availability of Monoclonal Antibody ( mAb ) Therapeutics and Prophylactics 1 https://www.fda.gov/emergency - preparedness - and - response/mcm - legal - regulatory - and - policy - framework/emergency - use - authorization#co viddrugs. 2 https://asm.org/Resource - Pages/COVID - 19 - Resources. Late 2019 First reported COVID - 19 cases 2 December 2020 COVID variants reported 2 June 2021 Delta becomes dominant variant worldwide 2 November 2021 Omicron variant identified 2 March 2020 WHO declares COVID - 19 a pandemic 2 Casirivimab /Imdevimab Bamlanivimab / Etesevimab Tixagevimab / Cilgavimab Bebtelovimab Sotrovimab January 2022 Omicron overtakes Delta as the dominant variant 2 US regulators have relied on emergency use authorizations (EUAs) to accelerate the availability of mAbs for COVID - 19 1
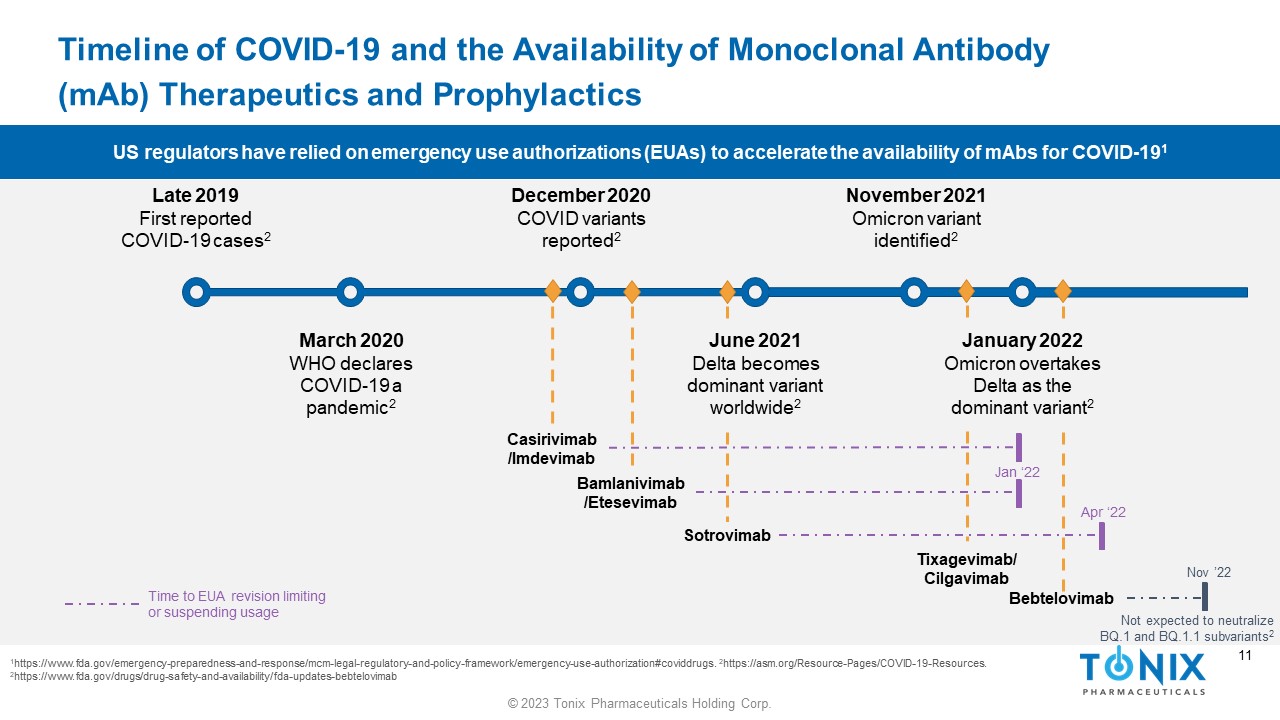
11 © 2023 Tonix Pharmaceuticals Holding Corp. 11 Timeline of COVID - 19 and the Availability of Monoclonal Antibody ( mAb ) Therapeutics and Prophylactics 1 https://www.fda.gov/emergency - preparedness - and - response/mcm - legal - regulatory - and - policy - framework/emergency - use - authorization#co viddrugs. 2 https://asm.org/Resource - Pages/COVID - 19 - Resources. 2 https://www.fda.gov/drugs/drug - safety - and - availability/fda - updates - bebtelovimab US regulators have relied on emergency use authorizations (EUAs) to accelerate the availability of mAbs for COVID - 19 1 Late 2019 First reported COVID - 19 cases 2 December 2020 COVID variants reported 2 June 2021 Delta becomes dominant variant worldwide 2 November 2021 Omicron variant identified 2 March 2020 WHO declares COVID - 19 a pandemic 2 Casirivimab /Imdevimab Bamlanivimab / Etesevimab Tixagevimab / Cilgavimab Bebtelovimab Sotrovimab January 2022 Omicron overtakes Delta as the dominant variant 2 Jan ‘22 Apr ‘22 Time to EUA revision limiting or suspending usage Nov ’22 Not expected to neutralize BQ.1 and BQ.1.1 subvariants 2
12 © 2023 Tonix Pharmaceuticals Holding Corp. 12 The Available anti - SARS - CoV - 2 Monoclonal Antibodies are Losing Their Activity as SARS - CoV - 2 Mutates and Evasive Variants Arise The efficacy of any mAb treatment varied as the dominant circulating variant changed 1,2 1 https://www.covid19treatmentguidelines.nih.gov/therapies/anti - sars - cov - 2 - antibody - products/anti - sars - cov - 2 - monoclonal - antibodies / - download Jan 4, 2023 2 Gardner, L. Jan 1, 2023. Politico . Once - favored Covid drugs ineffective on Omicron may be putting millions at risk - Once - favored Covid drugs ineffective on Omicron may be putting millions at risk (msn.com) 3 Wu, K.J. October 29, 2022. The Atlantic. “ The End of Evusheld : If you’re immunocompromised, this … isn’t great. www.theatlantic.com/health/archive/2022/10/covid - variants - antibody - treatments - immunocompromised/671929/ 4 CDC Dec 20, 2022 - HAN Archive - 00483 | Health Alert Network (HAN) (cdc.gov) 5 Vir isolated sotrovimab from the blood of a SARS - CoV - 1 patent 6 Regeneron used both convalescent patient cells and a humanized mouse platform: Hansen J et al. Science . 2020 Aug 21;369(6506):1010 - 1014. doi : 10.1126/science.abd0827 Most therapeutic and prophylactic mAbs have originated from COVID - convalescent patient bloods 5,6 Therapeutic Monoclonal antibodies ( mAbs ) – none remaining with active US Emergency Use Authorization (EUA) endorsed by NIH Guidelines Panel 1,2 ‒ AbCellera /NIAID - VRC/Eli Lilly - bebtelovimab ‒ Regeneron/Genentech - REGEN - COV® Casirivimab/imdevimab ‒ Eli Lilly/ AbCellera /NIAID/ Junshi - China Academy of Sciences – B amlanivimab / etesevimab ‒ Vir /GSK – XEVURDY® (sotrovimab) Concerns about efficacy of the only preventative mAb product against new variants ‒ AstraZeneca/Vanderbilt – Evusheld ® ( Tixagevimab / cilgavimab ) – EUA for long term prophylaxis ▪ CDC reports 82% prevalence of resistant strain s 3,4

13 © 2023 Tonix Pharmaceuticals Holding Corp. 13 Need for a Strategy to Frequently Update Monoclonal Antibodies Current and prior mAb therapeutics were developed in collaborations A platform to quickly develop and test novel SARS - CoV - 2 neutralizing mAbs may represent a significant advancement in the ability to update the pool of mAb treatments available to protect the immunocompromised population Dr. Luciana Borio is former National Security Council director for medical and biodefense preparedness and current senior fellow for global health at the think tank Council on Foreign Relations. She is a venture partner at ARCH.
© 2023 Tonix Pharmaceuticals Holding Corp. 14 Fully Human anti - SARS - CoV - 2 Monoclonal Antibody Platform TNX - 3600 1 : COVID - 1 9 Therapeutic and Preventive Agents 14 Collaboration with Columbia University Fully human mAbs generated from SARS - CoV - 2 + asymptomatic individuals or COVID - 19 convalescent patients 3 Potential monotherapies or preventives • Plan to seek indication similar to current EUA therapeutic mAbs for treating individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease Potential combination therapy with other mAbs as therapeutics or prophylactics • Combination therapies for other anti - SARS - CoV - 2 monoclonal antibodies are believed to have reduced the emergence of drug resistant viral strains 4 1 TNX - 3600 is the designation for a series of monoclonal antibodies; each is in the pre - IND stage of development and has not been approved for any indication 2 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https://www.nature.com/articles/d41586 - 022 - 00199 - z 3 Volunteers participated in an IRB - approved research protocol 4 Baum, A. et al. Science . 2020 Aug 21;369(6506):1014 - 1018. doi : 10.1126/science.abd0831. Epub 2020 Jun 15. Given the unpredictable trajectory of the SARS - CoV - 2 virus and new variants 2 , we seek to contribute to a broad set of monoclonal antibodies from a variety of SARS - CoV - 2 + volunteers and convalescent patients, that can be scaled up quickly and potentially combined with other monoclonal antibodies
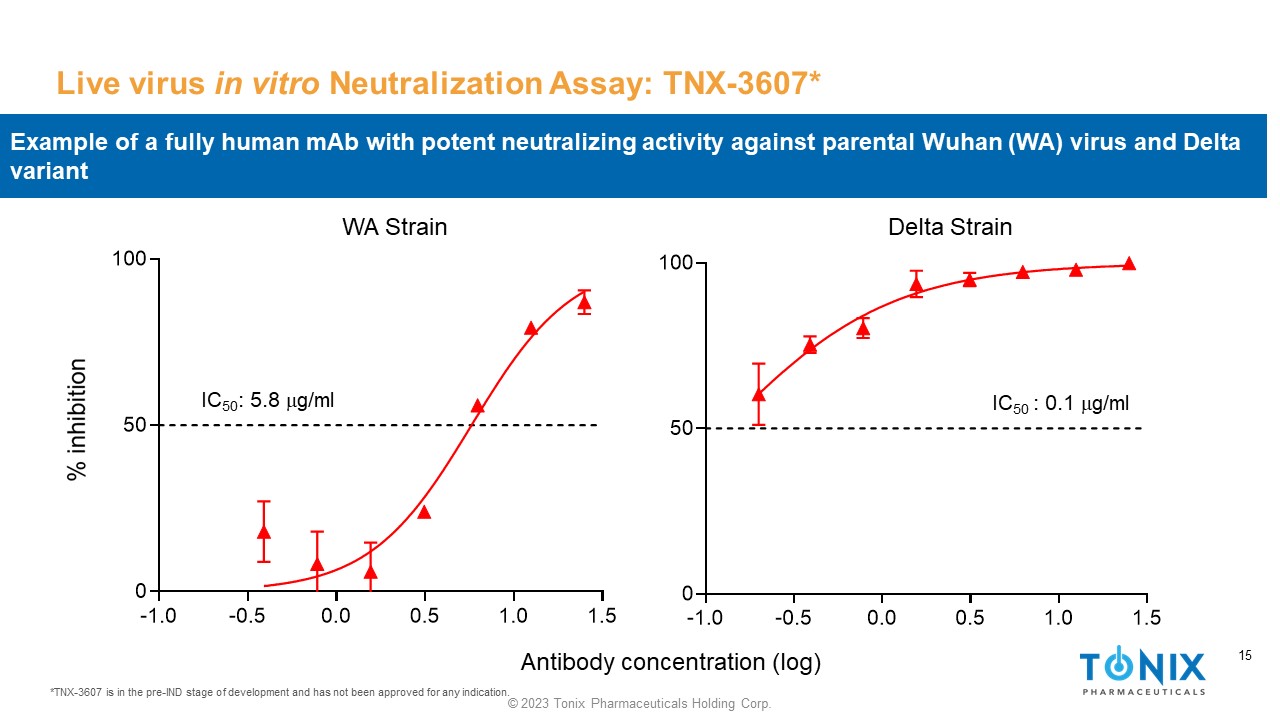
15 © 2023 Tonix Pharmaceuticals Holding Corp. 15 Live virus in vitro Neutralization Assay: TNX - 3607* *TNX - 3607 is in the pre - IND stage of development and has not been approved for any indication. -1.0 -0.5 0.0 0.5 1.0 1.5 0 50 100 IC 50 : 5.8 m g/ml -1.0 -0.5 0.0 0.5 1.0 1.5 0 50 100 IC 50 : 0.1 m g/ml WA Strain Delta Strain Antibody concentration (log) Example of a fully human mAb with potent neutralizing activity against parental Wuhan (WA) virus and Delta variant
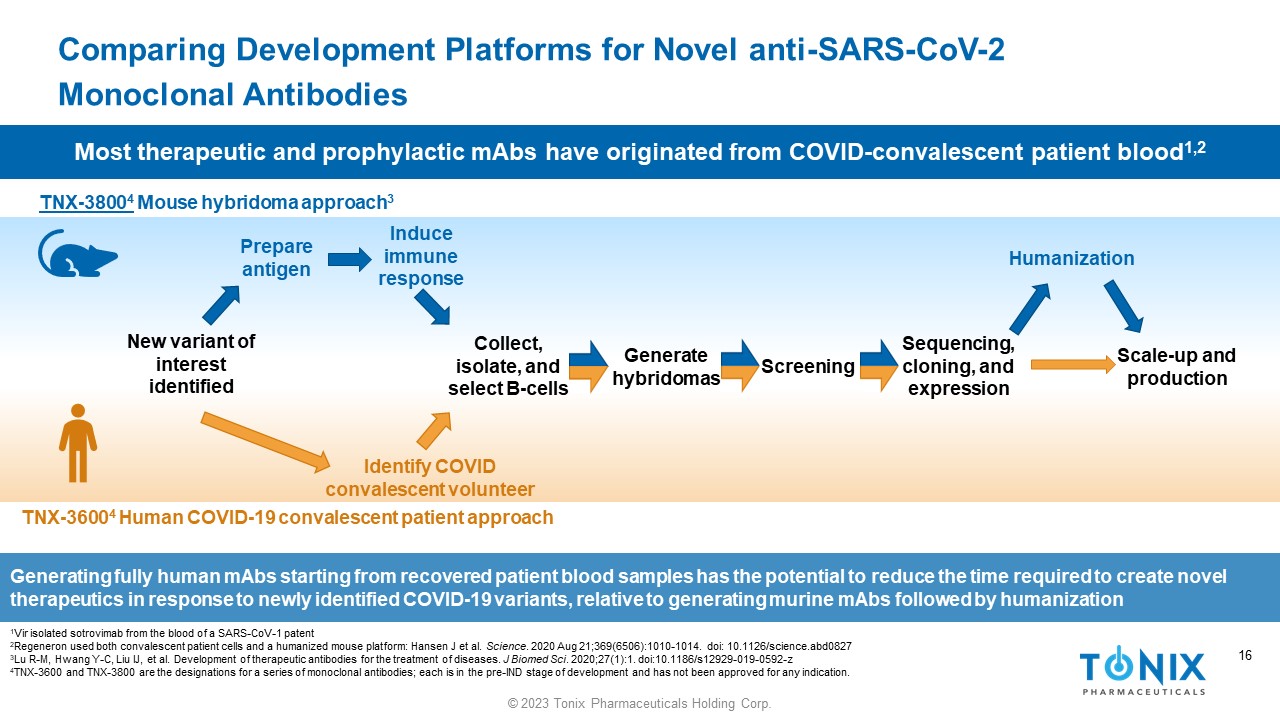
16 © 2023 Tonix Pharmaceuticals Holding Corp. 16 Comparing Development Platforms for Novel anti - SARS - CoV - 2 Monoclonal Antibodies 1 Vir isolated sotrovimab from the blood of a SARS - CoV - 1 patent 2 Regeneron used both convalescent patient cells and a humanized mouse platform: Hansen J et al. Science . 2020 Aug 21;369(6506):1010 - 1014. doi : 10.1126/science.abd0827 3 Lu R - M, Hwang Y - C, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci . 2020;27(1):1. doi:10.1186/s12929 - 019 - 0592 - z 4 TNX - 3600 and TNX - 3800 are the designations for a series of monoclonal antibodies; each is in the pre - IND stage of development an d has not been approved for any indication. Generating fully human mAbs starting from recovered patient blood samples has the potential to reduce the time required to create novel therapeutics in response to newly identified COVID - 19 variants, relative to generating murine mAbs followed by humanization TNX - 3800 4 Mouse hybridoma approach 3 TNX - 3600 4 Human COVID - 19 convalescent patient approach Collect, isolate, and select B - cells Generate hybridomas Screening Sequencing, cloning, and expression Scale - up and production Prepare antigen Induce immune response Humanization Identify COVID convalescent volunteer New variant of interest identified Most therapeutic and prophylactic mAbs have originated from COVID - convalescent patient blood 1,2
© 2023 Tonix Pharmaceuticals Holding Corp. 17 Humanized Murine anti - SARS - CoV - 2 Monoclonal Antibodies TNX - 3800 1 : COVID - 1 9 Therapeutic and Preventive Agents 17 Licensed from Curia Global Humanized mAbs generated from SARS - CoV - 2 + mice immunized with SARS - CoV - 2 spike protein 3 Potential monotherapies or preventives • Plan to seek indication similar to current EUA therapeutic mAbs for treating individuals with mild - to - moderate COVID - 19 who are at high risk for progression to severe disease Potential combination therapy with other mAbs as therapeutics or prophylactics • Combination therapies for other anti - SARS - CoV - 2 monoclonal antibodies are believed to have reduced the emergence of drug resistant viral strains 4 1 TNX - 3800 is the designation for a series of monoclonal antibodies; each is in the pre - IND stage of development and has not been approved for any indication 2 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https://www.nature.com/articles/d41586 - 022 - 00199 - z 3 Volunteers participated in an IRB - approved research protocol 4 Baum, A. et al. Science . 2020 Aug 21;369(6506):1014 - 1018. doi : 10.1126/science.abd0831. Epub 2020 Jun 15. Given the unpredictable trajectory of the SARS - CoV - 2 virus and new variants 2 , we seek to contribute to a broad set of monoclonal antibodies from a variety of SARS - CoV - 2 + volunteers and convalescent patients, that can be scaled up quickly and potentially combined with other monoclonal antibodies
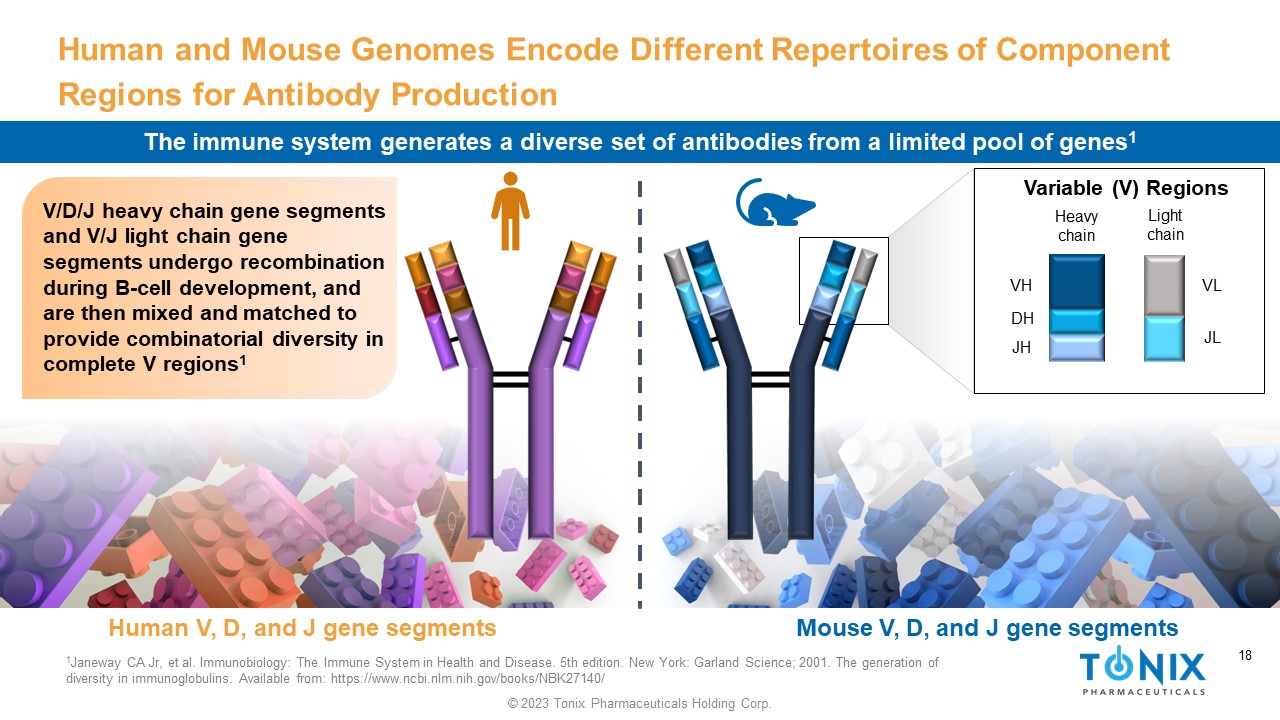
18 © 2023 Tonix Pharmaceuticals Holding Corp. 18 Human and Mouse Genomes Encode Different Repertoires of Component Regions for Antibody Production V/D/J heavy chain gene segments and V/J light chain gene segments undergo recombination during B - cell development, and are then mixed and matched to provide combinatorial diversity in complete V regions 1 1 Janeway CA Jr, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. T he generation of diversity in immunoglobulins. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27140/ Variable (V) Regions Heavy chain Light chain VL JL VH JH DH Human V, D, and J gene segments Mouse V, D, and J gene segments The immune system generates a diverse set of antibodies from a limited pool of genes 1
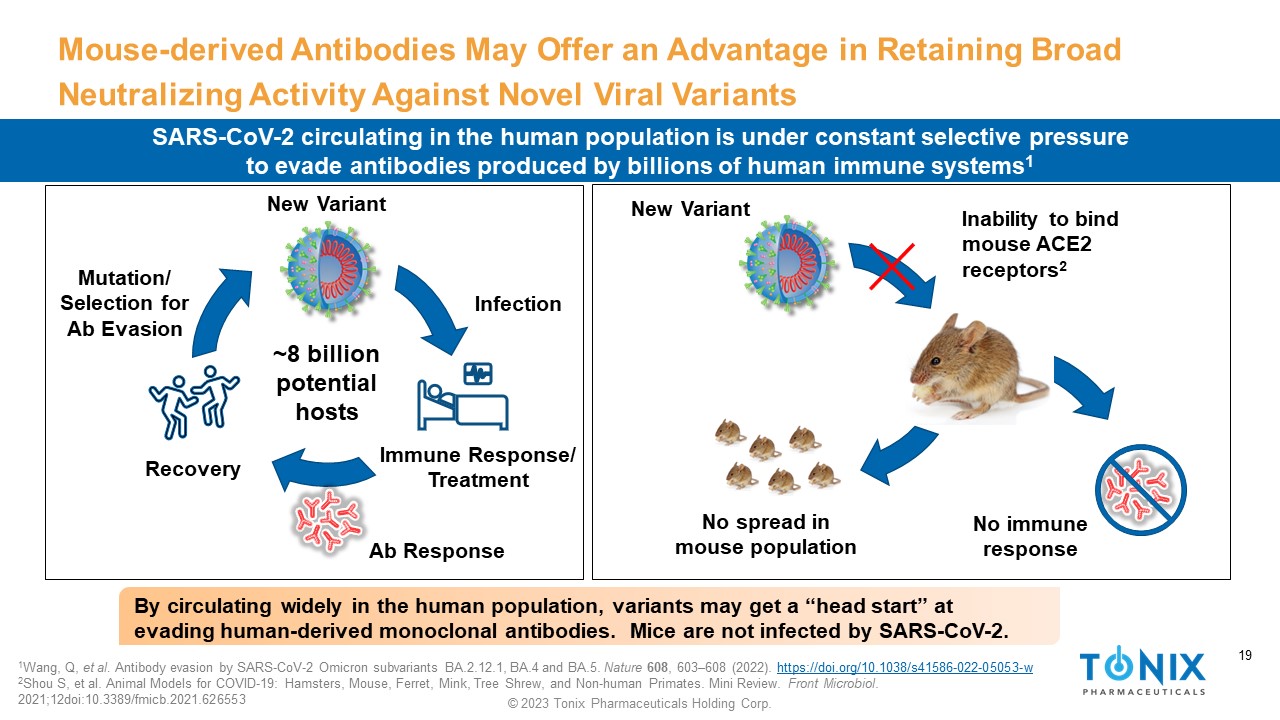
19 © 2023 Tonix Pharmaceuticals Holding Corp. 19 Mouse - derived Antibodies May Offer an Advantage in Retaining Broad Neutralizing Activity Against Novel Viral Variants SARS - CoV - 2 circulating in the human population is under constant selective pressure to evade antibodies produced by billions of human immune systems 1 New Variant Infection Immune Response/ Treatment Recovery Mutation/ Selection for Ab Evasion Ab Response New Variant Inability to bind mouse ACE2 receptors 2 No immune response ~8 billion potential hosts No spread in mouse population 1 Wang, Q, et al. Antibody evasion by SARS - CoV - 2 Omicron subvariants BA.2.12.1, BA.4 and BA.5. Nature 608 , 603 – 608 (2022). https://doi.org/10.1038/s41586 - 022 - 05053 - w 2 Shou S, et al. Animal Models for COVID - 19: Hamsters, Mouse, Ferret, Mink, Tree Shrew, and Non - human Primates. Mini Review. Front Microbiol . 2021;12doi:10.3389/fmicb.2021.626553 By circulating widely in the human population, variants may get a “head start” at evading human - derived monoclonal antibodies. Mice are not infected by SARS - CoV - 2.
© 2023 Tonix Pharmaceuticals Holding Corp. 20 • Mice have a different repertoire of antibodies 1 ‒ Bind to different epitopes than human - derived antibodies • Widespread, global COVID and SARS - CoV - 2 infection are putting selective pressure on SARS - CoV - 2 to evade human antibody repertoire ‒ Rapid evasion confounds the durability of individual mAb therapeutic products ‒ Potentially speeded by recombination between variants ‒ Both new products are needed and potentially new combinations of new with existing mAbs • Mice are not infected by SARS - CoV - 2, so SARS - CoV - 2 is not under selective pressure to evade murine antibody responses ‒ Mice are resistant to SARS - CoV - 2 for a variety of reasons, including that their AC2 receptor homologue does not bind SARS - CoV - 2 spike protein 2 ‒ For “updated” mRNA booster vaccines encoding omicron spike antigen, FDA approvals were granted without human efficacy data consistent with a “cartridge” approach Potential for Longer Period of Time for Mouse - Derived anti - SARS - CoV - 2 Spike Protein Antibodies to be Useful 20 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 2 https://www.covid19treatmentguidelines.nih.gov/therapies/anti - sars - cov - 2 - antibody - products/anti - sars - cov - 2 - monoclonal - antibodies / - accessed Nov 3, 2022
21 © 2023 Tonix Pharmaceuticals Holding Corp. 21 Therapeutic Monoclonal Antibody Development for COVID - 19 has been Focused on a “Whack - a - Mole” 1x1 Monoclonal Antibody v. Variant Battle Delta Omicron 1 Waltz, E. Nature. “Does the World Need an Omicron Vaccine?” 28 Jan 2022 https://www.nature.com/articles/d41586 - 022 - 00199 - z 2 https://www.fda.gov/emergency - preparedness - and - response/mcm - legal - regulatory - and - policy - framework/emergency - use - authorization#co viddrugs. • As new variants emerge, mAbs that were highly effective against older variants may quickly lose their place in the treatment landscape 1 • Antibodies receiving Emergency Use Authorizations (EUAs) may only have a lifespan of 1 - 2 years before shifts in the dominant circulating variant reduce their clinical utility 2
22 © 2023 Tonix Pharmaceuticals Holding Corp. 22 As the Circulating Mix of SARS - CoV - 2 Variants Changes, it Seems Prudent to Assemble a Diverse Inventory of Monoclonal Antibodies to Match It 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 Inventory of available anti - SARS - CoV - 2 mAbs Currently circulating “Variant Soup” SARS - CoV - 2 variants

23 © 2023 Tonix Pharmaceuticals Holding Corp. 23 The Platform is Designed to Develop and Maintain a Diverse Inventory of Monoclonal Antibodies to Keep Up with SARS - CoV - 2 “Variant Soup” 1 Currently circulating “Variant Soup” New variants Biotech industry Inventory of available anti - SARS - CoV - 2 mAbs Diabolical SARS - CoV - 2 Generator of Diversity Therapeutic mAb cocktail 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6
24 © 2023 Tonix Pharmaceuticals Holding Corp. 24 Desired Inventory Current Situation As the Circulating Mix of SARS - CoV - 2 Variants Changes, it Seems Prudent to Assemble a Diverse Inventory of Monoclonal Antibodies to Match It 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 Currently circulating “Variant Soup” 1 New variants Inventory of available anti - SARS - CoV - 2 mAbs
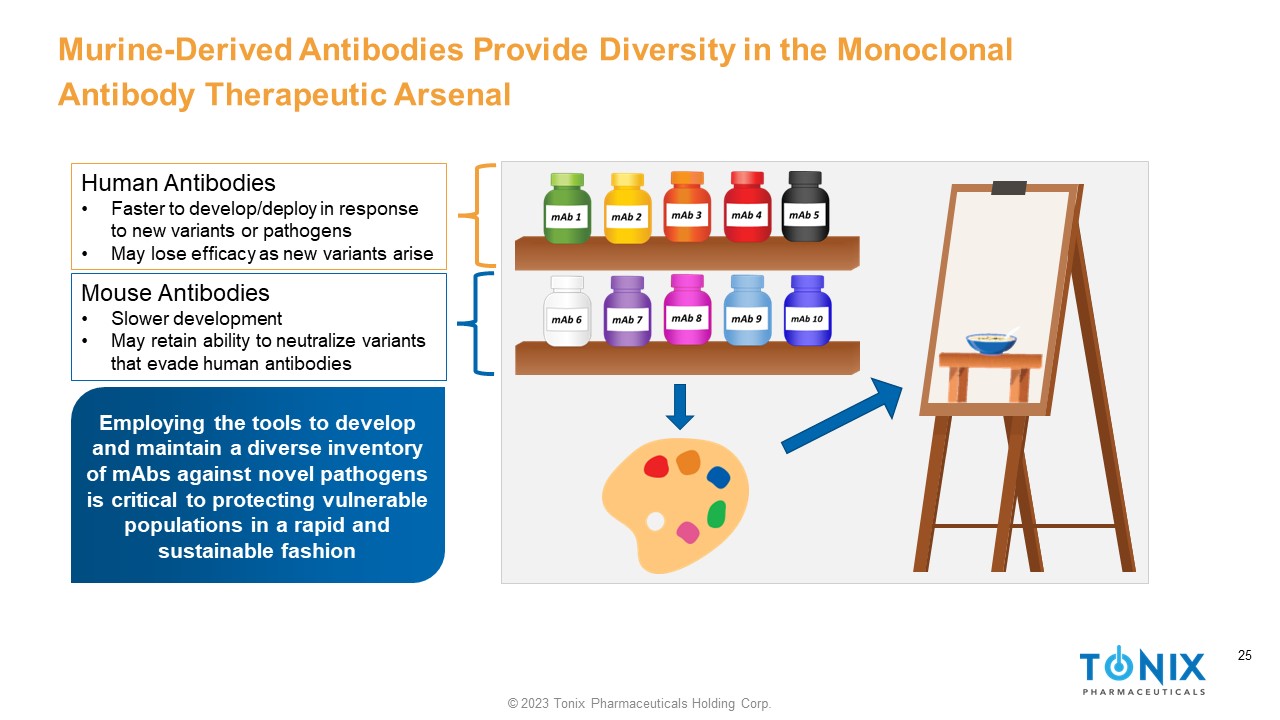
25 © 2023 Tonix Pharmaceuticals Holding Corp. 25 Murine - Derived Antibodies Provide Diversity in the Monoclonal Antibody Therapeutic Arsenal Human Antibodies • Faster to develop/deploy in response to new variants or pathogens • May lose efficacy as new variants arise Mouse Antibodies • Slower development • May retain ability to neutralize variants that evade human antibodies Employing the tools to develop and maintain a diverse inventory of mAbs against novel pathogens is critical to protecting vulnerable populations in a rapid and sustainable fashion
© 2023 Tonix Pharmaceuticals Holding Corp. 26 • Immune - evading SARS - CoV - 2 variants are arising by divergent and convergent evolutionary processes 1 ‒ Potentially speeded by recombination between variants • To protect immuno - compromised individuals from a changing “soup” of SARS - CoV - 2 variants, we need an extensive palate of mAbs ‒ Rapid evasion confounds the durability of individual mAb therapeutic products ‒ Both new products are needed and potentially new combinations of new with existing mAbs • For life - saving, but short - lived products, we expect FDA to regulate with commensurate speed ‒ Joint EMA/FDA meeting held on Dec 15, 2022 to discuss criteria for approving new mAbs 2 ‒ For “updated” mRNA booster vaccines encoding omicron spike antigen, FDA approvals were granted without human efficacy data consistent with a “cartridge” approach Future of COVID - 19 mAb Therapeutics and Prophylactics 26 1 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron are proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 2 Mast, J. Dec 15, 2022. STAT News. “ Drugmakers ask regulators to change standards on new Covid antibody drugs for most vulnerable ” www.statnews.com/2022/12/15/drugmakers - seek - standards - new - covid - antibody - drugs/

© 2023 Tonix Pharmaceuticals Holding Corp. FUTURE OUTLOOK

28 © 2023 Tonix Pharmaceuticals Holding Corp. Milestones: Recently Completed and Upcoming* Expected Clinical Trial Initiations □ 1 st Quarter 2023 Phase 2 study start of TNX - 1900 for the treatment of migraine □ 1 st Quarter 2023 Phase 2 study start of TNX - 1300 for the treatment of cocaine intoxication □ 1 st Quarter 2023 Phase 2 study start of TNX - 601 ER for the treatment of major depressive disorder □ 2 nd Quarter 2023 Phase 2 study start of TNX - 102 SL for the treatment of PTSD □ 2 nd Quarter 2023 Phase 1 study start of TNX - 1500 for prevention of allograft rejection □ 2 nd Half 2023 Phase 1 study start of TNX - 801 for prevention of monkeypox and smallpox * We cannot predict whether the global COVID - 19 pandemic will impact the timing of these milestones. □ 2 nd Quarter 2022 Phase 3 RESILIENT study start of TNX - 102 SL for the management of fibromyalgia □ 3rd Quarter 2022 Phase 2 PREVAIL study start of TNX - 102 SL for the treatment of Long COVID x x Expected Data □ 2 nd Quarter 2023 Interim analysis results of Phase 3 RESILIENT study of TNX - 102 SL in fibromyalgia □ 3 rd Quarter 2023 Interim analysis results of Phase 2 PREVAIL study of TNX - 102 SL in Long COVID
29 © 2023 Tonix Pharmaceuticals Holding Corp. Management Team Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU