
Exhibit 99.01

© 2023 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P0419 March 13 , 2023 (Doc 1177 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2023 Tonix Pharmaceuticals Holding Corp. Who We Are Tonix Pharmaceuticals is committed to improving population health by inventing and developing innovative therapies and vaccines, through broad in - house capabilities and creative collaborations , to help address important unmet needs. OUR MISSION Tonix strives to be a leader in providing novel drug therapies and vaccines to improve population health around the world. OUR VISION

4 © 2023 Tonix Pharmaceuticals Holding Corp. Investment Highlights DIVERSE PIPELINE Tonix’s c ore focus is on central nervous system disorders , but we also target unmet needs across multiple therapeutic areas including immunology, infectious disease and rare disease. STRATEGIC PARTNERSHIPS Partnering strategically with other biotech companies , world - class academic and non - profit research organizations to bring innovative therapeutics to market faster. IN - HOUSE CAPABILITIES Investment in domestic , in - house, R&D and manufacturing to accelerate development timelines and improve the ability to respond to pandemics. FINANCIAL POSITION Tonix had approximately $120 M in cash and cash equivalents as of 12/31/22. Tonix has no debt .
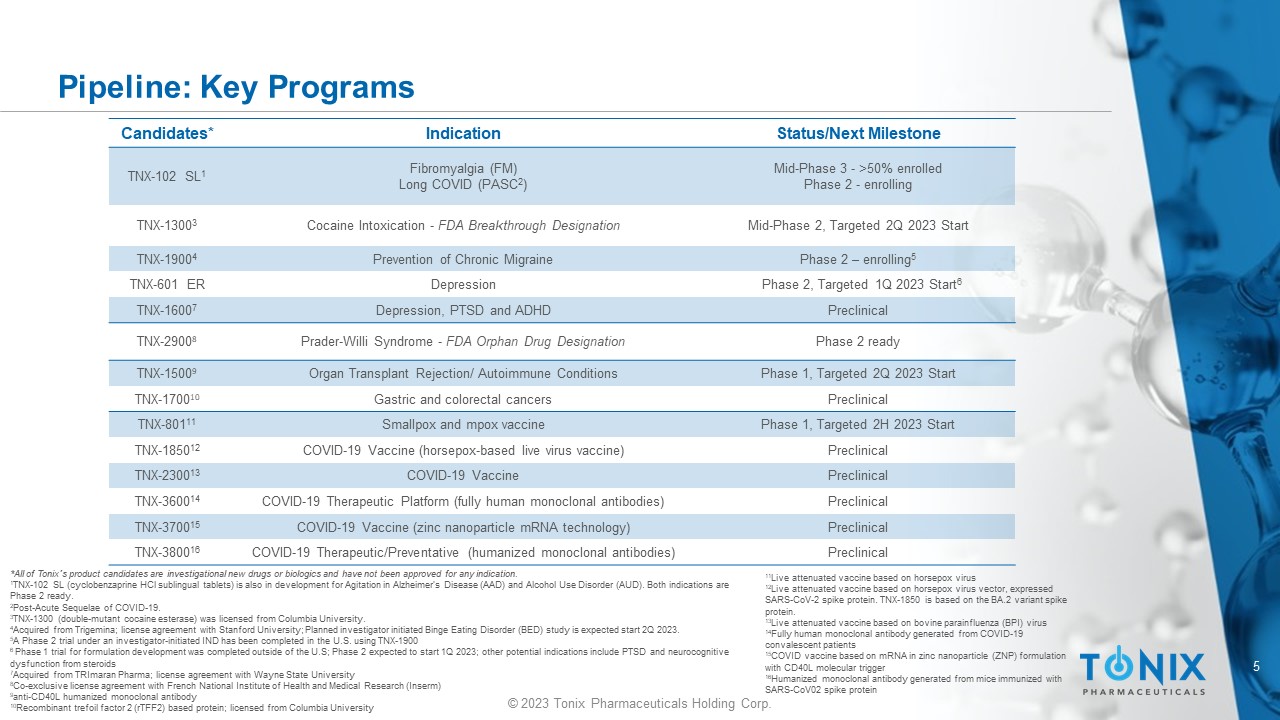
5 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Programs Candidates* Indication Status/Next Milestone TNX - 102 SL 1 Fibromyalgia (FM) Long COVID (PASC 2 ) Mid - Phase 3 - >50% enrolled Phase 2 - enrolling TNX - 1300 3 Cocaine Intoxication - FDA Breakthrough Designation Mid - Phase 2, Targeted 2Q 2023 Start TNX - 1900 4 Prevention of Chronic Migraine Phase 2 – enrolling 5 TNX - 601 ER Depression Phase 2, Targeted 1Q 2023 Start 6 TNX - 1600 7 Depression, PTSD and ADHD Preclinical TNX - 2900 8 Prader - Willi Syndrome - FDA Orphan Drug Designation Phase 2 ready TNX - 1500 9 Organ Transplant Rejection/ Autoimmune Conditions Phase 1, Targeted 2Q 2023 Start TNX - 1700 10 Gastric and colorectal cancers Preclinical TNX - 801 11 Smallpox and mpox vaccine Phase 1, Targeted 2H 2023 Start TNX - 1850 12 COVID - 19 Vaccine (horsepox - based live virus vaccine) Preclinical TNX - 2300 13 COVID - 19 Vaccine Preclinical TNX - 3600 14 COVID - 19 Therapeutic Platform (fully human monoclonal antibodies) Preclinical TNX - 3700 15 COVID - 19 Vaccine (zinc nanoparticle mRNA technology) Preclinical TNX - 3800 16 COVID - 19 Therapeutic/Preventative (humanized monoclonal antibodies) Preclinical *All of Tonix’s product candidates are investigational new drugs or biologics and have not been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) is also in development for Agitation in A lzheimer’s Disease (AAD) and Alcohol Use D isorder (AUD). Both indications are Phase 2 ready. 2 Post - Acute Sequelae of COVID - 19. 3 TNX - 1300 (double - mutant cocaine esterase) was licensed from Columbia University . 4 Acquired from Trigemina ; license agreement with Stanford University; Planned investigator initiated Binge Eating Disorder (BED) study is expected st art 2Q 2023. 5 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 6 Phase 1 trial for formulation development was completed outside of the U.S; Phase 2 expected to start 1Q 2023; other potentia l i ndications include PTSD and neurocognitive d ysfunction from steroids 7 Acquired from TRImaran Pharma; license agreement with Wayne State University 8 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) 9 anti - CD40L humanized monoclonal antibody 10 Recombinant trefoil factor 2 (rTFF2) based protein; licensed from Columbia University 11 Live attenuated vaccine based on horsepox virus 12 Live attenuated vaccine based on horsepox virus vector, expressed SARS - CoV - 2 spike protein. TNX - 1850 is based on the BA.2 variant spike protein. 13 Live attenuated vaccine based on bovine parainfluenza (BPI) virus 14 Fully human monoclonal antibody generated from COVID - 19 convalescent patients 15 COVID vaccine based on mRNA in zinc nanoparticle (ZNP) formulation with CD40L molecular trigger 16 Humanized monoclonal antibody generated from mice immunized with SARS - CoV02 spike protein

© 2023 Tonix Pharmaceuticals Holding Corp. CNS: KEY CANDIDATES
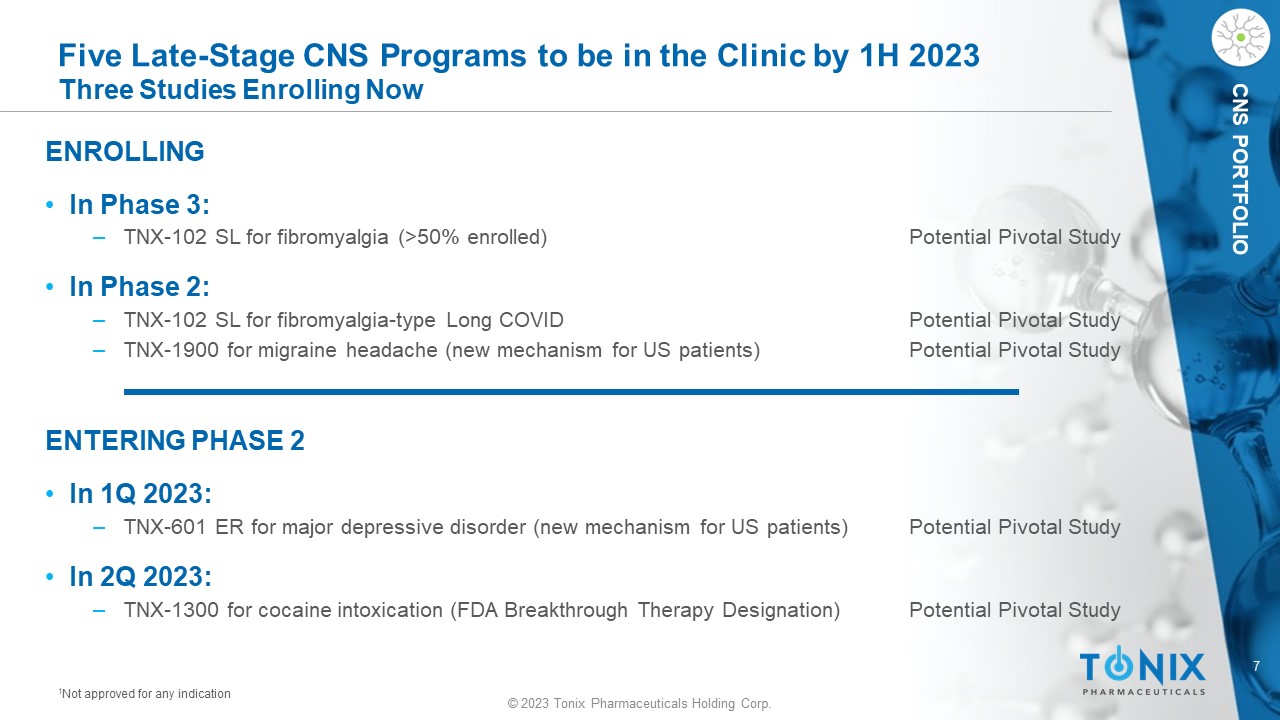
7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Five Late - Stage CNS Programs to be in the Clinic by 1H 2023 Three Studies Enrolling Now ENROLLING • In Phase 3: ‒ TNX - 102 SL for fibromyalgia (>50% enrolled) Potential Pivotal Study • In Phase 2: ‒ TNX - 102 SL for fibromyalgia - type Long COVID Potential Pivotal Study ‒ TNX - 1900 for migraine headache (new mechanism for US patients) Potential Pivotal Study ENTERING PHASE 2 • In 1Q 2023: ‒ TNX - 601 ER for major depressive disorder (new mechanism for US patients) Potential Pivotal Study • In 2Q 2023: ‒ TNX - 1300 for cocaine intoxication (FDA Breakthrough Therapy Designation) Potential Pivotal Study 1 Not approved for any indication
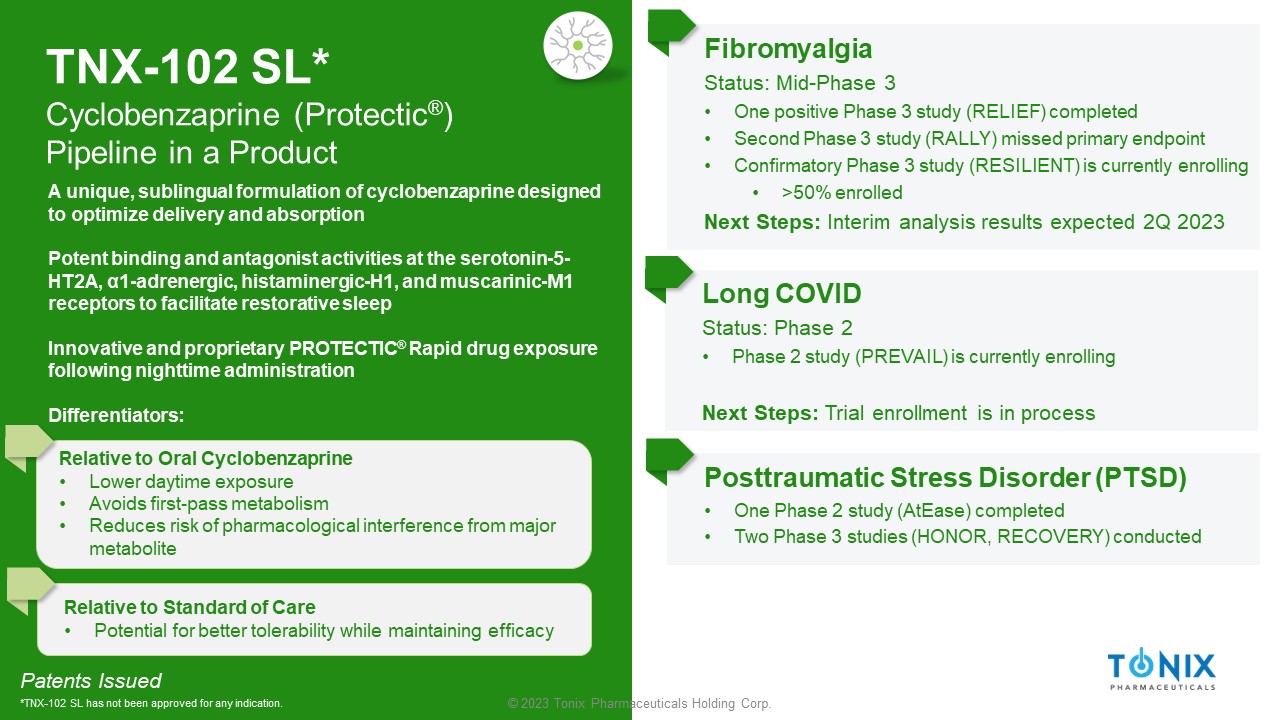
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* Cyclobenzaprine ( Protectic ® ) Pipeline in a Product Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) is currently enrolling • >50% enrolled Next Steps: Interim analysis results expected 2Q 2023 Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) is currently enrolling Next Steps: Trial enrollment is in process Patents Issued *TNX - 102 SL has not been approved for any indication. Posttraumatic Stress Disorder (PTSD) • One Phase 2 study ( AtEase ) completed • Two Phase 3 studies (HONOR, RECOVERY) conducted A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption Potent binding and antagonist activities at the serotonin - 5 - HT2A, α1 - adrenergic, histaminergic - H1, and muscarinic - M1 receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy

9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets CNS PORTFOLIO Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • A fflicts an estimated 6 - 12 million adults in the U.S., approximately 90% of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry: Fibromyalgia Additional Indications: Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status: One Positive Phase 3 study RELIEF completed Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT is currently enrolling Next Steps: Interim analysis results expected 2Q 2023 *TNX - 102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) When the check engine light malfunctions, the light is on even though the car is not malfunctioning
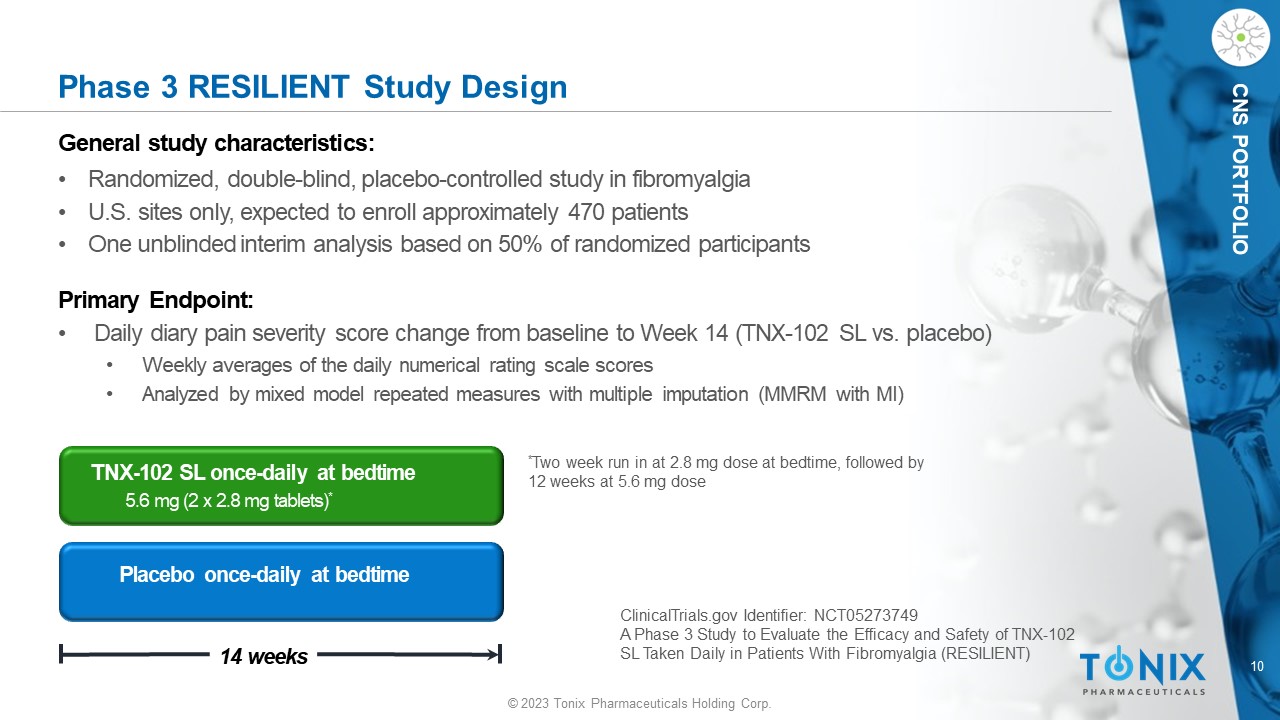
10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Phase 3 R ESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia • U.S. sites only, expected to enroll approximately 470 patients • One unblinded interim analysis based on 50% of randomized participants Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores • Analyzed by mixed model repeated measures with multiple imputation (MMRM with MI) Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT)
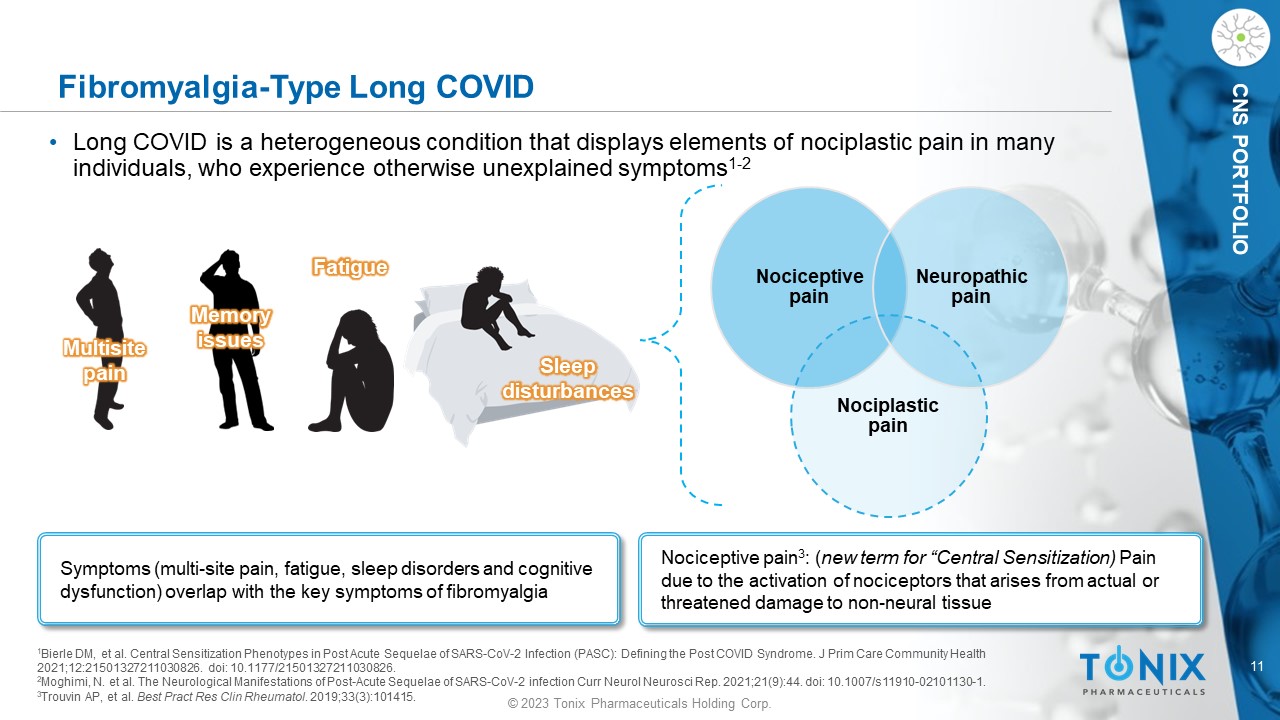
11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia - Type Long COVID • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained symptoms 1 - 2 Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia Multisite pain Memory issues Fatigue Sleep disturbances 1 Bierle DM, et al. Central Sensitization Phenotypes in Post Acute Sequelae of SARS - CoV - 2 Infection (PASC): Defining the Post COVI D Syndrome. J Prim Care Community Health 2021 ;12:21501327211030826. doi : 10.1177/21501327211030826. 2 Moghimi, N. et al. The Neurological Manifestations of Post - Acute Sequelae of SARS - CoV - 2 infection Curr Neurol Neurosci Rep. 2021;21(9):44. doi : 10.1007/s11910 - 02101130 - 1. 3 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Nociceptive pain Nociplastic pain Neuropathic pain Nociceptive pain 3 : ( new term for “Central Sensitization) Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue
12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia - Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 13% of recovered COVID - 19 patients 1 • As many as 40% of Long COVID patients experience multi - site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and chronic fatigue syndrome Market Entry : Fibromyalgia - Type Long COVID (PASC) Status: Phase 2 study PREVAIL is currently enrolling Next Steps: PREVAIL trial enrollment is in process 1 September 1, 2022 - CDC - https://www.cdc.gov/coronavirus/2019 - ncov/long - term - effects/index.html 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO Additional Indications: Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder
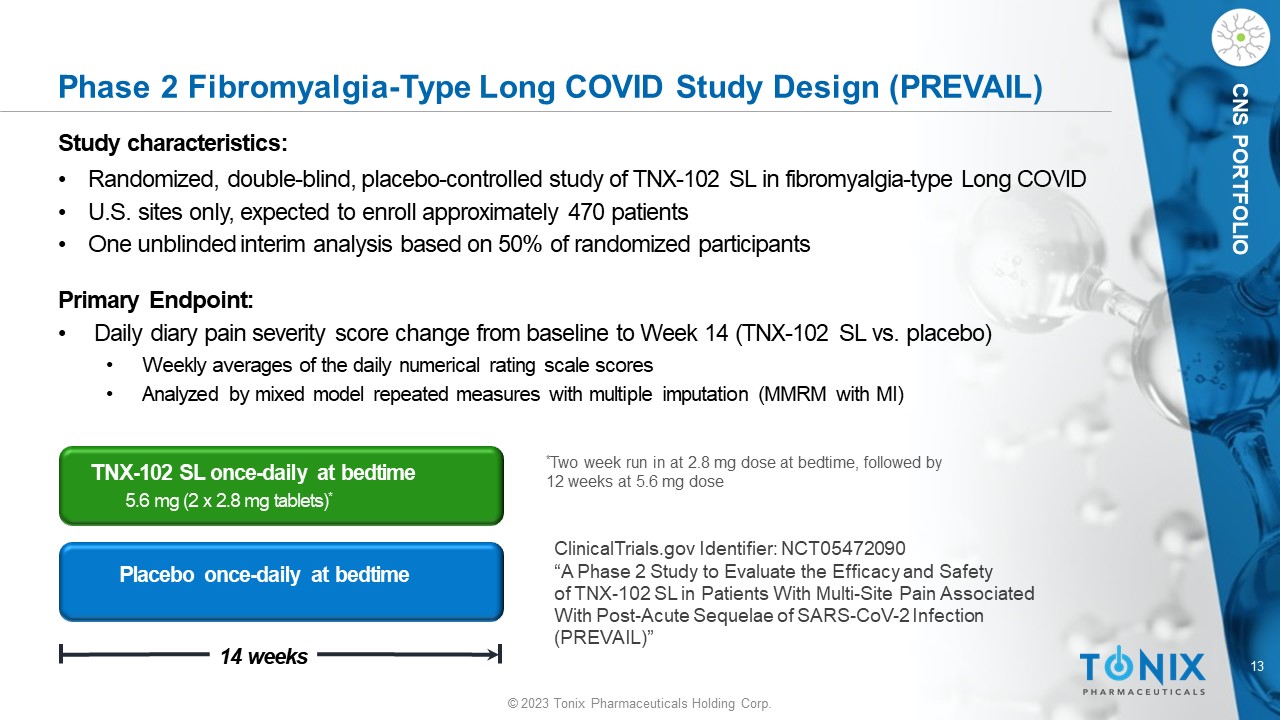
13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Phase 2 Fibromyalgia - Type Long COVID Study Design (PREVAIL) Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, expected to enroll approximately 470 patients • One unblinded interim analysis based on 50% of randomized participants Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores • Analyzed by mixed model repeated measures with multiple imputation (MMRM with MI) Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL in Patients With Multi - Site Pain Associated With Post - Acute Sequelae of SARS - CoV - 2 Infection (PREVAIL)”
14 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 601 E R*: Depression Tianeptine Hemioxalate Extended - Release Tablets (39.4 mg) CNS PORTFOLIO • A novel, oral, extended - release once - daily tablet • Treatment effect of tianeptine sodium immediate release t.i.d. in depression is well - established • Tianeptine restores neuroplasticity in animal models • Indirectly modulates the glutamatergic system • Does not interact with AMPA, NMDA or Kainate receptors 1 Differentiators: Relative to Tianeptine IR: • Once daily dosing Relative to traditional anti - depressants: • U nique mechanism of action – beyond neurotransmitter modulation • Tianeptine sodium IR has similar efficacy but fewer side effects than traditional anti - depressants Market Entry: Major Depressive Disorder Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids Status: Phase 2 ready Next Steps: Initiate a Phase 2 potentially pivotal study 1Q 2023 Interim analysis results expected 4Q 2023 1 AMPA= α - amino - 3 - hydroxy - 5 - methyl - 4 - isoxazolepropionic acid; NMDA=N - methyl - D - aspartate *TNX - 601 ER has not been approved for any indication.
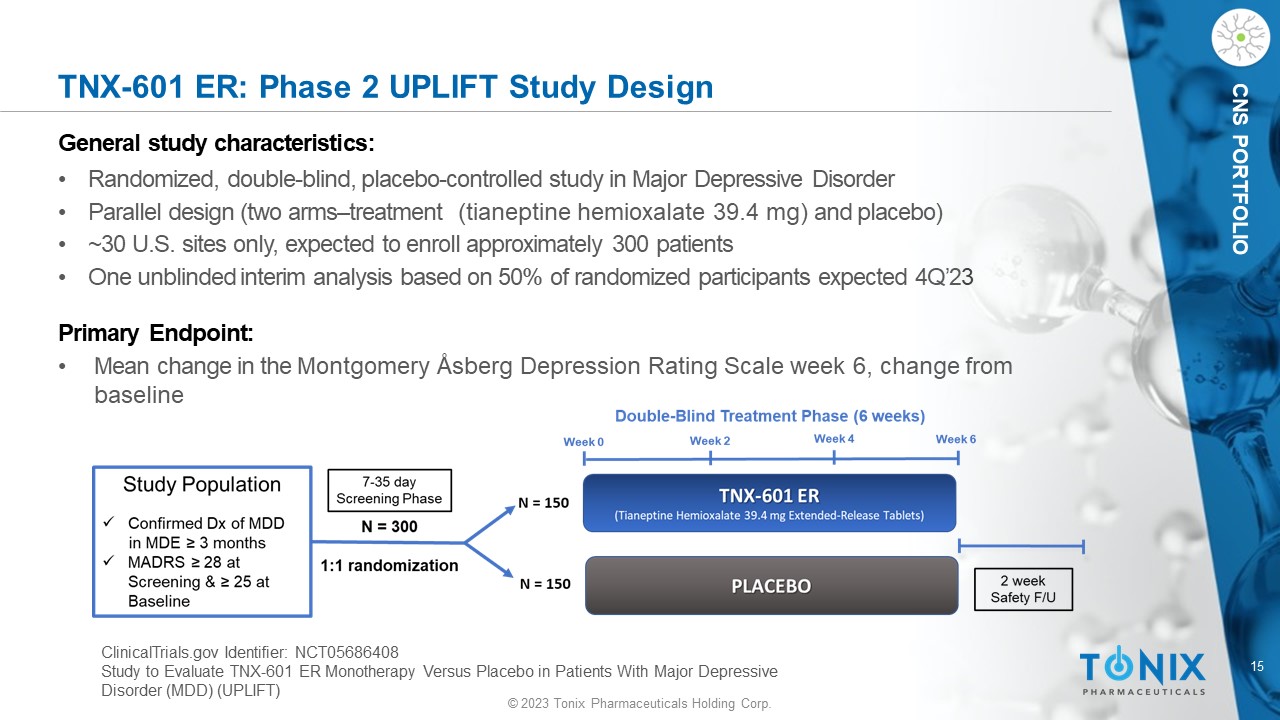
15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER: Phase 2 UPLIFT Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Major Depressive Disorder • Parallel design (two arms – treatment (tianeptine hemioxalate 39.4 mg) and placebo) • ~30 U.S. sites only, expected to enroll approximately 300 patients • One unblinded interim analysis based on 50% of randomized participants expected 4Q’2 3 Primary Endpoint: • M ean change in the Montgomery Åsberg Depression Rating Scale week 6, change from baseline ClinicalTrials.gov Identifier: NCT05686408 Study to Evaluate TNX - 601 ER Monotherapy Versus Placebo in Patients With Major Depressive Disorder (MDD) (UPLIFT)
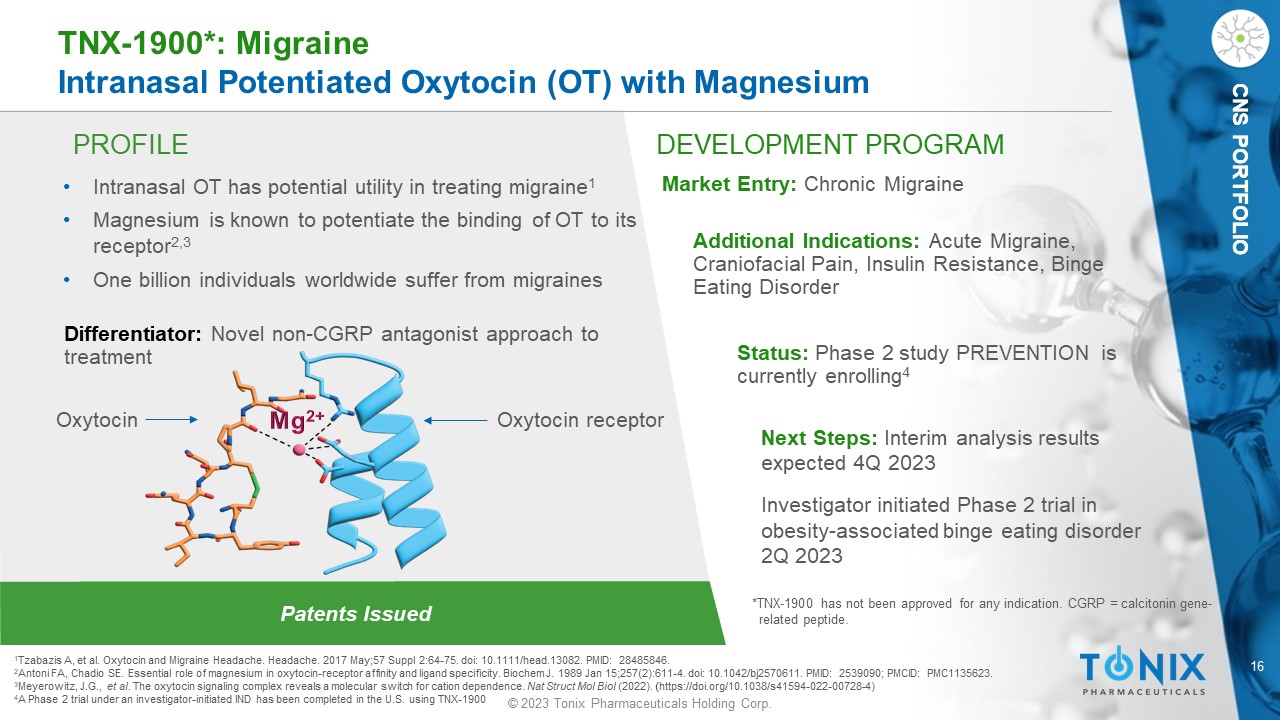
16 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1900*: Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium CNS PORTFOLIO • Intranasal OT has potential utility in treating migraine 1 • Magnesium is known to potentiate the binding of OT to its receptor 2,3 • One billion individuals worldwide suffer from migraines Differentiator: Novel non - CGRP antagonist approach to treatment Market Entry: Chronic Migraine Additional Indications: Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status: Phase 2 study PREVENTION is currently enrolling 4 Next Steps: Interim analysis results expected 4Q 2023 Investigator initiated Phase 2 t rial in obesity - associated binge e ating disorder 2Q 2023 1 Tzabazis A, et al. Oxytocin and Migraine Headache. Headache. 2017 May;57 Suppl 2:64 - 75. doi : 10.1111/head.13082. PMID: 28485846. 2 Antoni FA, Chadio SE. Essential role of magnesium in oxytocin - receptor affinity and ligand specificity. Biochem J. 1989 Jan 15;257(2):611 - 4. doi : 10.1042/bj2570611. PMID: 2539090; PMCID: PMC1135623. 3 Meyerowitz , J.G., et al. The oxytocin signaling complex reveals a molecular switch for cation dependence. Nat Struct Mol Biol (2022). ( https://doi.org/10.1038/s41594 - 022 - 00728 - 4) 4 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 *TNX - 1900 has not been approved for any indication. CGRP = calcitonin gene - related peptide. Oxytocin receptor Oxytocin
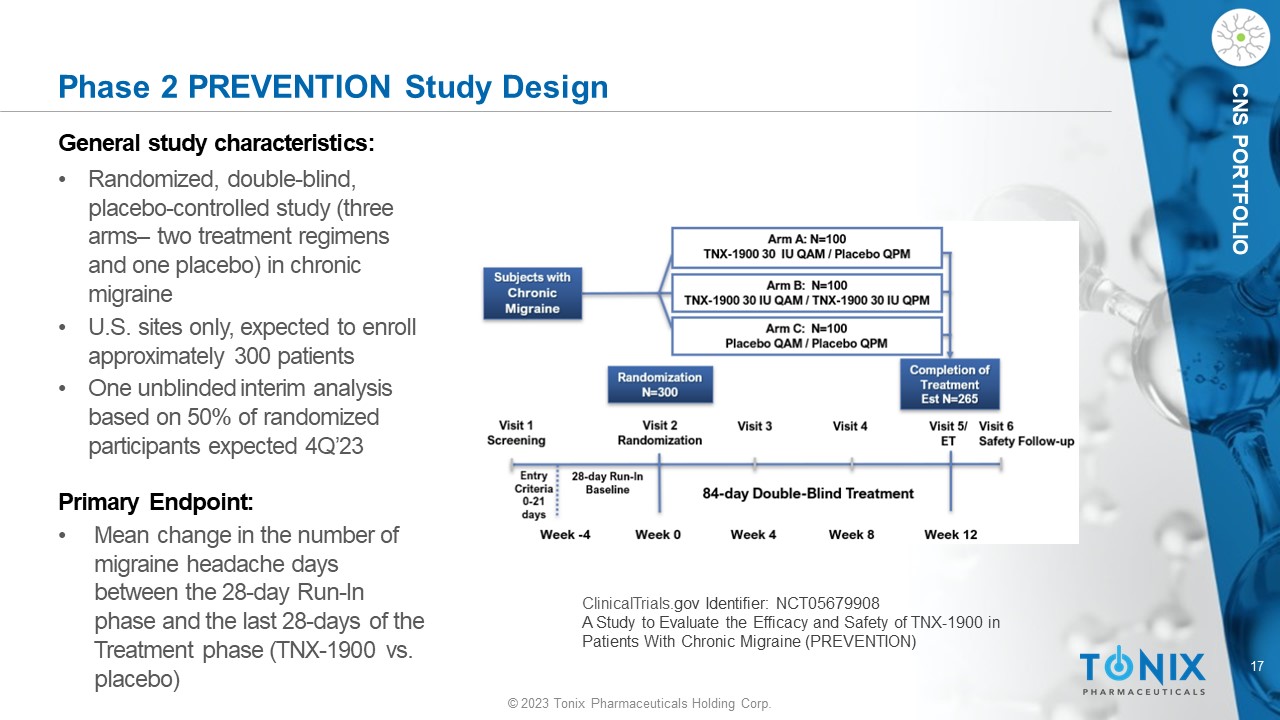
17 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Phase 2 PREVENTION Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study (three arms – two treatment regimens and one placebo) in chronic migraine • U.S. sites only, expected to enroll approximately 300 patients • One unblinded interim analysis based on 50% of randomized participants expected 4Q’23 Primary Endpoint: • M ean change in the number of migraine headache days between the 28 - day Run - In phase and the last 28 - days of the Treatment phase (TNX - 1900 vs. placebo) ClinicalTrials. gov Identifier: NCT05679908 A Study to Evaluate the Efficacy and Safety of TNX - 1900 in Patients With Chronic Migraine (PREVENTION)
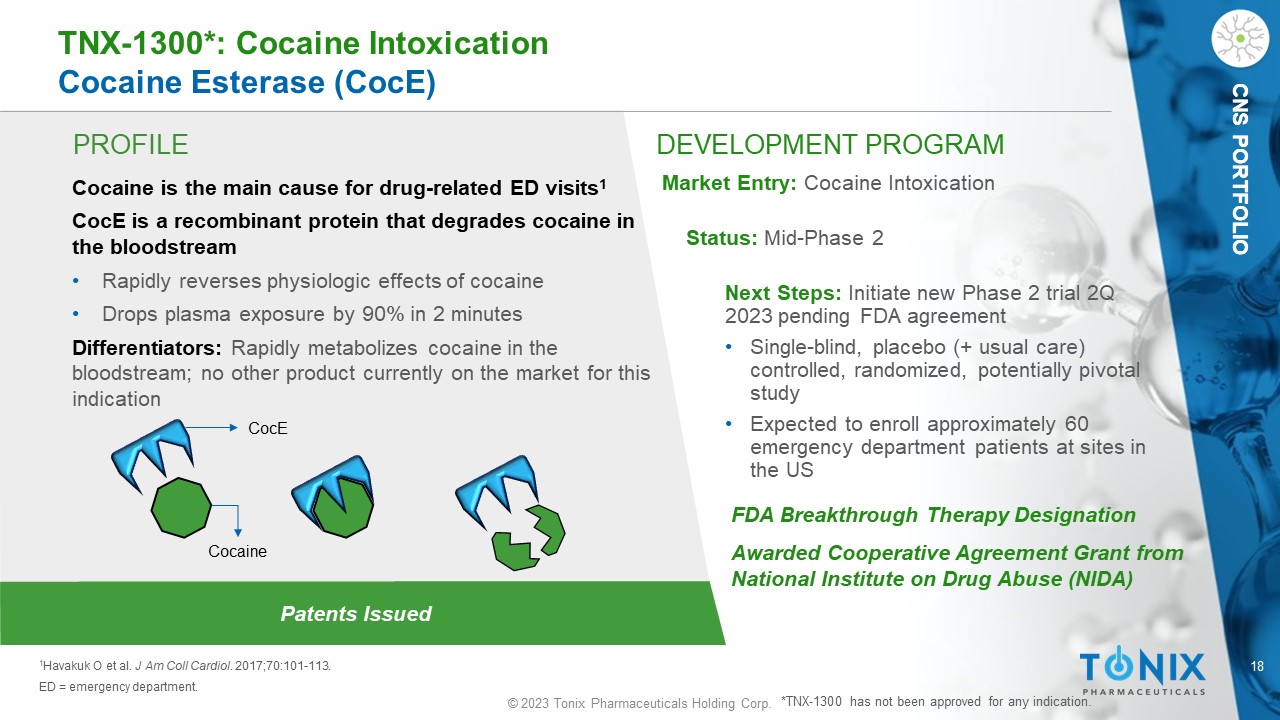
18 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 1300*: Cocaine Intoxication Cocaine Esterase ( CocE ) Cocaine is the main cause for drug - related ED visits 1 CocE is a recombinant protein that degrades cocaine in the bloodstream • Rapidly reverses physiologic effects of cocaine • Drops plasma exposure by 90% in 2 minutes Differentiators: Rapidly metabolizes cocaine in the bloodstream; no other product currently on the market for this indication Market Entry: Cocaine Intoxication Status: Mid - Phase 2 Next Steps: Initiate new Phase 2 trial 2Q 2023 pending FDA agreement • S ingle - blind, placebo (+ usual care) controlled, randomized, potentially pivotal study • Expected to enroll approximately 60 emergency department patients at sites in the US 1 Havakuk O et al. J Am Coll Cardiol . 2017;70:101 - 113. ED = emergency department. FDA Breakthrough Therapy Designation Awarded Cooperative Agreement Grant from National Institute on Drug Abuse (NIDA) *TNX - 1300 has not been approved for any indication. CNS PORTFOLIO CocE Cocaine

© 2023 Tonix Pharmaceuticals Holding Corp. RARE DISEASE: KEY CANDIDATES
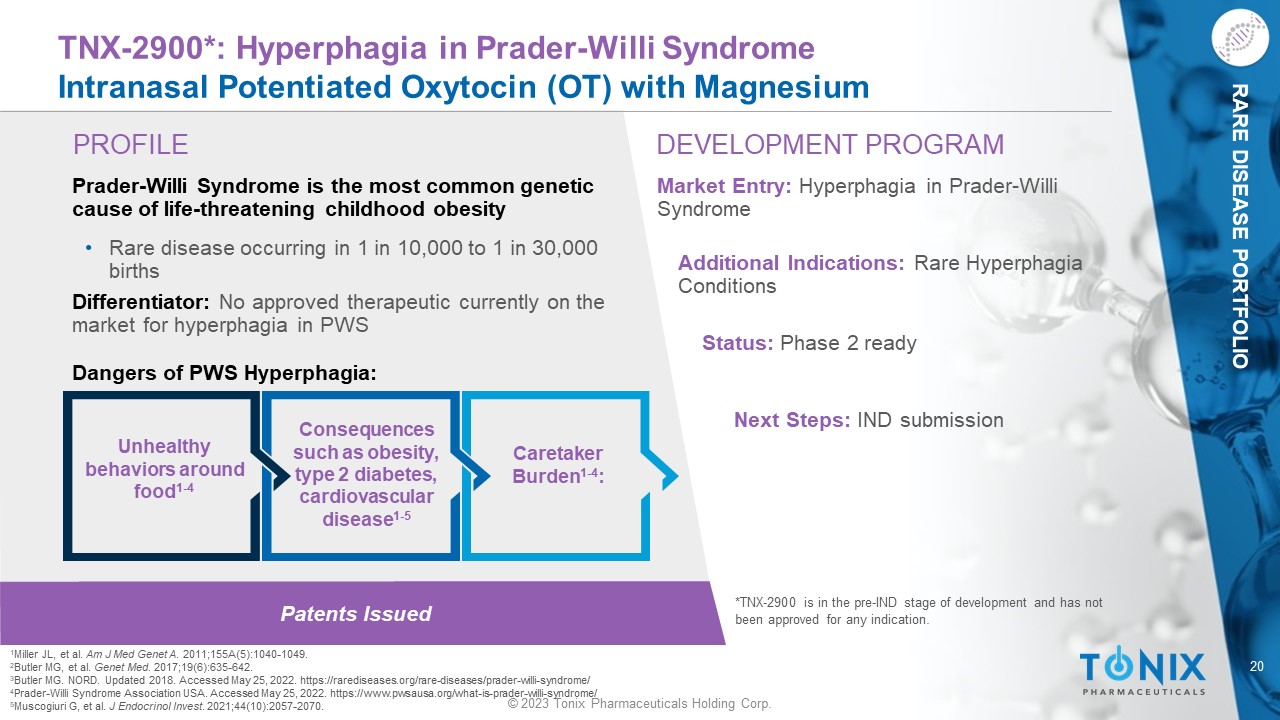
20 © 2023 Tonix Pharmaceuticals Holding Corp. RARE DISEASE PORTFOLIO Patents Issued PROFILE DEVELOPMENT PROGRAM TNX - 2900*: Hyperphagia in Prader - Willi Syndrome Intranasal Potentiated Oxytocin (OT) with Magnesium Prader - Willi Syndrome is the most common genetic cause of life - threatening childhood obesity • Rare disease occurring in 1 in 10,000 to 1 in 30,000 births Differentiator: No approved therapeutic currently on the market for hyperphagia in PWS Dangers of PWS Hyperphagia: Market Entry: Hyperphagia in Prader - Willi Syndrome Additional Indications: Rare Hyperphagia Conditions Status: Phase 2 ready Next Steps: IND submission *TNX - 2900 is in the pre - IND stage of development and has not been approved for any indication. Caretaker Burden 1 - 4 : Unhealthy behaviors around food 1 - 4 Consequences such as obesity, type 2 diabetes, cardiovascular disease 1 - 5 1 Miller JL, et al. Am J Med Genet A . 2011;155A(5):1040 - 1049. 2 Butler MG, et al. Genet Med. 2017;19(6):635 - 642. 3 Butler MG. NORD. Updated 2018. Accessed May 25, 2022. https://rarediseases.org/rare - diseases/prader - willi - syndrome/ 4 Prader - Willi Syndrome Association USA. Accessed May 25, 2022. https://www.pwsausa.org/what - is - prader - willi - syndrome/ 5 Muscogiuri G, et al. J Endocrinol Invest . 2021;44(10):2057 - 2070.

© 2023 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY: KEY CANDIDATES
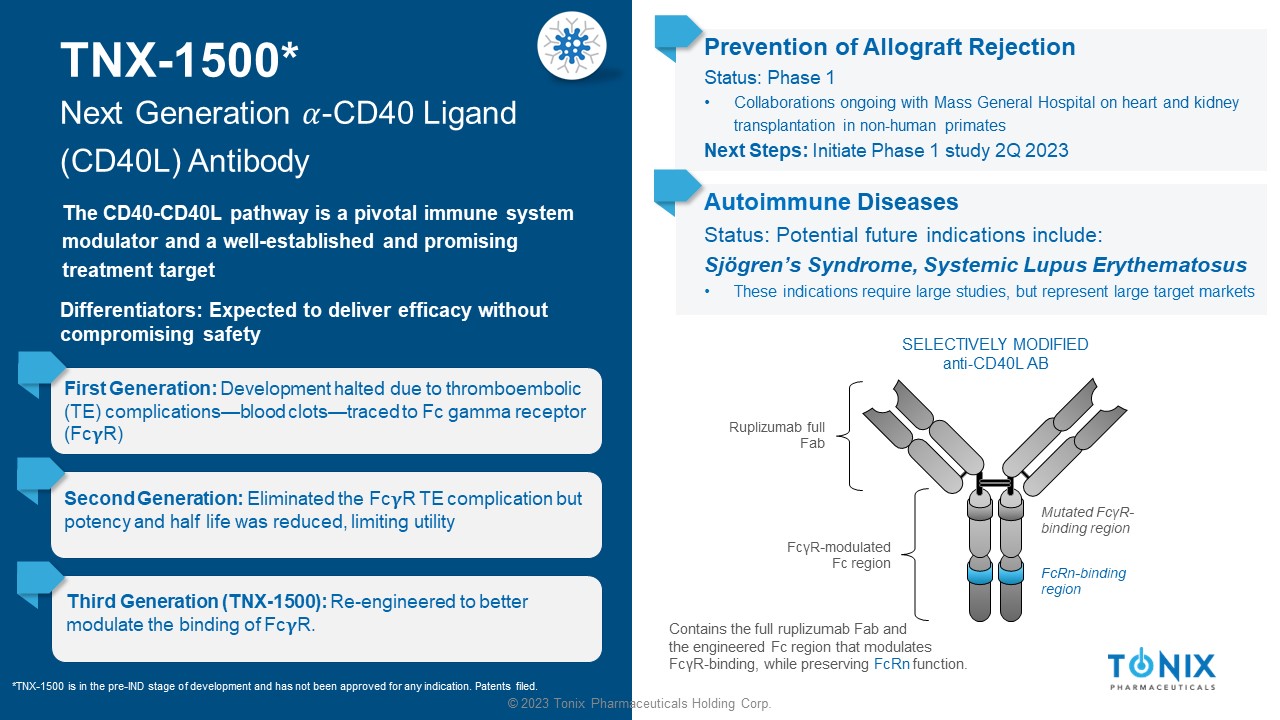
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 1500* Next Generation ߙ - CD40 Ligand (CD40L) Antibody The CD40 - CD40L pathway is a pivotal immune system modulator and a well - established and promising treatment target First Generation: Development halted due to thromboembolic (TE) complications — blood clots — traced to Fc gamma receptor (Fc R) Prevention of Allograft Rejection Status: Phase 1 • Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non - human primates Next Steps: Initiate Phase 1 study 2Q 2023 SELECTIVELY MODIFIED anti - CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc γ R - binding, while preserving FcRn function. Mutated Fc γ R - binding region FcRn - binding region Fc γ R - modulated Fc region Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX - 1500): Re - engineered to better modulate the binding of Fc R. *TNX - 1500 is in the pre - IND stage of development and has not been approved for any indication. Patents filed. Differentiators: Expected to deliver efficacy without compromising safety Autoimmune Diseases Status: Potential future indications include: Sjögren’s Syndrome, Systemic Lupus Erythematosus • These indications require large studies, but represent large target markets
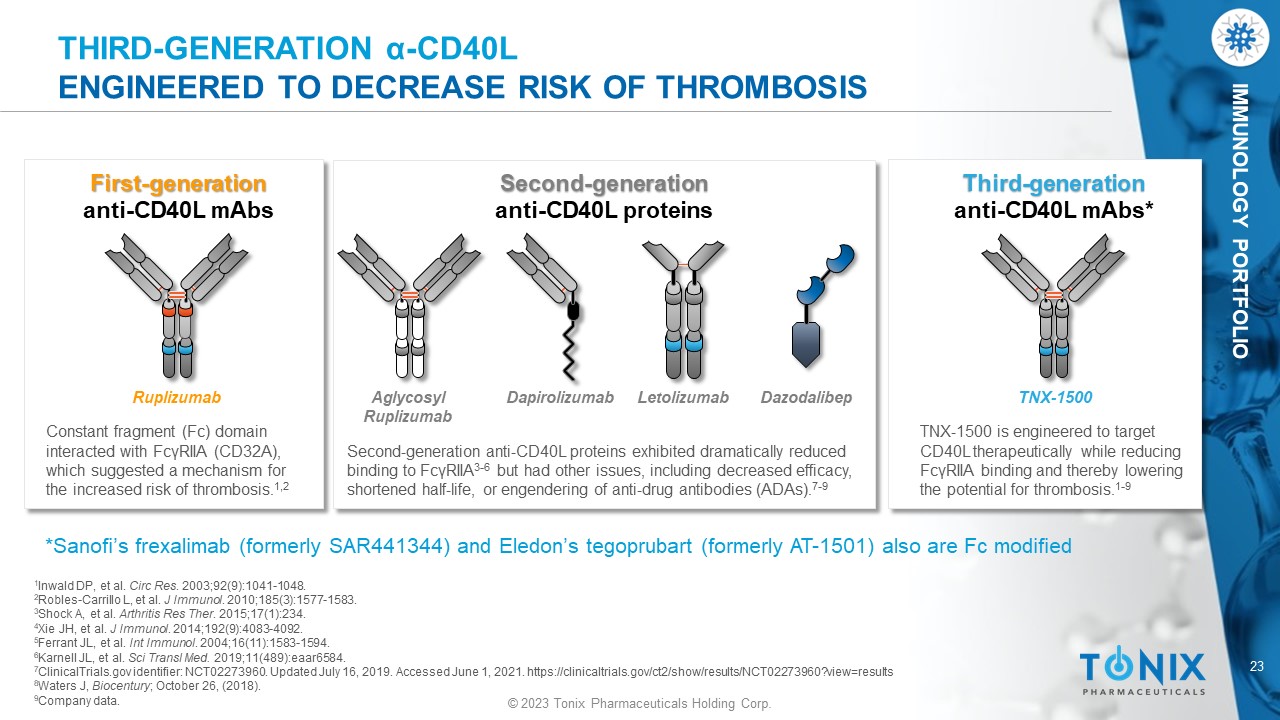
23 © 2023 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO THIRD - GENERATION α - CD40L ENGINEERED TO DECREASE RISK OF THROMBOSIS First - generation anti - CD40L mAbs Constant fragment (Fc) domain interacted with FcγRIIA (CD32A), which suggested a mechanism for the increased risk of thrombosis. 1,2 Ruplizumab Second - generation anti - CD40L proteins Second - generation anti - CD40L proteins exhibited dramatically reduced binding to FcγRIIA 3 - 6 but had other issues, including decreased efficacy, shortened half - life, or engendering of anti - drug antibodies (ADAs). 7 - 9 Dapirolizumab Letolizumab Aglycosyl Ruplizumab Third - generation anti - CD40L mAbs * TNX - 1500 is engineered to target CD40L therapeutically while reducing FcγRIIA binding and thereby lowering the potential for thrombosis. 1 - 9 TNX - 1500 *Sanofi’s frexalimab (formerly SAR441344) and Eledon’s tegoprubart (formerly AT - 1501) also are Fc modified 1 Inwald DP, et al. Circ Res . 2003;92(9):1041 - 1048. 2 Robles - Carrillo L, et al. J Immunol . 2010;185(3):1577 - 1583. 3 Shock A, et al. Arthritis Res Ther . 2015;17(1):234. 4 Xie JH, et al. J Immunol . 2014;192(9):4083 - 4092. 5 Ferrant JL, et al. Int Immunol . 2004;16(11):1583 - 1594. 6 Karnell JL, et al. Sci Transl Med. 2019;11(489):eaar6584. 7 ClinicalTrials.gov identifier: NCT02273960. Updated July 16, 2019. Accessed June 1, 2021. https://clinicaltrials.gov/ct2/show /re sults/NCT02273960?view=results 8 Waters J, Biocentury ; October 26, (2018). 9 Company data. Dazodalibep

24 © 2023 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Other anti - CD40L Monoclonal Antibodies in Development UCB (Co - developed with Biogen) – Systemic Lupus Erythematosus (SLE) • Phase 3 Trial Currently Enrolling (NCT04294667) − Topline results expected 1H 2024 1 • Dapirolizumab pegol (pegylated Fab) Horizon (Agreed to be acquired by Amgen) – Sjögren's Syndrome ( SjS ) • Two Positive Phase 2 studies reported 2,3 • Dazodalibep (tn03 fusion protein) Sanofi – Sjögren's Syndrome ( SjS ), Multiple Sclerosis (MS), Systemic Lupus Erythematosus (SLE) • Phase 2 Trial Currently Enrolling in SjS (NCT04572841) and SLE (NCT05039840) • Active Phase 2 Trial in Relapsing MS (NCT04879628) • SAR441344 (Fc - modified) Eledon – Amyotrophic Lateral Sclerosis (ALS) and Kidney Transplant • Phase 2 Trial Completed in ALS (NCT04322149) • Phase 1/2 Trial Currently Enrolling in Kidney Transplant (NCT05027906) • T egoprubart , f.k.a . AT - 1501 (Fc - modified) 1 https://www.ucb.com/our - science/pipeline 2 https://ir.horizontherapeutics.com/news - releases/news - release - details/horizon - therapeutics - plc - announces - phase - 2 - trial - evaluatin g 3 https://ir.horizontherapeutics.com/news - releases/news - release - details/horizon - therapeutics - plc - announces - phase - 2 - trial - evaluatin g - 0 Lundbeck and AprilBio – Neurology • Phase 1 Trial Currently Enrolling in Healthy Adults (NCT05136053) • APB - A1 or Lu AG22515 (HAS fusion protein)
25 © 2023 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY PORTFOLIO Patents Filed TNX - 1700*: Gastric and Colorectal Cancers Recombinant Trefoil Factor 2 (rTFF2 - HSA) Fusion Protein Potential New Cancer Treatment • TNX - 1700 (rTFF2) has effects on cancer by altering the tumor micro - environment • Mechanism of action: suppresses myeloid - derived suppressor cells and activates anti - cancer CD8+ T cells • Potential synergy with anti - PD - 1 or anti - PD - L1 monoclonal antibodies ( mAbs ) Preclinical Evidence for Inhibiting Growth of Cancer Cells • Data showed that mTFF2 - CTP augmented the efficacy of mAb anti - PD - 1 therapy. Anti - PD - 1 in combination with TFF2 - CTP showed greater anti - tumor activity in PD - L1 - overexpressing mice • mTNX - 1700 (mTFF2 - MSA fusion protein) and anti - PD - 1 monotherapy each was able to evoke anti - tumor immunity in the MC38 model of colorectal cancer 1 • mTNX - 1700 augmented the anti - tumor efficacy of anti - PD - 1 therapy in both the MC38 and the CT26.wt models 1 Market Entry: Immuno - oncology, combination therapy with PD1 blockers for gastric and colorectal cancer Status: Preclinical Next Steps: Animal studies ongoing *TNX - 1700 is in the pre - IND stage of development and has not been approved for any indication. Differentiator: No product yet identified consistently augments PD1 effects on cold tumors Licensed from Columbia University • Developing in partnership under sponsored research agreement 1 Daugherty, B. et al. March 6, 2023 Keystone Poster; www.tonixpharma.com/wp - content/uploads/2023/03/mTFF2 - MSA_mTNX - 1700_Suppresses - Tumor - Growth - and - Increases - Survival - in - an - Anti - PD - 1 - Treated - MC38 - Colorectal - Cancer - Model - by - Targeting - MDSCs.pdf

© 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: KEY CANDIDATES

© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 801 & TNX - 1850* Recombinant Pox Vaccine (RPV) Platform Using Live Virus Technology Mpox and Smallpox Vaccine Status: Preclinical • TNX - 801 is a cloned version of horsepox 1 (without any insert) purified from cell culture Next Steps: Developing GMP manufacturing; Initiate Phase 1 Trial 2H 2023 COVID - 19 Vaccine Status: Preclinical • First version TNX - 1800 encodes spike protein from SARS - CoV - 2, Wuhan strain • Planned new version TNX - 1850 encode spike protein from SARS - CoV - 2 BA.2 strain 2 Next Steps: Developing TNX - 1850 (BA.2) version *TNX - 801 and TNX - 1800/TNX - 1850 are in the pre - IND stage of development and has not been approved for any indication. Patents filed. • Live virus vaccines are the most established vaccine technology ‒ Starting with Edward Jenner’s smallpox vaccine, the first vaccine, which eradicated smallpox ‒ Prevents forward transmission ‒ Effective in eliciting durable or long - term immunity • Economical to manufacture at scale ‒ Low dose because replication amplifies dose in vivo ‒ Single shot administration • Standard refrigeration required for shipping and storage 1 Noyce RS, et al. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One. 2018 Jan 19;13(1):e0188453. 2 Brennan, Z. Endpoints March 2, 2022 (https://endpts.com/weaker - omicron - variant - is - great - news - for - the - world - but - bad - news - for - covid - related - clinical - trials/) Differentiators: TNX - 801* scHPXV (Horsepox) 212,811 bp TNX - 1800* rHPXV /SARS - CoV - 2 S 210,963 bp
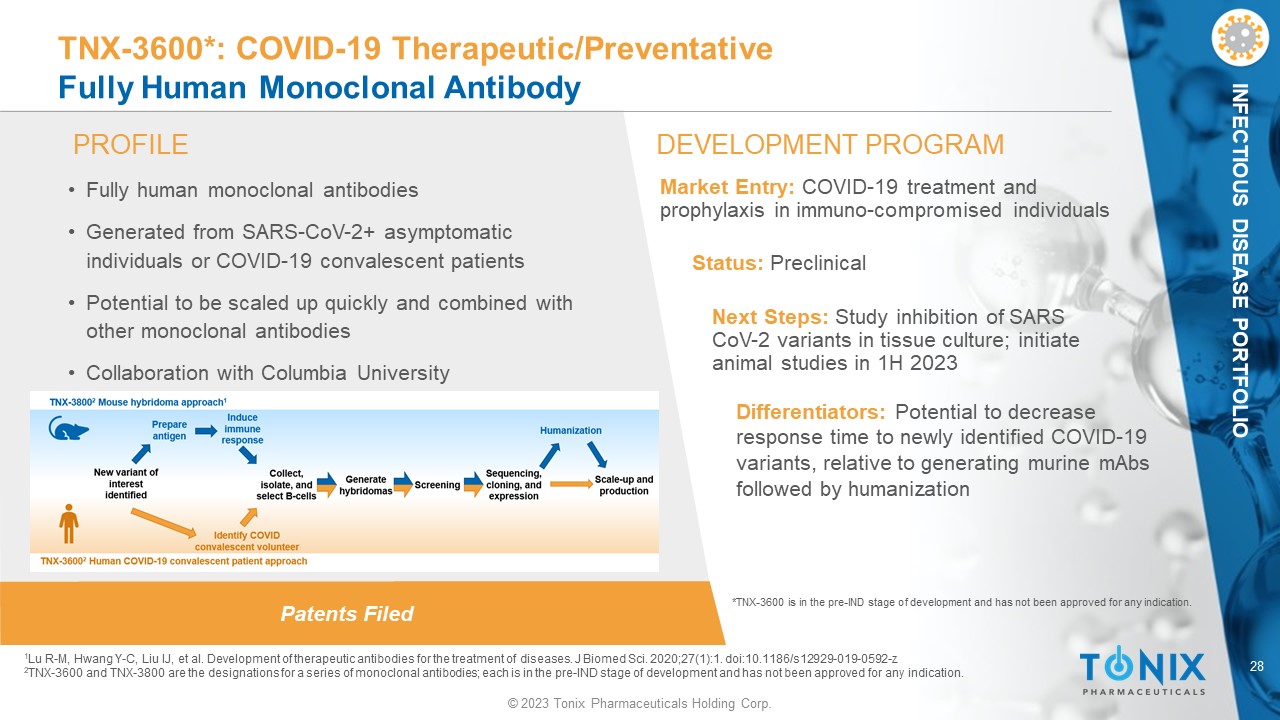
28 © 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Patents Filed PROFILE DEVELOPMENT PROGRAM TNX - 3600*: COVID - 19 Therapeutic/Preventative Fully Human Monoclonal Antibody • Fully human monoclonal antibodies • Generated from SARS - CoV - 2+ asymptomatic individuals or COVID - 19 convalescent patients • Potential to be scaled up quickly and combined with other monoclonal antibodies • Collaboration with Columbia University Differentiators: Potential to decrease response time to newly identified COVID - 19 variants, relative to generating murine mAbs followed by humanization Market Entry: COVID - 19 treatment and prophylaxis in immuno - compromised individuals Status: Preclinical Next Steps: Study inhibition of SARS CoV - 2 variants in tissue culture; initiate animal studies in 1H 2023 1 Lu R - M, Hwang Y - C, Liu IJ, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020;27(1): 1. doi:10.1186/s12929 - 019 - 0592 - z 2 TNX - 3600 and TNX - 3800 are the designations for a series of monoclonal antibodies; each is in the pre - IND stage of development an d has not been approved for any indication. *TNX - 3600 is in the pre - IND stage of development and has not been approved for any indication.

29 © 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Patents Filed PROFILE DEVELOPMENT PROGRAM TNX - 3800*: COVID - 19 Therapeutic/Preventative Humanized Mouse Monoclonal Antibodies Market Entry: COVID - 19 treatment and prophylaxis in immuno - compromised individuals Status: Preclinical Next Steps: Study inhibition of SARS CoV - 2 variants in tissue culture; initiate animal studies in 1H 2023 • Humanized monoclonal antibodies • Generated from mice immunized with SARS - CoV - 2 spike protein • Exclusive license from Curia Global, Inc. Differentiators: To date, EUA - approved products have been derived from the blood of COVID - convalescent patients or a humanized mouse 1,2 Relative to fully humanized mAbs : • Murine mAbs discovered by Curia and licensed by Tonix represent a potential new approach to treating SARS - CoV - 2 infection • Murine mAbs have the potential to neutralize a broader spectrum of SARS - CoV - 2 variants and may be more difficult to evade as we face expanding variant pool from both convergent and divergent evolution 3 1 Hansen J et al. Science. 2020 Aug 21;369(6506):1010 - 1014. Doi: 10.1126/science.abd0827 2 Asdaq, S.M.B. et al. A Patent Review on the Therapeutic Application of Monoclonal Antibodies in COVID - 19. Int. J. Mol. Sci. 2021 , 22, 11953. https://doi.org/10.3390/ijms222111953 3 Callaway, E. Oct 28 2022. Nature (News). COVID ‘variant soup’ is making winter surges hard to predict: Descendants of Omicron ar e proliferating worldwide — and the same mutations are coming up again and again. www.nature.com/articles/d41586 - 022 - 03445 - 6 *TNX - 3800 is in the pre - IND stage of development and has not been approved for any indication.
30 © 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Additional Infectious Disease Therapeutics in Development TNX - 2300*: Live Virus Vaccine Based on Bovine Parainfluenza (BPI) Virus Market Entry: COVID - 19 Vaccine Status: Preclinical Next Steps: Animal studies with Kansas State University (KSU) to test the effect of co - expression of CD40 - ligand to stimulate T cell immunity TNX - 3700*: Zinc Nanoparticle (ZNP) Formulation for mRNA Vaccines Market Entry: Booster for COVID - 19 Vaccines Status: Preclinical Next Steps: Research at KSU on CoV - 2 spike based vaccine in tissue culture and animals; initiate animal studies in 1H 2023 *TNX - 2300 and TNX - 3700 are in the pre - IND stage of development and have not been approved for any indication.
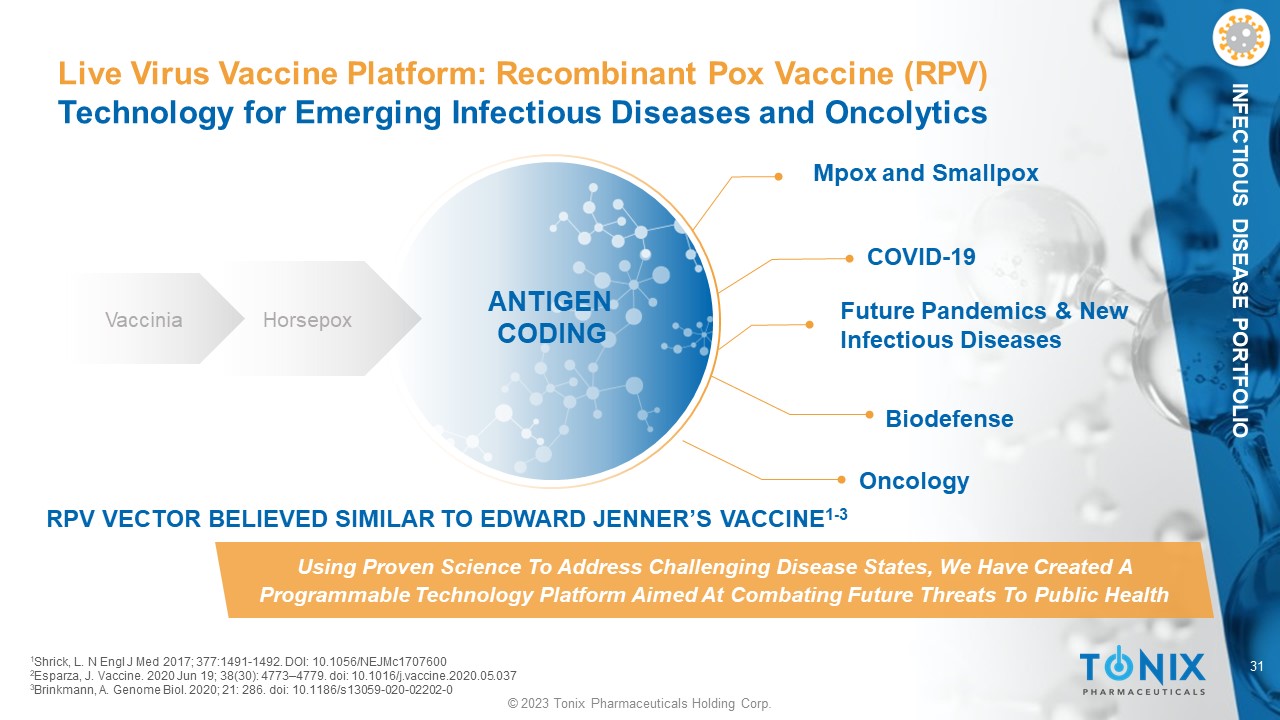
31 © 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Live Virus Vaccine Platform: Recombinant Pox Vaccine (RPV) Technology for Emerging Infectious Diseases and Oncolytics Mpox and Smallpox Future Pandemics & New Infectious Diseases COVID - 19 Biodefense Using Proven Science To Address Challenging Disease States, We Have Created A Programmable Technology Platform Aimed At Combating Future Threats To Public Health Vaccinia Horsepox ANTIGEN CODING Oncology RPV VECTOR BELIEVED SIMILAR TO EDWARD JENNER’S VACCINE 1 - 3 1 Shrick, L. N Engl J Med 2017; 377:1491 - 1492. DOI: 10.1056/NEJMc1707600 2 Esparza, J. Vaccine. 2020 Jun 19; 38(30): 4773 – 4779. doi : 10.1016/j.vaccine.2020.05.037 3 Brinkmann, A. Genome Biol. 2020; 21: 286. doi : 10.1186/s13059 - 020 - 02202 - 0

32 © 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE PORTFOLIO Internal Development & Manufacturing Capabilities R&D Center (RDC) – Frederick, MD • Functions: ‒ Accelerated development of vaccines and antiviral drugs against COVID - 19, its variants and other infectious diseases ‒ Research advancing CNS and immunology drugs • Description: ~48,000 square feet, BSL - 2 with some areas designated BSL - 3 • Status: Operational Advanced Development Center (ADC) – North Dartmouth, MA • Function: Development and clinical scale manufacturing of biologics • Description: ~45,000 square feet, BSL - 2 • Status: Operational Commercial Manufacturing Center (CMC) – Hamilton, MT • Function : Phase 3 and Commercial scale manufacturing of biologics • Description: ~44 - acre green field site, planned BSL - 2 • Status: Planning for site enabling work in 2023 Architectural Rendering

© 2023 Tonix Pharmaceuticals Holding Corp. FUTURE OUTLOOK
34 © 2023 Tonix Pharmaceuticals Holding Corp. Management Team Seth Lederman, MD Co - Founder, CEO & Chairman Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer

35 © 2023 Tonix Pharmaceuticals Holding Corp. Milestones: Recently Completed and Upcoming Expected Clinical Trial Initiations □ 1 st Quarter 2023 Phase 2 UPLIFT study start of TNX - 601 ER for major depressive disorder □ 2 nd Quarter 2023 Phase 1 study start of TNX - 1500 for prevention of allograft rejection □ 2 nd Quarter 2023 Phase 2 study start of TNX - 1300 for the treatment of cocaine intoxication □ 2 nd Half 2023 Phase 1 study start of TNX - 801 for prevention of mpox and smallpox □ 2 nd Quarter 2022 Phase 3 RESILIENT study start of TNX - 102 SL for the management of fibromyalgia □ 3rd Quarter 2022 Phase 2 PREVAIL study start of TNX - 102 SL for the treatment of Long COVID □ 1 st Quarter 2023 Phase 2 study start of TNX - 1900 for the treatment of migraine x x Expected Data □ 2 nd Quarter 2023 Interim Analysis results of Phase 3 RESILIENT study of TNX - 102 SL for fibromyalgia □ 4 th Quarter 2023 Interim Analysis results of Phase 2 PREVENTION study of TNX - 1900 for chronic migraine □ 4 th Quarter 2023 Interim Analysis results of Phase 2 UPLIFT study of TNX - 601 ER for major depressive disorder □ 4 th Quarter 2023 topline results of Phase 3 RESILIENT study of TNX - 102 SL for fibromyalgia x

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU