
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99-02

© 2023 Tonix Pharmaceuticals Holding Corp. The 5th International Congress on Controversies in Fibromyalgia NASDAQ: TNXP Version P0427 March 30 , 2023 (Doc 1185 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
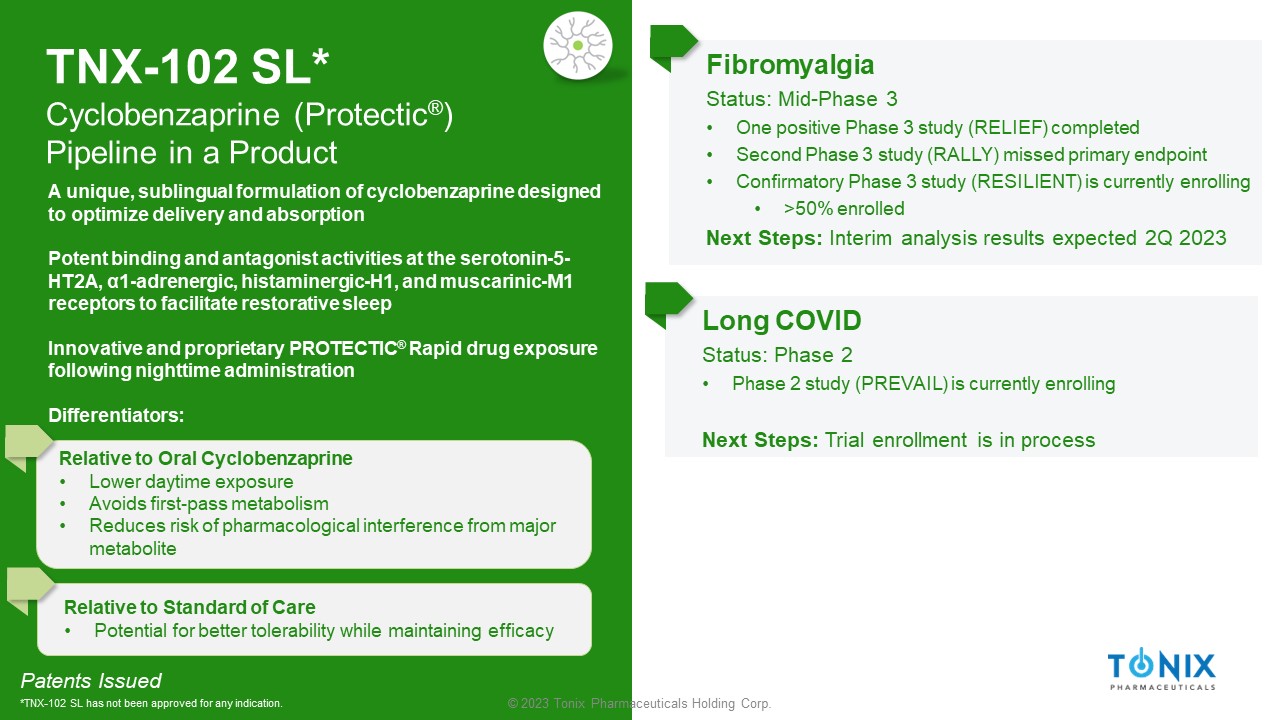
© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL* Cyclobenzaprine ( Protectic ® ) Pipeline in a Product Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) is currently enrolling • >50% enrolled Next Steps: Interim analysis results expected 2Q 2023 Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) is currently enrolling Next Steps: Trial enrollment is in process Patents Issued *TNX - 102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption Potent binding and antagonist activities at the serotonin - 5 - HT2A, α1 - adrenergic, histaminergic - H1, and muscarinic - M1 receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy

4 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: F304 (RELIEF) Phase 3 Study • General Study Design: Phase 3, Randomized, Multicenter (39), Parallel Group, Double - Blind, Placebo - Controlled 14 Week Study • Objectives: To evaluate efficacy and safety of bedtime TNX - 102 SL in fibromyalgia (FM) • Investigational Product (IP): TNX - 102 SL (sublingual cyclobenzaprine) is a tricyclic drug that potently binds and antagonizes: hydroxytryptamine - 2A, α1 - adrenergic, H1 - histaminergic, and M1 - muscarinic acetylcholine receptors • Study Visits: Screening, Baseline, and four treatment (Weeks 2, 6, 10 & 14/ET) visits • IP Dosage: first 2 weeks on 1 tablet (TNX - 102 SL 2.8 mg); at Week 2 visit the dose is increased to 2 tablets providing 5.6 mg of TNX - 102 SL at bedtime for 12 weeks • Patient Population: diagnosis of primary FM as defined by 2016 Revision to the 2010/2011 FM diagnostic criteria (ACR Preliminary Diagnostic Criteria) • Exclusionary Medications: duloxetine, milnacipran, pregabalin, gabapentin, tramadol, tapentadol, muscle relaxants, tricyclic antidepressants, MAOIs, trazodone, narcotics/opioids, naltrexone, benzodiazepines, anticonvulsants (exception for migraine), sodium oxybate, ketamine, CGRP/CGRP - R meds, and all other cyclobenzaprine
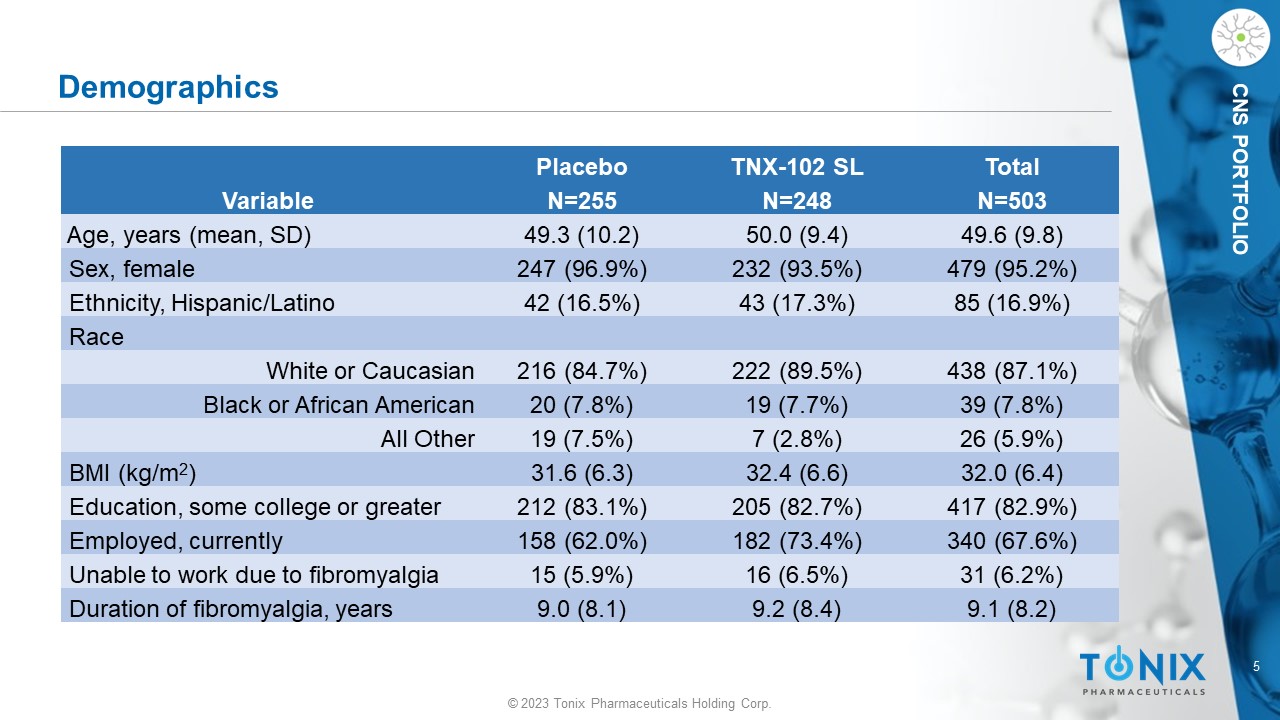
5 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Demographics Total TNX - 102 SL Placebo N=503 N=248 N=255 Variable 49.6 (9.8) 50.0 (9.4) 49.3 (10.2) Age, years (mean, SD) 479 (95.2%) 232 (93.5%) 247 (96.9%) Sex, female 85 (16.9%) 43 (17.3%) 42 (16.5%) Ethnicity, Hispanic/Latino Race 438 (87.1%) 222 (89.5%) 216 (84.7%) White or Caucasian 39 (7.8%) 19 (7.7%) 20 (7.8%) Black or African American 26 (5.9%) 7 (2.8%) 19 (7.5%) All Other 32.0 (6.4) 32.4 (6.6) 31.6 (6.3) BMI (kg/m 2 ) 417 (82.9%) 205 (82.7%) 212 (83.1%) Education, some college or greater 340 (67.6%) 182 (73.4%) 158 (62.0%) Employed, currently 31 (6.2%) 16 (6.5%) 15 (5.9%) Unable to work due to fibromyalgia 9.1 (8.2) 9.2 (8.4) 9.0 (8.1) Duration of fibromyalgia, years
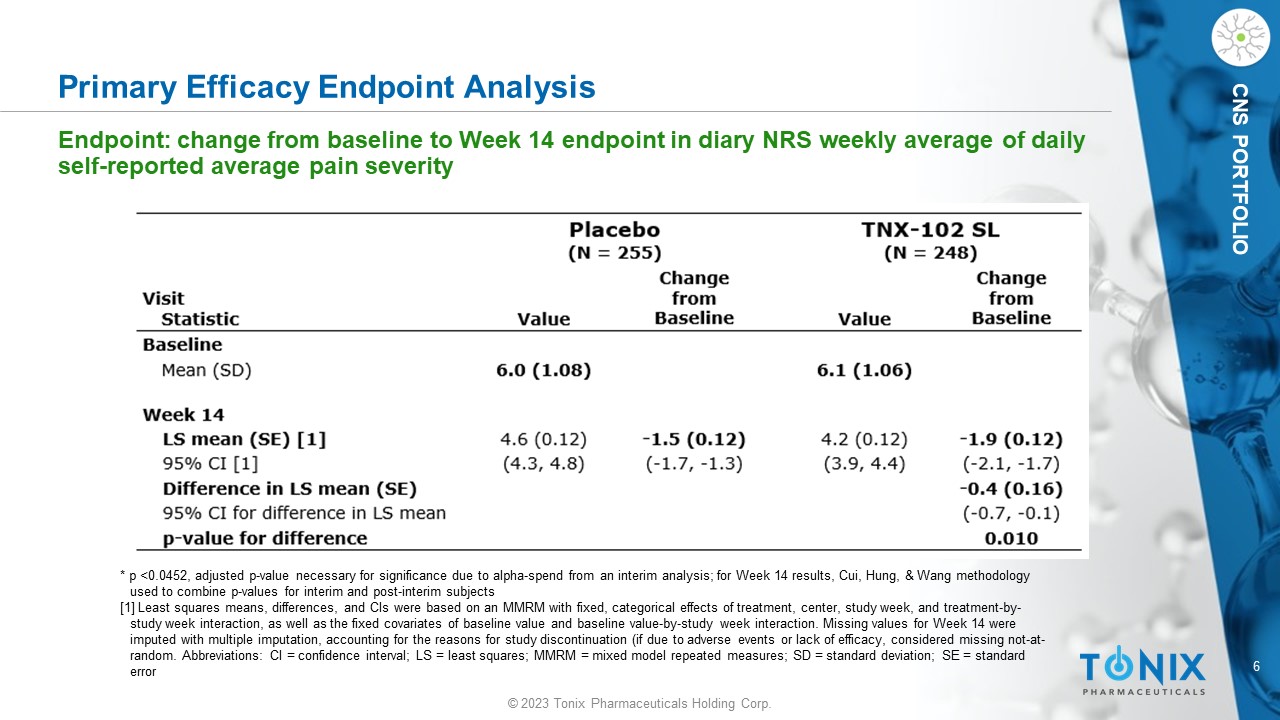
6 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Primary Efficacy Endpoint Analysis Endpoint: change from baseline to Week 14 endpoint in diary NRS weekly average of daily self - reported average pain severity * p <0.0452, adjusted p - value necessary for significance due to alpha - spend from an interim analysis; for Week 14 results, Cui, Hun g, & Wang methodology used to combine p - values for interim and post - interim subjects [1] Least squares means, differences, and CIs were based on an MMRM with fixed, categorical effects of treatment, center, stu dy week, and treatment - by - study week interaction, as well as the fixed covariates of baseline value and baseline value - by - study week interaction. Missing values for Week 14 were imputed with multiple imputation, accounting for the reasons for study discontinuation (if due to adverse events or lack of efficacy, considered missing not - at - random. Abbreviations: CI = confidence interval; LS = least squares; MMRM = mixed model repeated measures; SD = standard devi ati on; SE = standard error
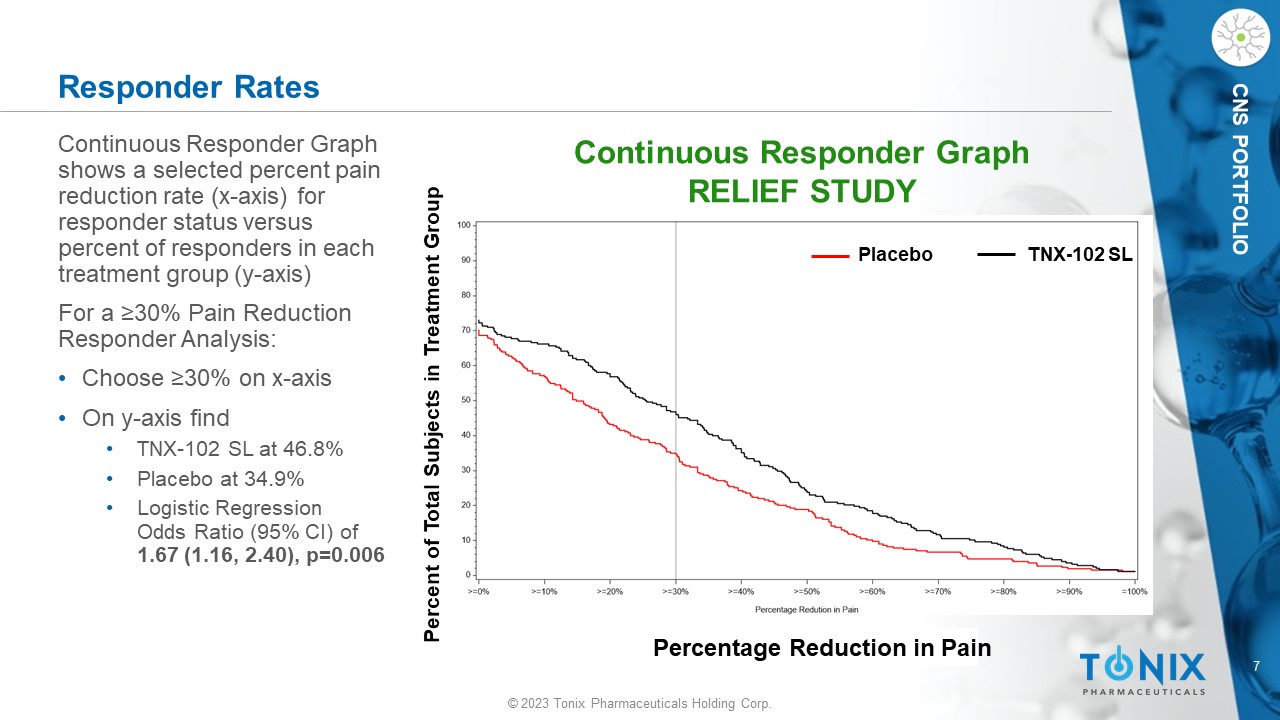
7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Responder Rates Continuous Responder Graph shows a selected percent pain reduction rate (x - axis) for responder status versus percent of responders in each treatment group (y - axis) For a ≥30% Pain Reduction Responder Analysis: • Choose ≥30% on x - axis • On y - axis find • TNX - 102 SL at 46.8% • Placebo at 34.9% • Logistic Regression Odds Ratio (95% CI) of 1.67 (1.16, 2.40), p=0.006 Continuous Responder Graph RELIEF STUDY Placebo TNX - 102 SL Percent of Total Subjects in Treatment Group Percentage Reduction in Pain
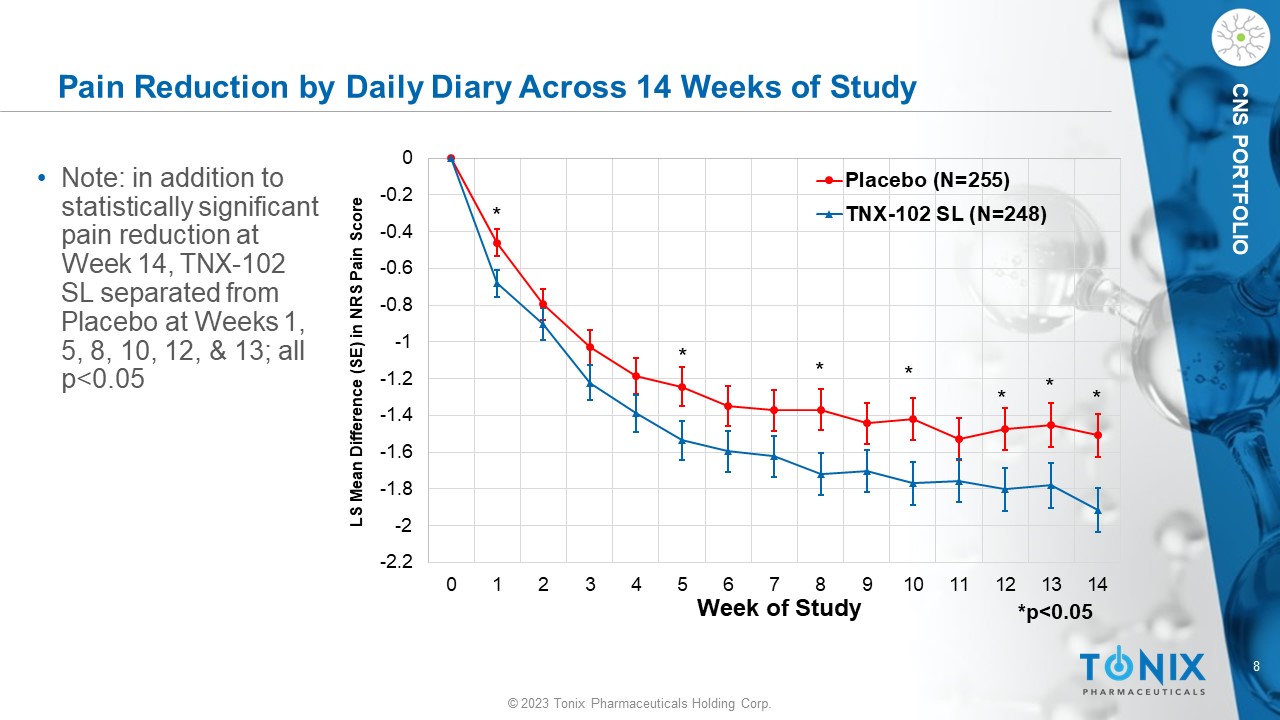
8 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Pain Reduction by Daily Diary Across 14 Weeks of Study • Note: in addition to statistically significant pain reduction at Week 14, TNX - 102 SL separated from Placebo at Weeks 1, 5, 8, 10, 12, & 13; all p<0.05 -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean Difference (SE) in NRS Pain Score Week of Study Placebo (N=255) TNX-102 SL (N=248) * * * * * * * *p<0.05
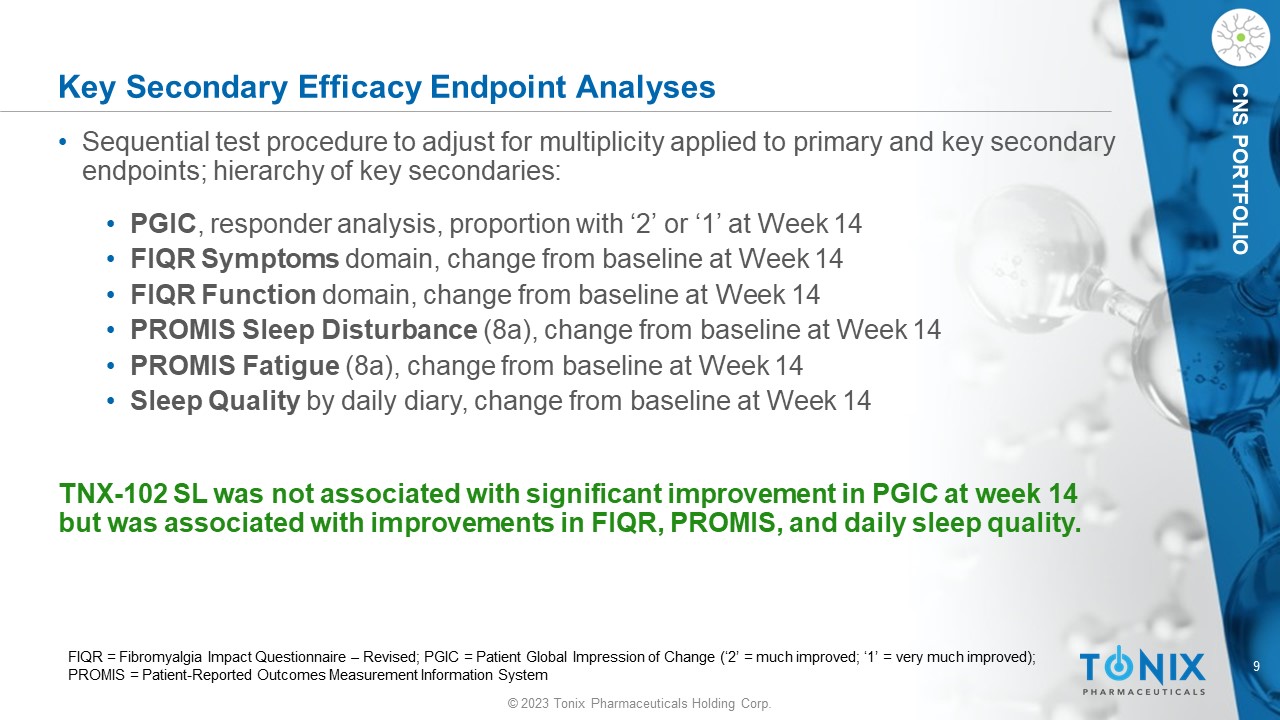
9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Key Secondary Efficacy Endpoint Analyses • Sequential test procedure to adjust for multiplicity applied to primary and key secondary endpoints; hierarchy of key secondaries: • PGIC , responder analysis, proportion with ‘2’ or ‘1’ at Week 14 • FIQR Symptoms domain, change from baseline at Week 14 • FIQR Function domain, change from baseline at Week 14 • PROMIS Sleep Disturbance (8a), change from baseline at Week 14 • PROMIS Fatigue (8a), change from baseline at Week 14 • Sleep Quality by daily diary, change from baseline at Week 14 TNX - 102 SL was not associated with significant improvement in PGIC at week 14 but was associated with improvements in FIQR, PROMIS, and daily sleep quality. FIQR = Fibromyalgia Impact Questionnaire – Revised; PGIC = Patient Global Impression of Change (‘2’ = much improved; ‘1’ = very much improved); PROMIS = Patient - Reported Outcomes Measurement Information System
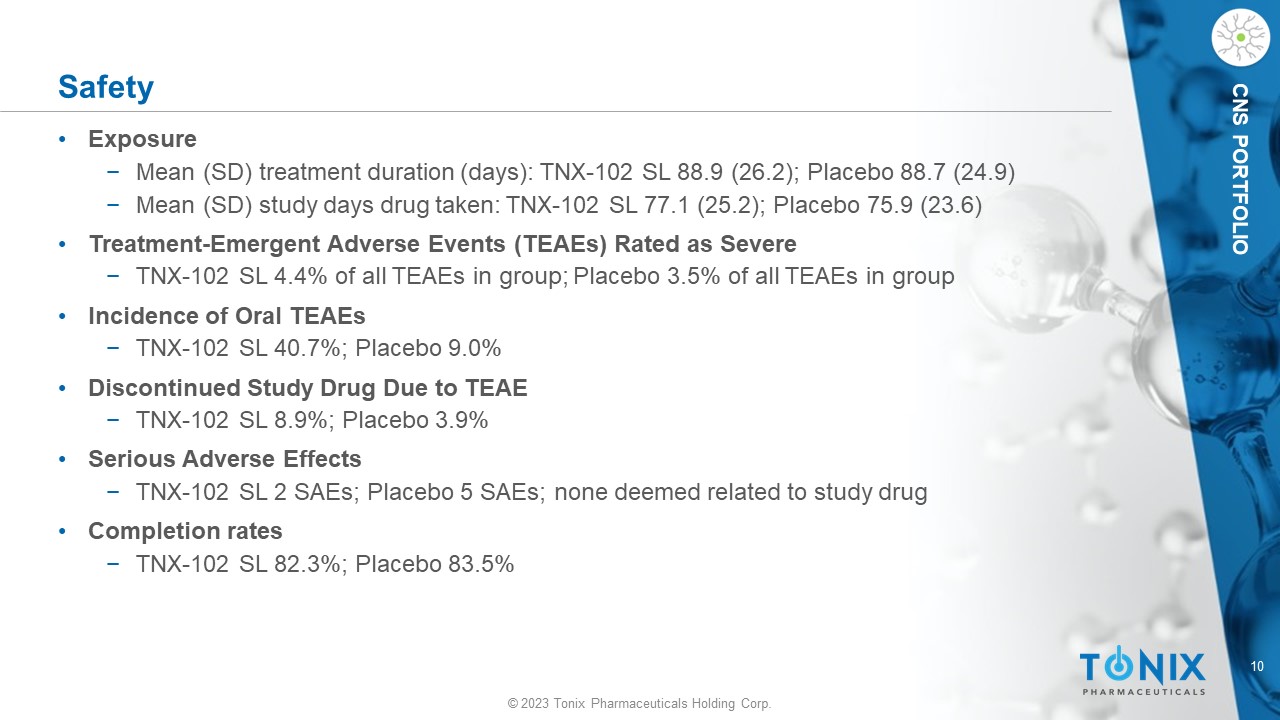
10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Safety • Exposure − Mean (SD) treatment duration (days): TNX - 102 SL 88.9 (26.2); Placebo 88.7 (24.9) − Mean (SD) study days drug taken: TNX - 102 SL 77.1 (25.2); Placebo 75.9 (23.6) • Treatment - Emergent Adverse Events (TEAEs) Rated as Severe − TNX - 102 SL 4.4% of all TEAEs in group; Placebo 3.5% of all TEAEs in group • Incidence of Oral TEAEs − TNX - 102 SL 40.7%; Placebo 9.0% • Discontinued Study Drug Due to TEAE − TNX - 102 SL 8.9%; Placebo 3.9% • Serious Adverse Effects − TNX - 102 SL 2 SAEs; Placebo 5 SAEs; none deemed related to study drug • Completion rates − TNX - 102 SL 82.3%; Placebo 83.5%
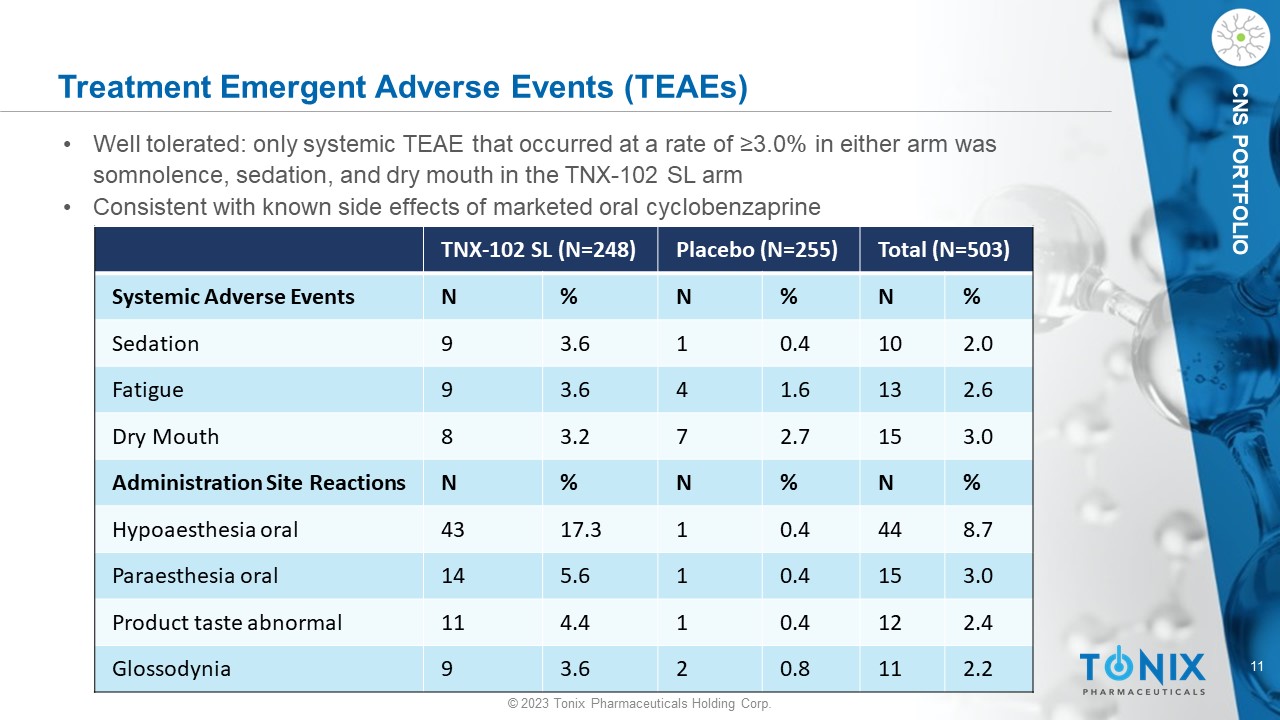
11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Treatment Emergent Adverse Events (TEAEs) • Well tolerated: only systemic TEAE that occurred at a rate of ≥3.0% in either arm was somnolence, sedation, and dry mouth in the TNX - 102 SL arm • Consistent with known side effects of marketed oral cyclobenzaprine Total (N=503) Placebo (N=255) TNX - 102 SL (N=248) % N % N % N Systemic Adverse Events 2.0 10 0.4 1 3.6 9 Sedation 2.6 13 1.6 4 3.6 9 Fatigue 3.0 15 2.7 7 3.2 8 Dry Mouth % N % N % N Administration Site Reactions 8.7 44 0.4 1 17.3 43 Hypoaesthesia oral 3.0 15 0.4 1 5.6 14 Paraesthesia oral 2.4 12 0.4 1 4.4 11 Product taste abnormal 2.2 11 0.8 2 3.6 9 Glossodynia

12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Conclusions • TNX - 102 SL reduced pain in fibromyalgia significantly more than placebo (p=0.010) over 14 weeks of treatment • 30% pain responder analysis demonstrated greater responders with TNX - 102 SL at 46.8% than with placebo at 34.9% (p=0.006) • TNX - 102 SL had broad syndromal effects across core fibromyalgia symptoms of widespread pain, fatigue, sleep disturbance, memory disturbance, mood disturbance, and sensory sensitivity • Most common adverse event from active treatment is oral hypoaesthesia, a sensory administration site reaction that is typically transient, never rated as severe , and lead to only 1 discontinuation • TNX - 102 SL was very well tolerated , with the two highest rates of systemic adverse events, sedation and fatigue, both at 3.6% • Only 17.7% of TNX - 102 SL group discontinued early (16.5% on Placebo)

13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Since the F304 “RELIEF” Study • Fibromyalgia ‒ F306 “RALLY” ▪ Second phase 3 study similar to RELIEF ▪ Enrollment was stopped at the interim ▪ Excess drop - outs in both drug - and placebo - arms ⁃ Delta wave of the COVID - 19 landscape may have contributed to terminations ‒ F307 “RESILIENT” ▪ Potentially confirmatory pivotal phase 3 study enrolling ▪ Design is similar to RELIEF and RALLY ▪ Expecting interim results in Q2 2023 • Fibromyalgia – like Long COVID ‒ PA201 “PREVAIL” ‒ Approximately two - thirds of Long COVID patients have multi - site pain
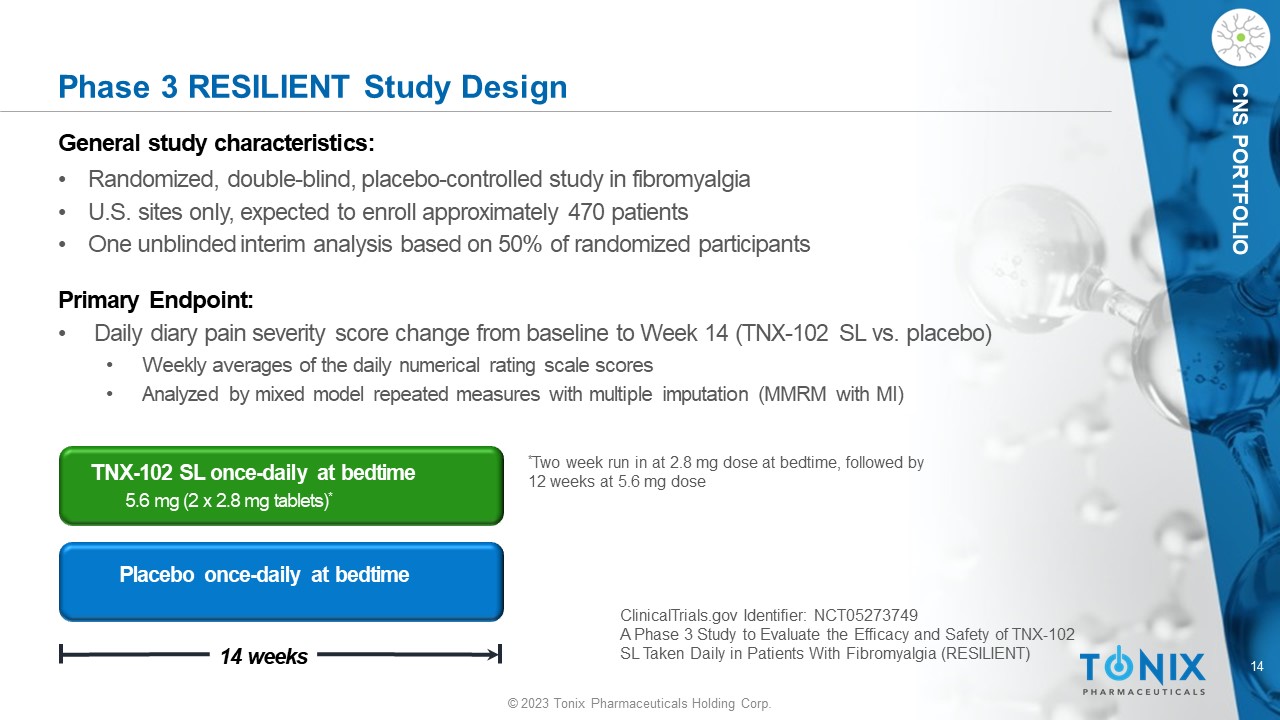
14 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Phase 3 RESILIENT Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in fibromyalgia • U.S. sites only, expected to enroll approximately 470 patients • One unblinded interim analysis based on 50% of randomized participants Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores • Analyzed by mixed model repeated measures with multiple imputation (MMRM with MI) Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 A Phase 3 Study to Evaluate the Efficacy and Safety of TNX - 102 SL Taken Daily in Patients With Fibromyalgia (RESILIENT)

15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Phase 2 PREVAIL Fibromyalgia - Type Long COVID Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores • Analyzed by mixed model repeated measures with multiple imputation (MMRM with MI) Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety of TNX - 102 SL in Patients With Multi - Site Pain Associated With Post - Acute Sequelae of SARS - CoV - 2 Infection (PREVAIL)”

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU