
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

The Development of Horsepox Virus as a Vaccine Platform: Evaluation of TNX - 1800 as a SARS - CoV - 2 Vaccine Farooq Nasar, Ph.D., M.P.H. Senior Principal Scientist World Vaccine Congress April 6, 2023 © 2023 Tonix Pharmaceuticals Holding Corp.

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
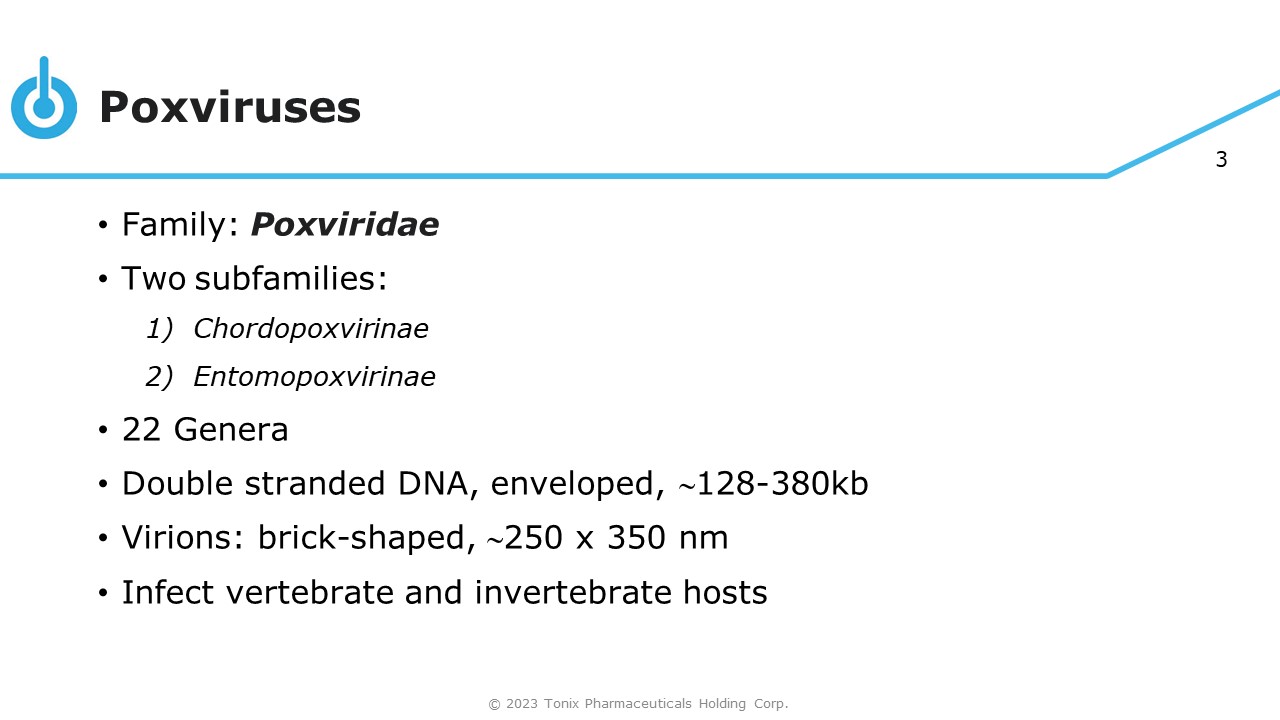
3 © 2023 Tonix Pharmaceuticals Holding Corp. Poxviruses • Family: Poxviridae • Two subfamilies: 1) Chordopoxvirinae 2) Entomopoxvirinae • 22 Genera • Double stranded DNA, enveloped, 128 - 380kb • Virions: brick - shaped, 250 x 350 nm • Infect vertebrate and invertebrate hosts
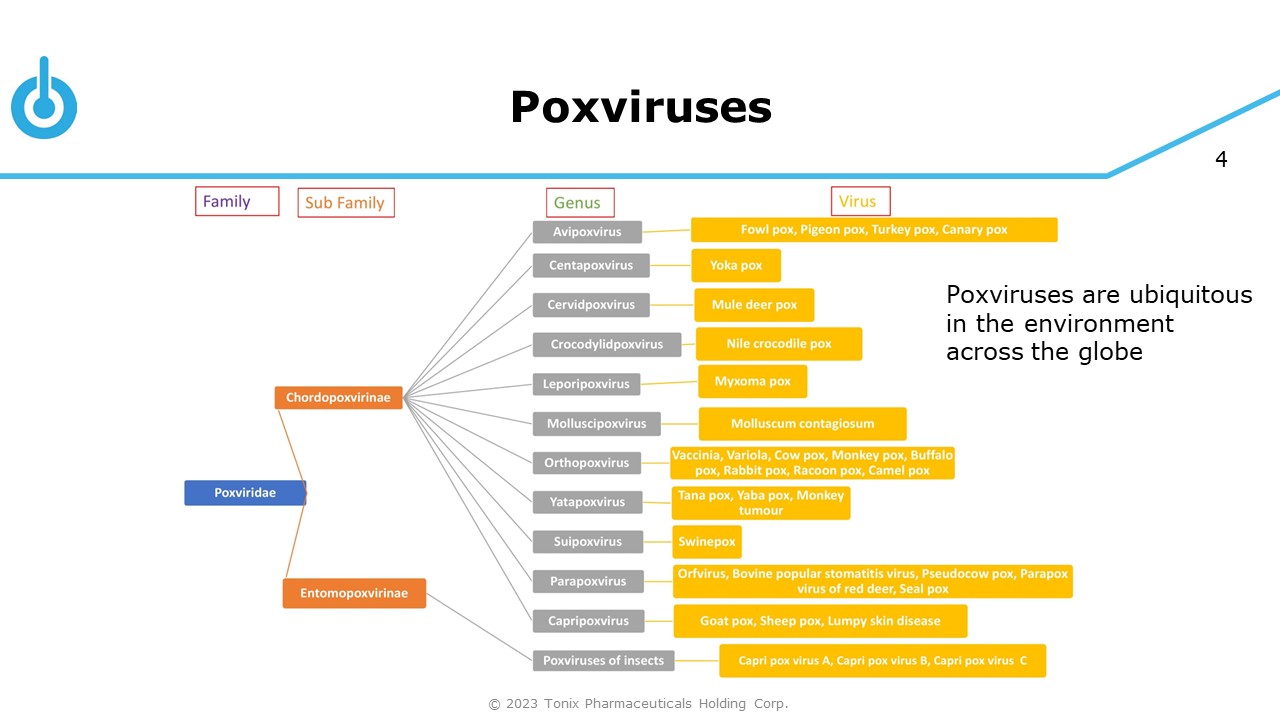
4 Poxviruses Poxviruses are ubiquitous in the environment across the globe © 2023 Tonix Pharmaceuticals Holding Corp.
5 Orthopox Viruses Smallpox Vaccine: CPXV, HPXV, VACV © 2023 Tonix Pharmaceuticals Holding Corp.

6 In 1796, Edward Jenner Successfully Used Vaccination to Protect Against Smallpox • Jenner observed milkmaids were protected from smallpox, reasoned that infection with an illness similar to smallpox but less deadly could protect one against smallpox “Cowpox” was the name of a disease in cows that could transfer to humans and cause sores Jenner “vaccinated” (from vacca , Latin for “cow”) a patient with pustule matter from “cowpox” sores on a milkmaid’s hands; that patient remained healthy when challenged with smallpox virus • Jenner suspected that the agent causing cowpox, which he called vaccinia , actually originated in horses and had been transferred from horses to cows’ udders by dirty hands The College of Physicians of Philadelphia. Accessed July 15, 2021. https:/ /w w w.historyofvaccines.org © 2023 Tonix Pharmaceuticals Holding Corp.
7 Phylogenetic Tree of Genus Orthopox Cowpox close orthopox relative to smallpox (Variola) Conferred protection against Variola © 2023 Tonix Pharmaceuticals Holding Corp.
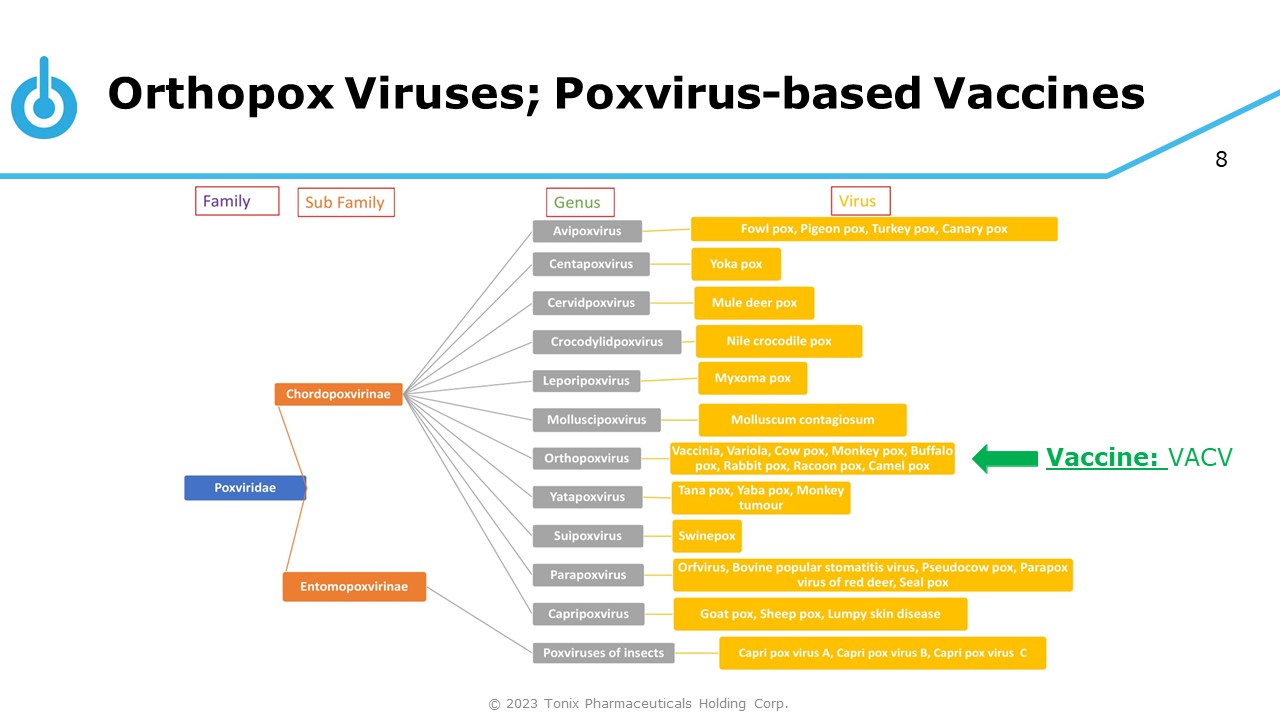
8 Orthopox Viruses; Poxvirus - based Vaccines Vaccine: VACV © 2023 Tonix Pharmaceuticals Holding Corp.
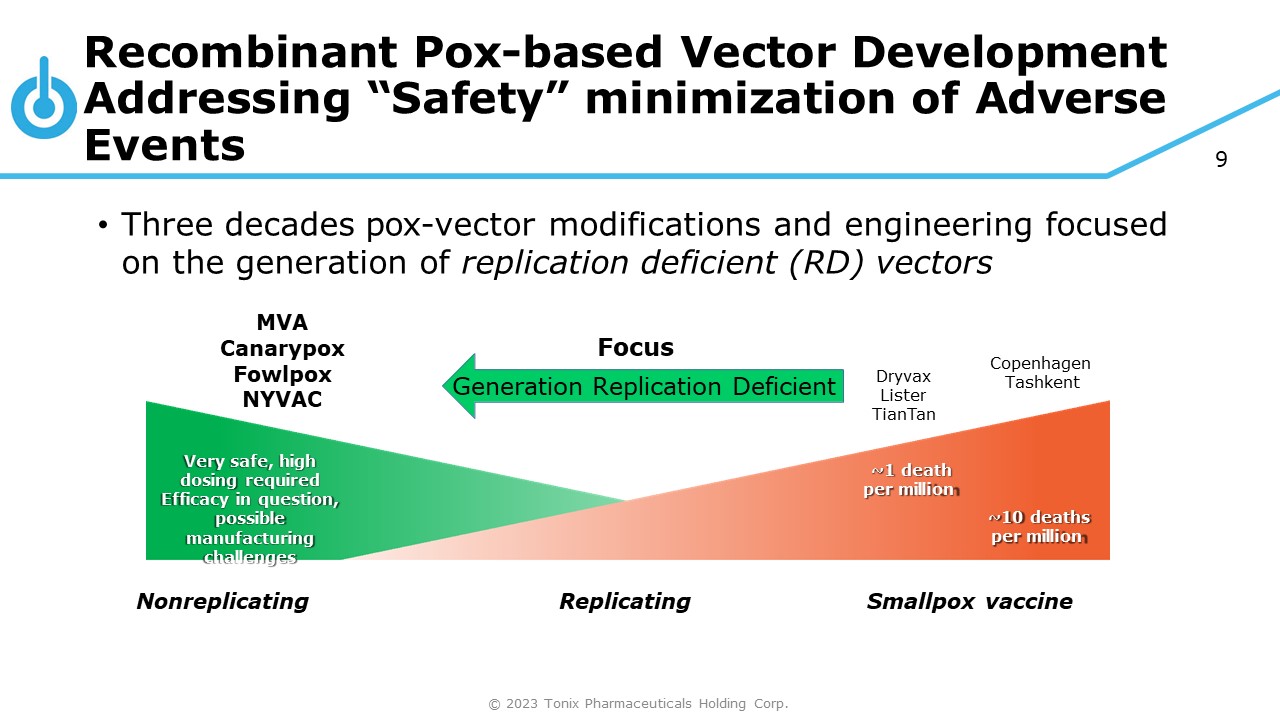
9 Recombinant Pox - based Vector Development Addressing “Safety” minimization of Adverse Events Very safe, high dosing required Efficacy in question, possible manufacturing challenges ~1 death per million ~10 deaths per million Nonreplicating © 2023 Tonix Pharmaceuticals Holding Corp. Replicating Smallpox vaccine Dryvax Lister TianTan Copenhagen Tashkent • Three decades pox - vector modifications and engineering focused on the generation of replication deficient (RD) vectors MVA Canarypox Fowlpox NYVAC Focus Generation Replication Deficient
10 Recombinant Pox - based Vector Development Addressing “Safety” minimization of Adverse Events Very safe, high dosing required ~1 death per million ~10 deaths per million Non - replicating Replicating Smallpox vaccine Dryvax Lister TianTan Copenhagen Tashkent Canarypox Fowlpox NYVAC Viral / Host Immune Modulation Some limited Replicative Capacity Optimal Replication © 2023 Tonix Pharmaceuticals Holding Corp. Considering the overall body of data from RD pox - based vectors Have we gone to far in vector engineering requiring RD ? • Safety data is great but immunological responses are typically weak or suboptimal immune responses • Some Replicative Capacity is essential, Horsepox TNX - 801* Focus MVA Horsepox TNX - 801 *TNX - 801 has not been approved for any indication.
11 Equination, Use of Vaccines From Horses, Was Also Effective Against Smallpox • Equination, the use of vaccines from horses ( equus in Latin), was successfully used in parallel with vaccination in Europe 1 • Vaccine producers may have propagated stocks by periodically supplementing or refreshing them with horsepox 2 A 1902 smallpox vaccine ( Mulford ) was found to be 99.7% identical to HPVX in core viral sequence, implicating a HPXV - like virus as a progenitor to modern vaccinia 3 Sequence Identity for the 1902 Mulford Vaccine Compared to HPVX 3 Similarity (%) 100 20 40 60 Core sequence 99.7% identical 80 100 120 Genome position (kb) 140 160 180 200 0 0 Distinct deletions seen in vaccinia virus strains 1. Esparza J, et al. Vaccine . 2017;35(52):7222 - 7230. 2. Esparza J, et al. Vaccine. 2020;38(30):4773 - 4779. 3. Schrick L, et al. N Engl J Med. 2017;377(15):1491 - 1492. © 2023 Tonix Pharmaceuticals Holding Corp.
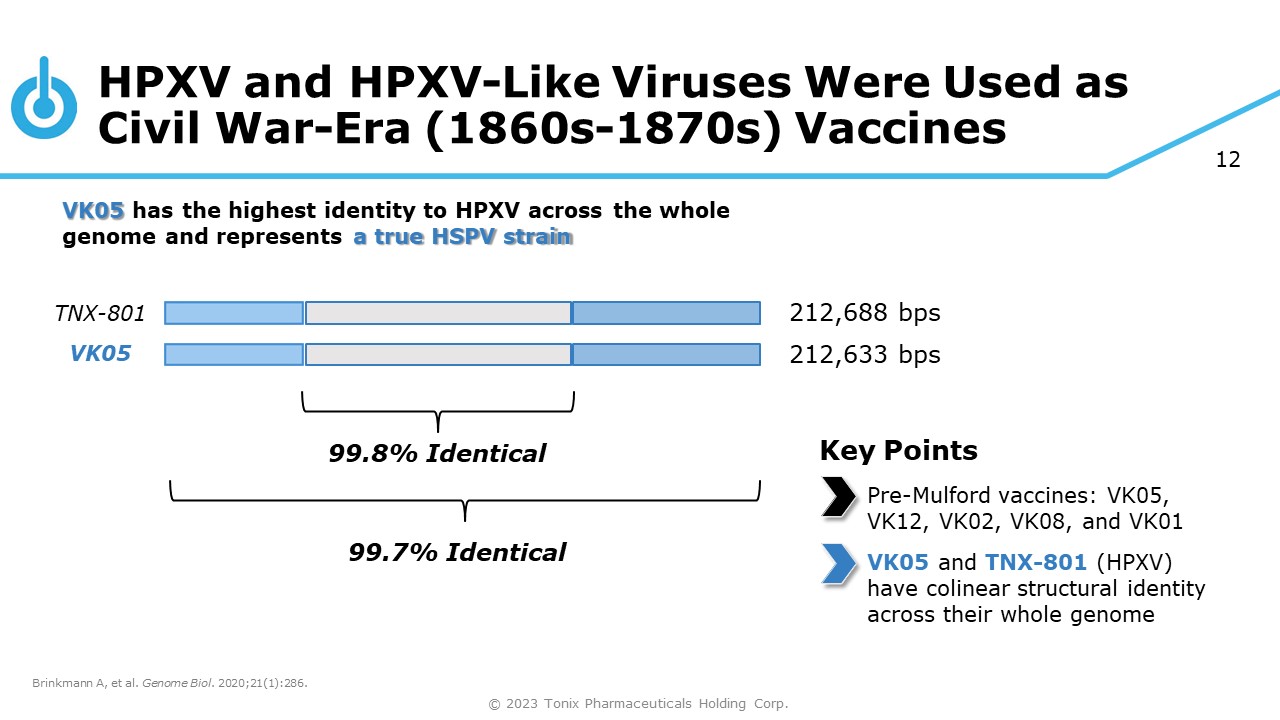
12 HPXV and HPXV - Like Viruses Were Used as Civil War - Era (1860s - 1870s) Vaccines TNX - 801 VK05 VK05 has the highest identity to HPXV across the whole genome and represents a true HSPV strain 99.8% Identical Brinkmann A, et al. Genome Biol . 2020;21(1):286. © 2023 Tonix Pharmaceuticals Holding Corp. 99.7% Identical 212,688 bps 212,633 bps Key Points Pre - Mulford vaccines: VK05, VK12, VK02, VK08, and VK01 VK05 and TNX - 801 (HPXV) have colinear structural identity across their whole genome
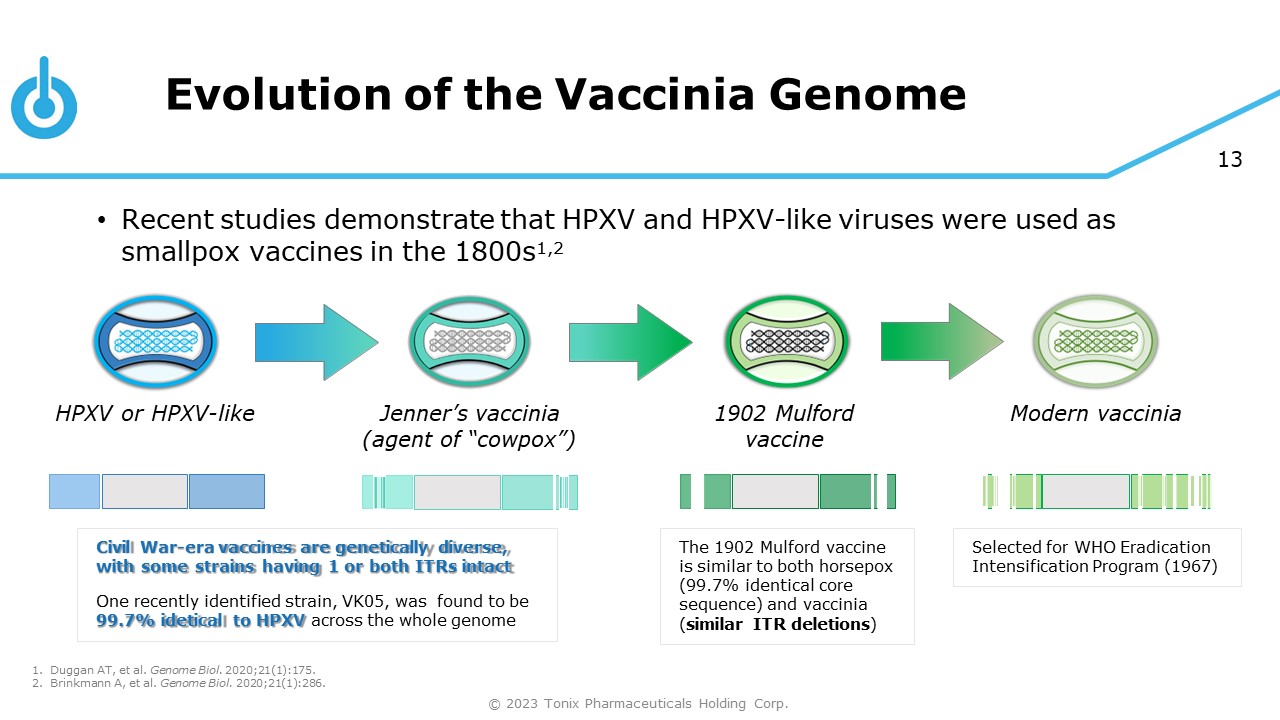
Evolution of the Vaccinia Genome 13 • Recent studies demonstrate that HPXV and HPXV - like viruses were used as smallpox vaccines in the 1800s 1,2 HPXV or HPXV - like Jenner’s vaccinia (agent of “cowpox”) 1902 Mulford vaccine Modern vaccinia The 1902 Mulford vaccine is similar to both horsepox (99.7% identical core sequence) and vaccinia ( similar ITR deletions ) Selected for WHO Eradication Intensification Program (1967) Civil War - era vaccines are genetically diverse, with some strains having 1 or both ITRs intact One recently identified strain, VK05, was found to be 99.7% idetical to HPXV across the whole genome 2. Brinkmann A, et al. Genome Biol. 2020;21(1):286. © 2023 Tonix Pharmaceuticals Holding Corp. 1. Duggan AT, et al. Genome Biol. 2020;21(1):175.
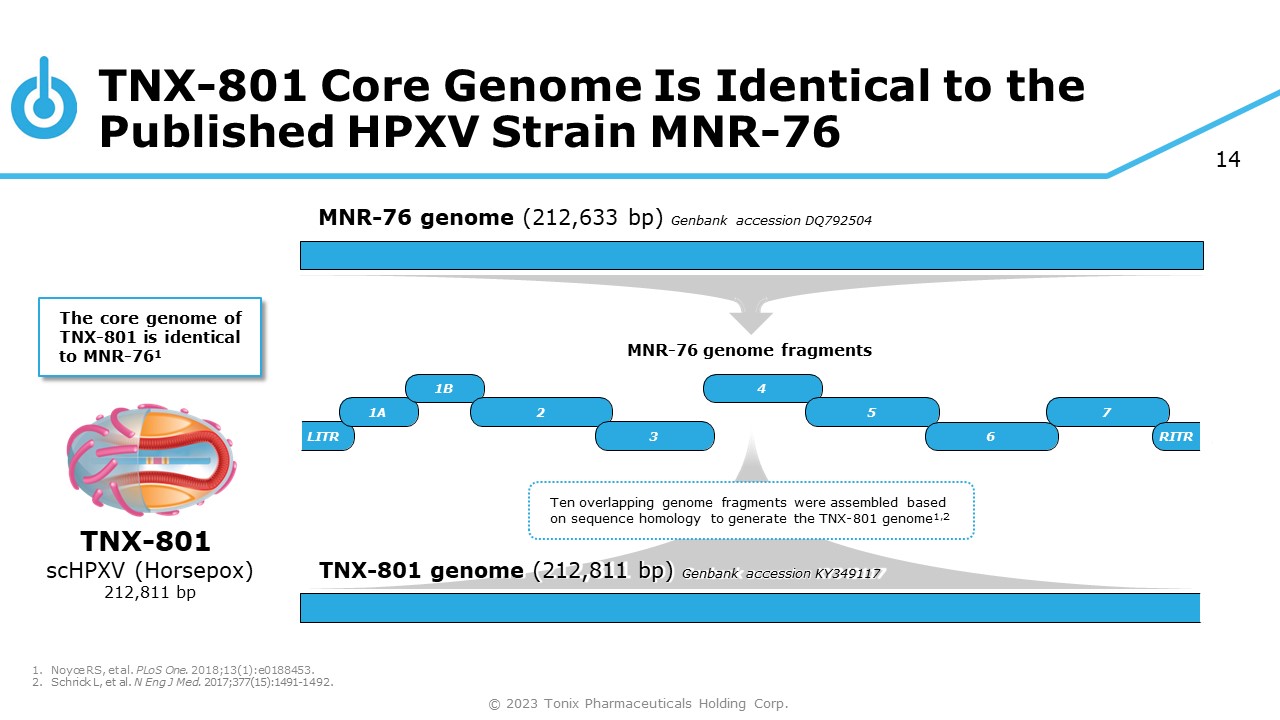
14 TNX - 801 Core Genome Is Identical to the Published HPXV Strain MNR - 76 1. Noyce RS, et al. PLoS One. 2018;13(1):e0188453. 2. Schrick L, et al. N Eng J Med. 2017;377(15):1491 - 1492. TNX - 801 scHPXV (Horsepox) 212,811 bp LITR 1A 1B 4 2 3 5 6 7 RITR MNR - 76 genome fragments MNR - 76 genome (212,633 bp) Genbank accession DQ792504 The core genome of TNX - 801 is identical to MNR - 76 1 TNX - 801 genome (212,811 bp) Genbank accession KY349117 Ten overlapping genome fragments were assembled based on sequence homology to generate the TNX - 801 genome 1,2 © 2023 Tonix Pharmaceuticals Holding Corp.
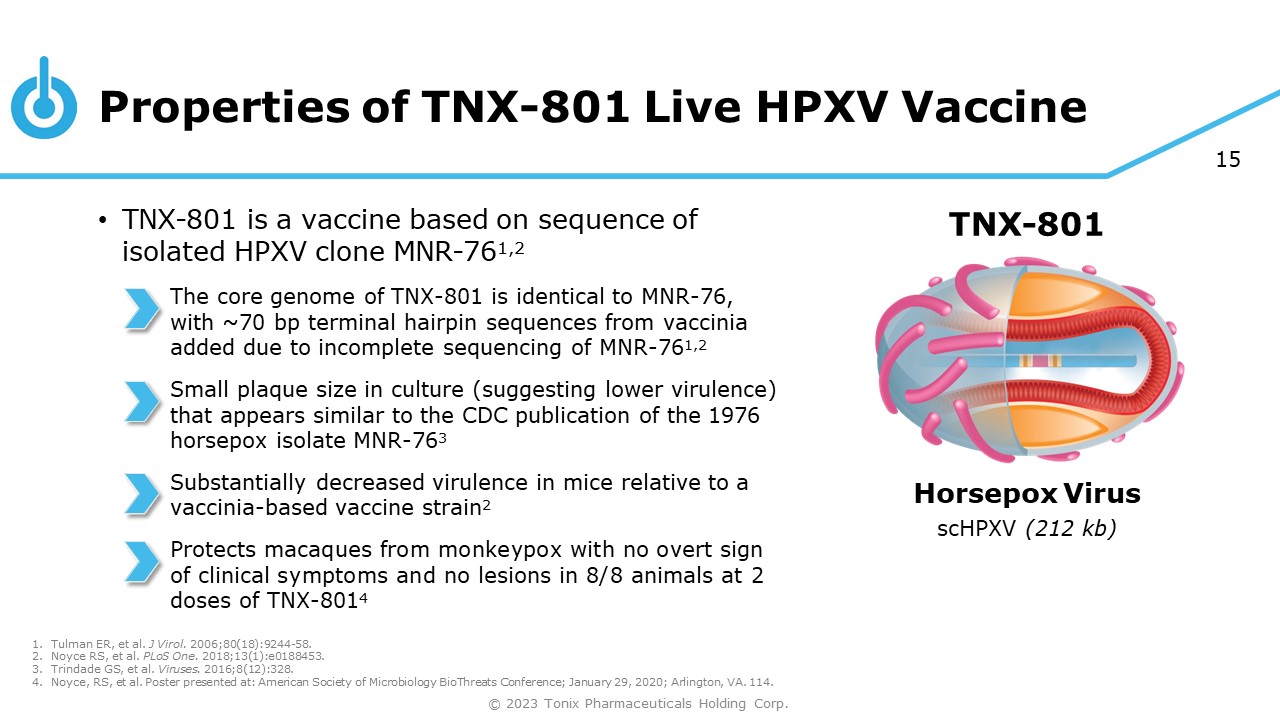
15 Properties of TNX - 801 Live HPXV Vaccine • TNX - 801 is a vaccine based on sequence of isolated HPXV clone MNR - 76 1,2 The core genome of TNX - 801 is identical to MNR - 76, with ~70 bp terminal hairpin sequences from vaccinia added due to incomplete sequencing of MNR - 76 1,2 Small plaque size in culture (suggesting lower virulence) that appears similar to the CDC publication of the 1976 horsepox isolate MNR - 76 3 Substantially decreased virulence in mice relative to a vaccinia - based vaccine strain 2 Protects macaques from monkeypox with no overt sign of clinical symptoms and no lesions in 8 / 8 animals at 2 doses of TNX - 801 4 Horsepox Virus scHPXV (212 kb) TNX - 801 1. Tulman ER, et al. J Virol. 2006;80(18):9244 - 58. 2. Noyce RS, et al. PLoS One. 2018;13(1):e0188453. 3. Trindade GS, et al. Viruses. 2016;8(12):328. 4. Noyce, RS, et al. Poster presented at: American Society of Microbiology BioThreats Conference; January 29, 2020; Arlington, VA. 114. © 2023 Tonix Pharmaceuticals Holding Corp.
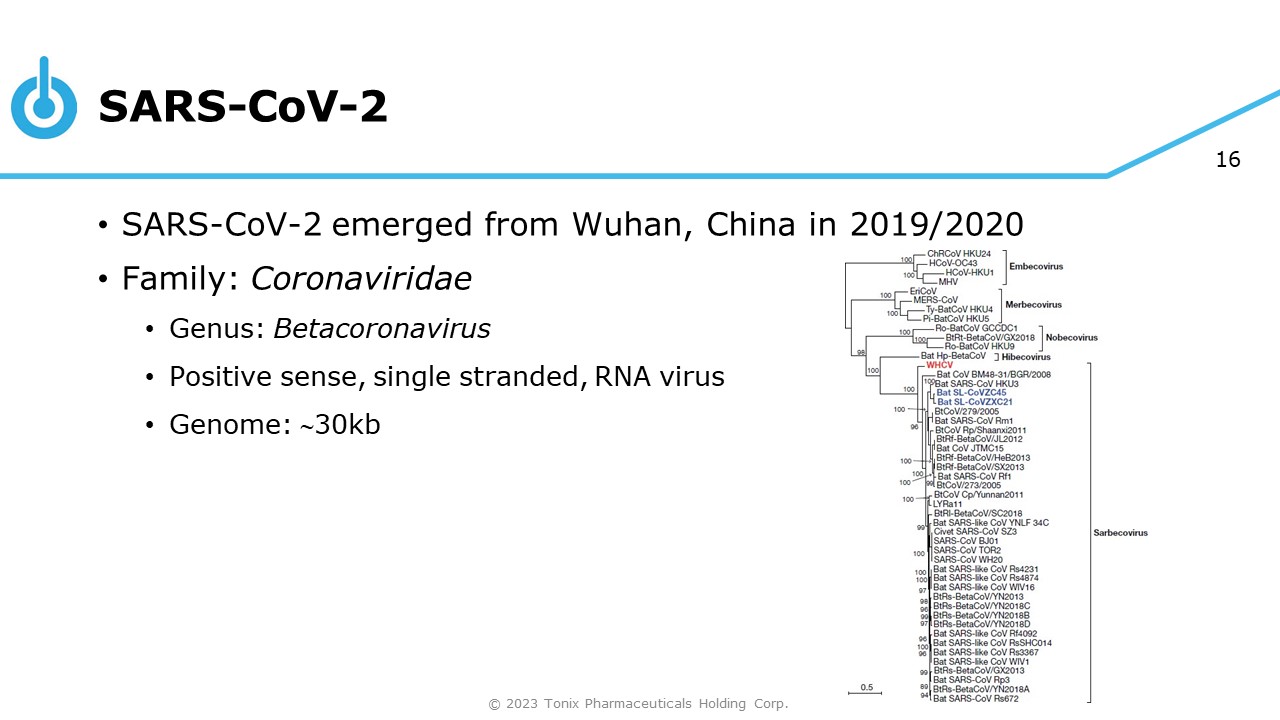
16 SARS - CoV - 2 • SARS - CoV - 2 emerged from Wuhan, China in 2019/2020 • Family: Coronaviridae • Genus: Betacoronavirus • Positive sense, single stranded, RNA virus • Genome: 30kb © 2023 Tonix Pharmaceuticals Holding Corp.
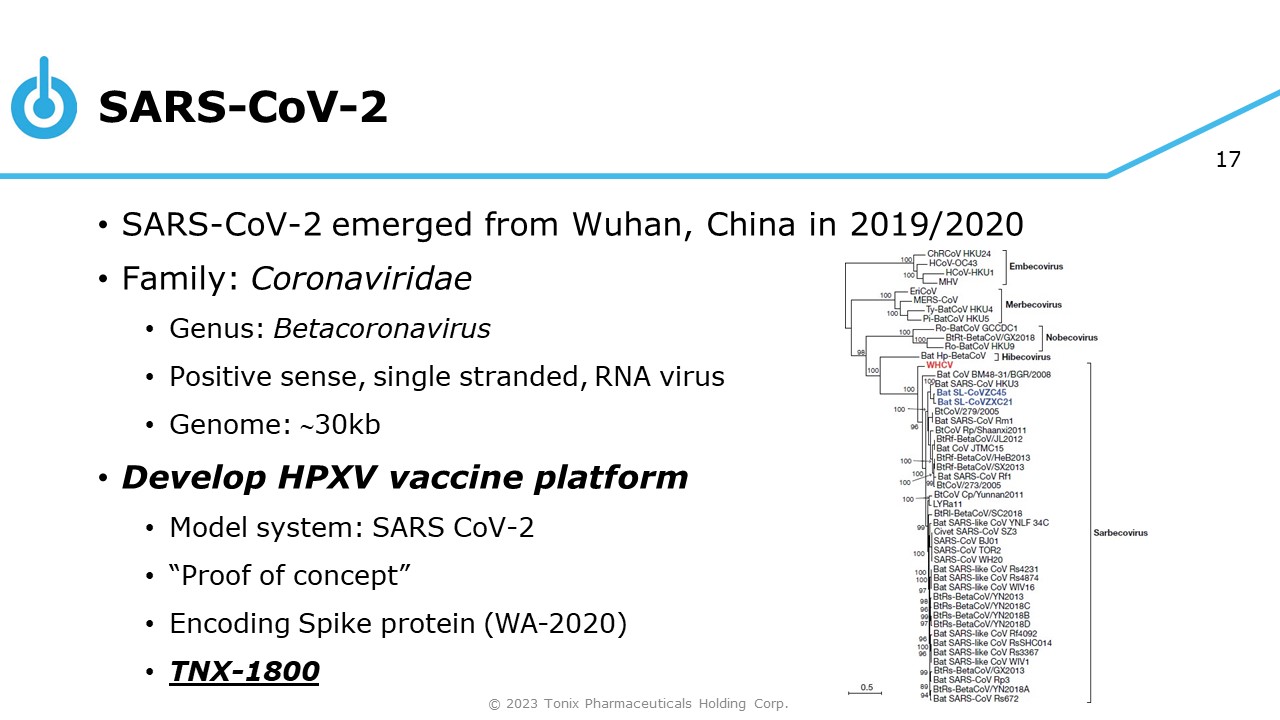
17 SARS - CoV - 2 • SARS - CoV - 2 emerged from Wuhan, China in 2019/2020 • Family: Coronaviridae • Genus: Betacoronavirus • Positive sense, single stranded, RNA virus • Genome: 30kb • Develop HPXV vaccine platform • Model system: SARS CoV - 2 • “Proof of concept” • Encoding Spike protein (WA - 2020) • TNX - 1800 © 2023 Tonix Pharmaceuticals Holding Corp.
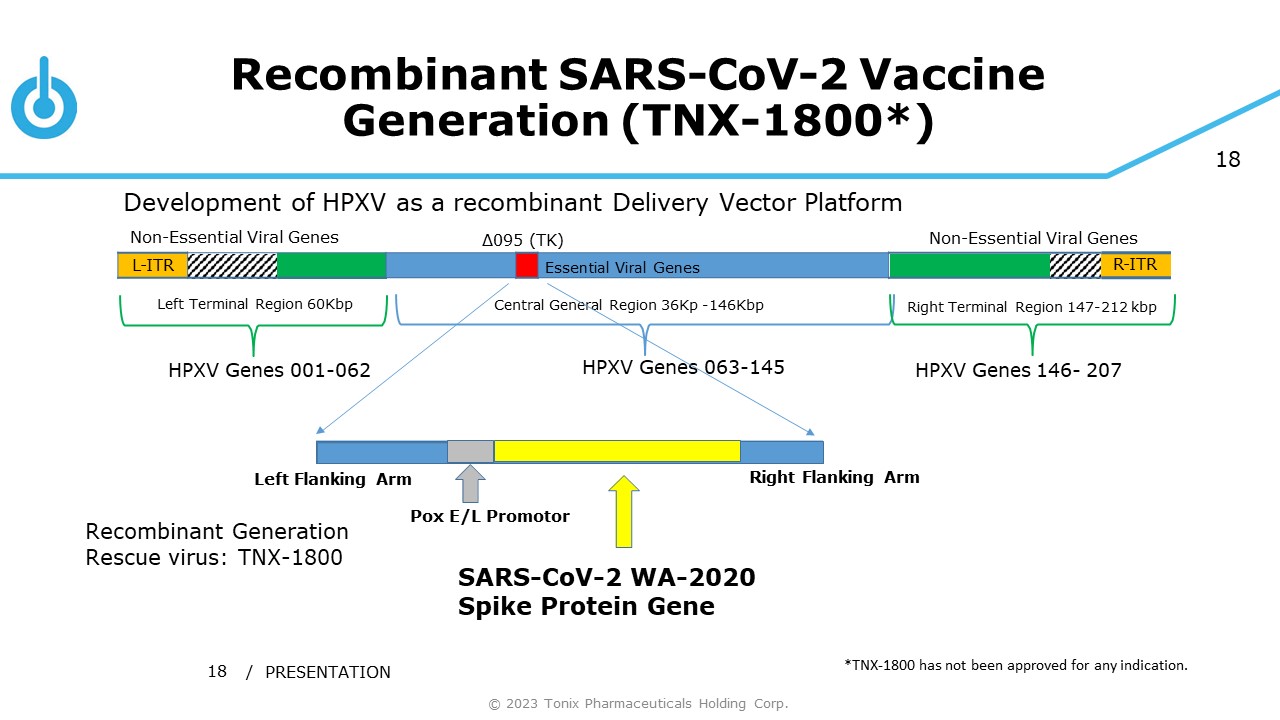
18 Recombinant SARS - CoV - 2 Vaccine Generation (TNX - 1800*) / PRESENTATION 18 Development of HPXV as a recombinant Delivery Vector Platform Central General Region 36Kp - 146Kbp Left Terminal Region 60Kbp Right Terminal Region 147 - 212 kbp Non - Essential Viral Genes Non - Essential Viral Genes L - ITR Essential Viral Genes R - ITR HPXV Genes 001 - 062 HPXV Genes 063 - 145 HPXV Genes 146 - 207 Δ095 (TK) Pox E/L Promotor Right Flanking Arm Left Flanking Arm Recombinant Generation Rescue virus: TNX - 1800 © 2023 Tonix Pharmaceuticals Holding Corp. SARS - CoV - 2 WA - 2020 Spike Protein Gene *TNX - 1800 has not been approved for any indication.
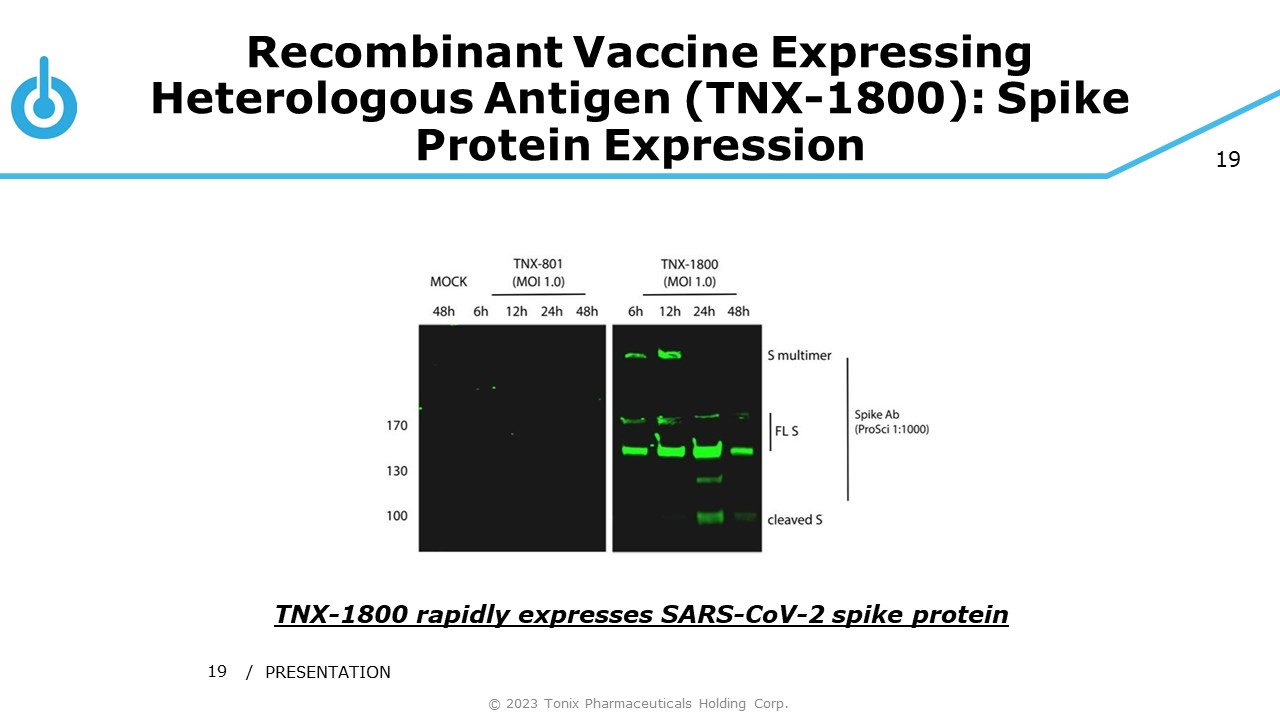
19 Recombinant Vaccine Expressing Heterologous Antigen (TNX - 1800): Spike Protein Expression / PRESENTATION 19 TNX - 1800 rapidly expresses SARS - CoV - 2 spike protein © 2023 Tonix Pharmaceuticals Holding Corp.
20 Preliminary Immunogenicity Studies © 2023 Tonix Pharmaceuticals Holding Corp. » Goal: Investigate immunogenicity and tolerability following administration of a single dose of TNX - 1800 » Two animal models: 1) Syrian Hamsters 2) New Zealand Rabbits
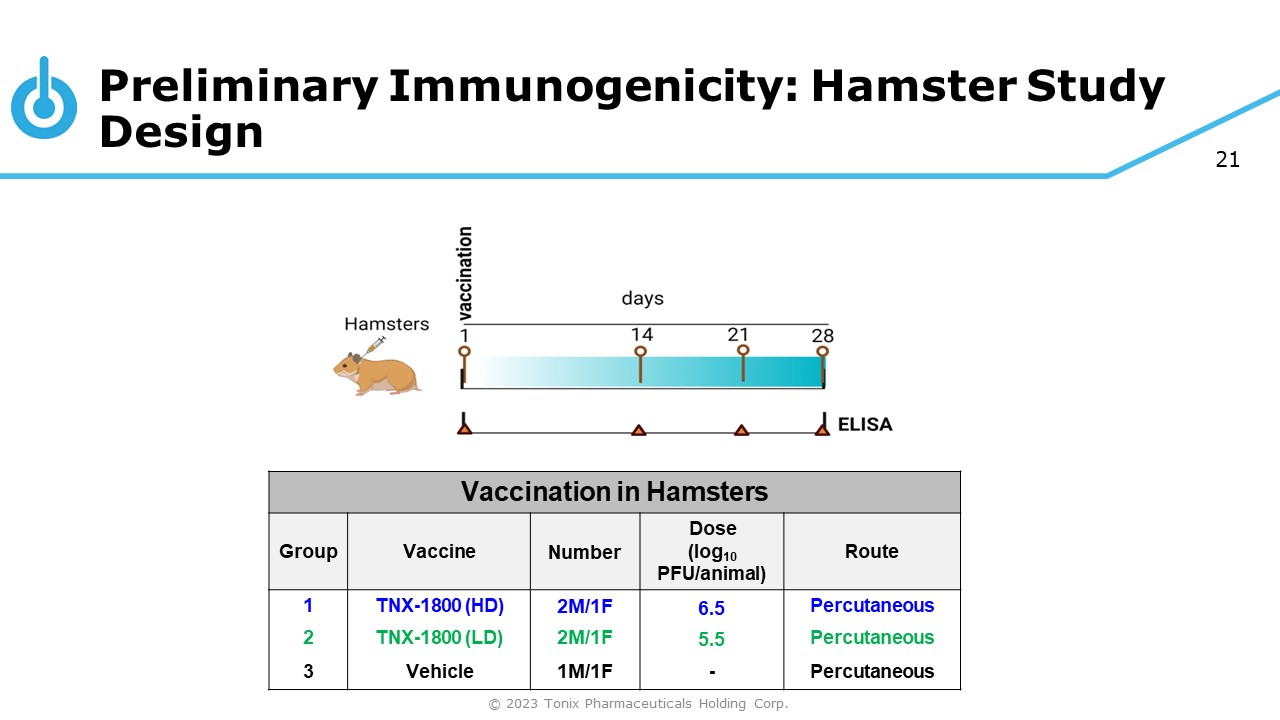
21 Preliminary Immunogenicity: Hamster Study Design Vaccination in Hamsters Group Vaccine Number Dose (log 10 PFU/animal) Route 1 TNX - 1800 (HD) 2M/1F 6.5 Percutaneous 2 TNX - 1800 (LD) 2M/1F 5.5 Percutaneous 3 Vehicle 1M/1F - Percutaneous © 2023 Tonix Pharmaceuticals Holding Corp.
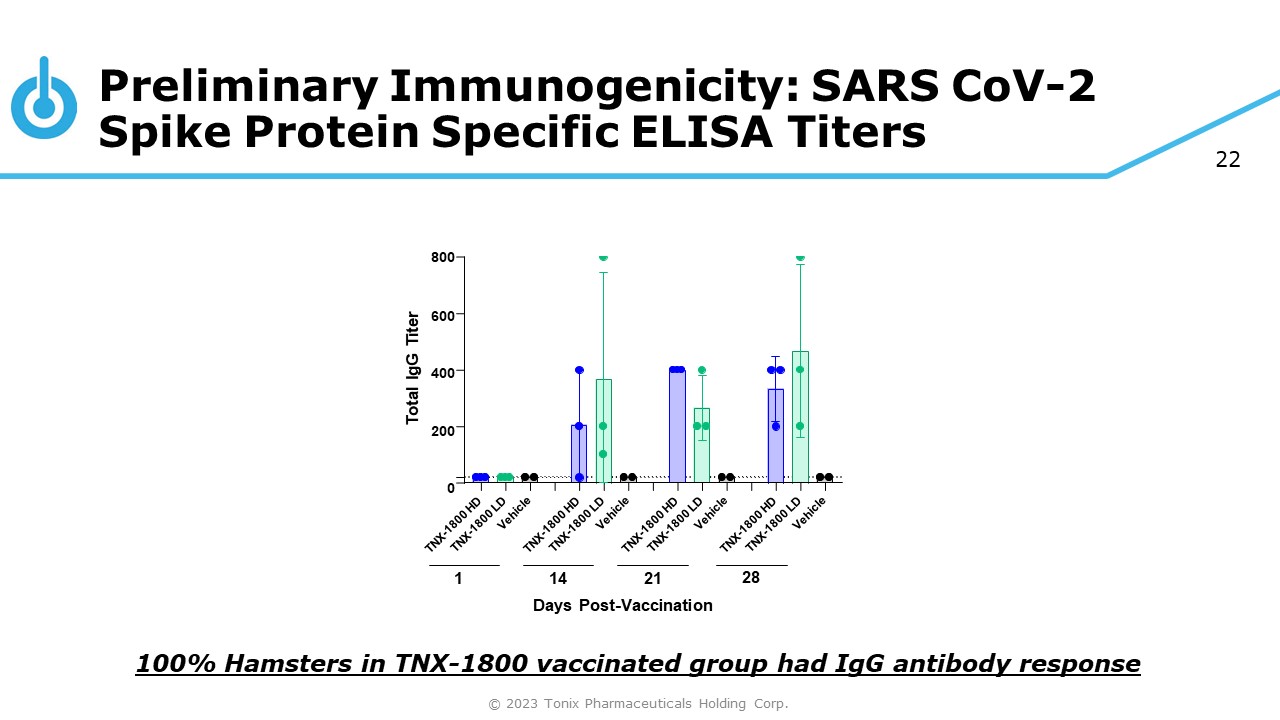
22 Preliminary Immunogenicity: SARS CoV - 2 Spike Protein Specific ELISA Titers 100% Hamsters in TNX - 1800 vaccinated group had IgG antibody response 800 600 400 200 0 Total IgG Titer 1 14 21 Days Post - Vaccination 28 © 2023 Tonix Pharmaceuticals Holding Corp.
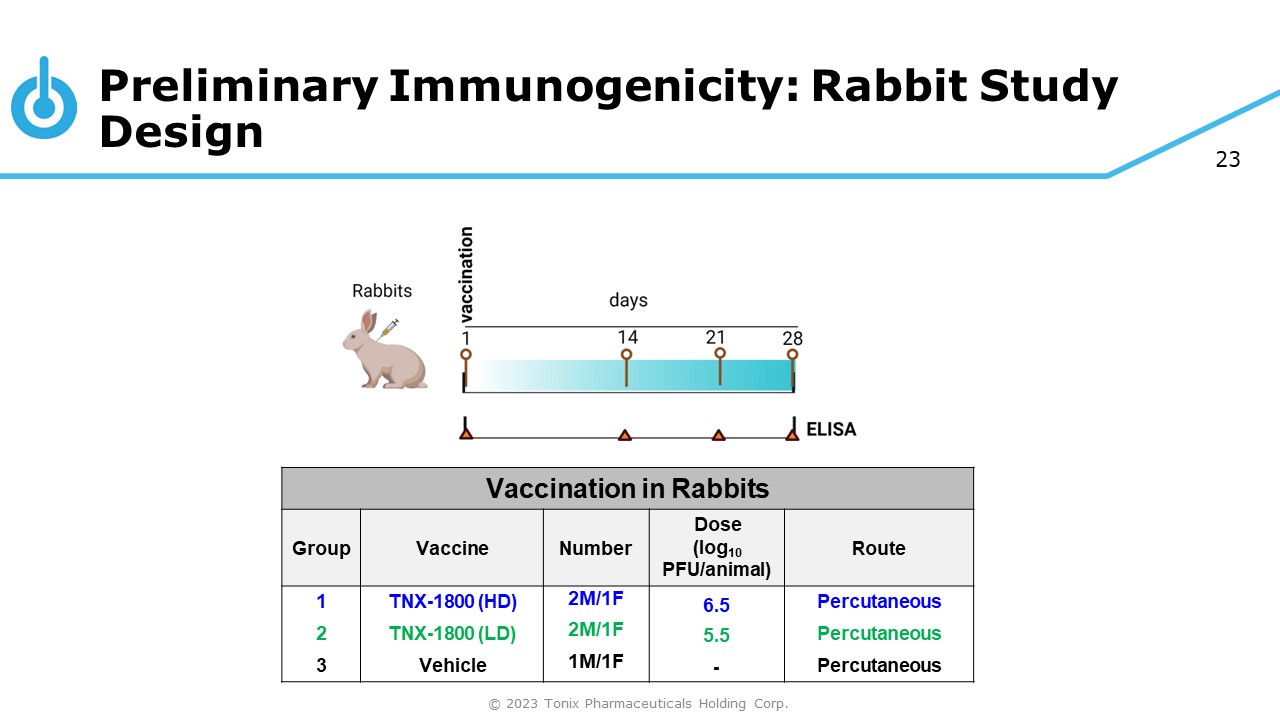
23 Preliminary Immunogenicity: Rabbit Study Design Vaccination in Rabbits Group Vaccine Number Dose (log 10 PFU/animal) Route 1 TNX - 1800 (HD) 2M/1F 6.5 Percutaneous 2 TNX - 1800 (LD) 2M/1F 5.5 Percutaneous 3 Vehicle 1M/1F - Percutaneous © 2023 Tonix Pharmaceuticals Holding Corp.
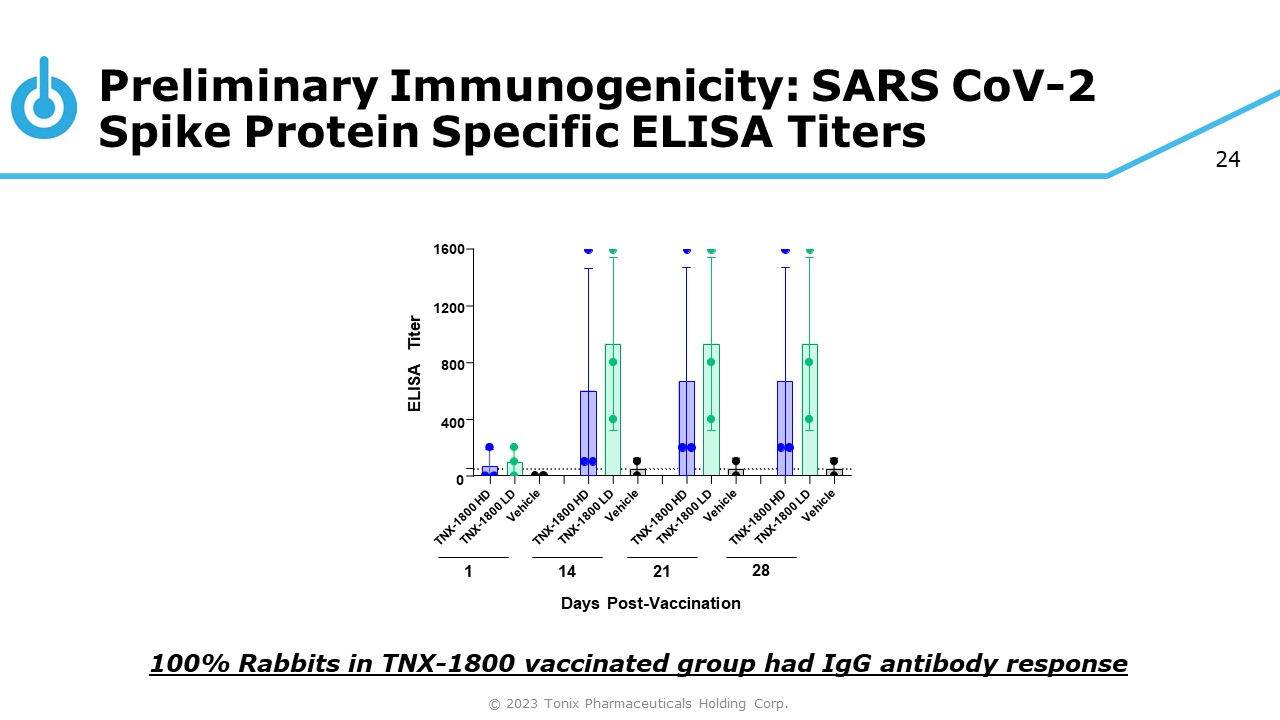
24 Preliminary Immunogenicity: SARS CoV - 2 Spike Protein Specific ELISA Titers 100% Rabbits in TNX - 1800 vaccinated group had IgG antibody response 1600 1200 800 400 0 ELISA Titer 1 14 21 Days Post - Vaccination 28 © 2023 Tonix Pharmaceuticals Holding Corp.
25 Preliminary Immunogenicity Studies: Conclusion © 2023 Tonix Pharmaceuticals Holding Corp. 1) 100% of animals generate an antibody response 2) Vaccine was well - tolerated ▪ No adverse events ▪ No disseminated horsepox virus infection » Proceeded to efficacy studies in NHPs
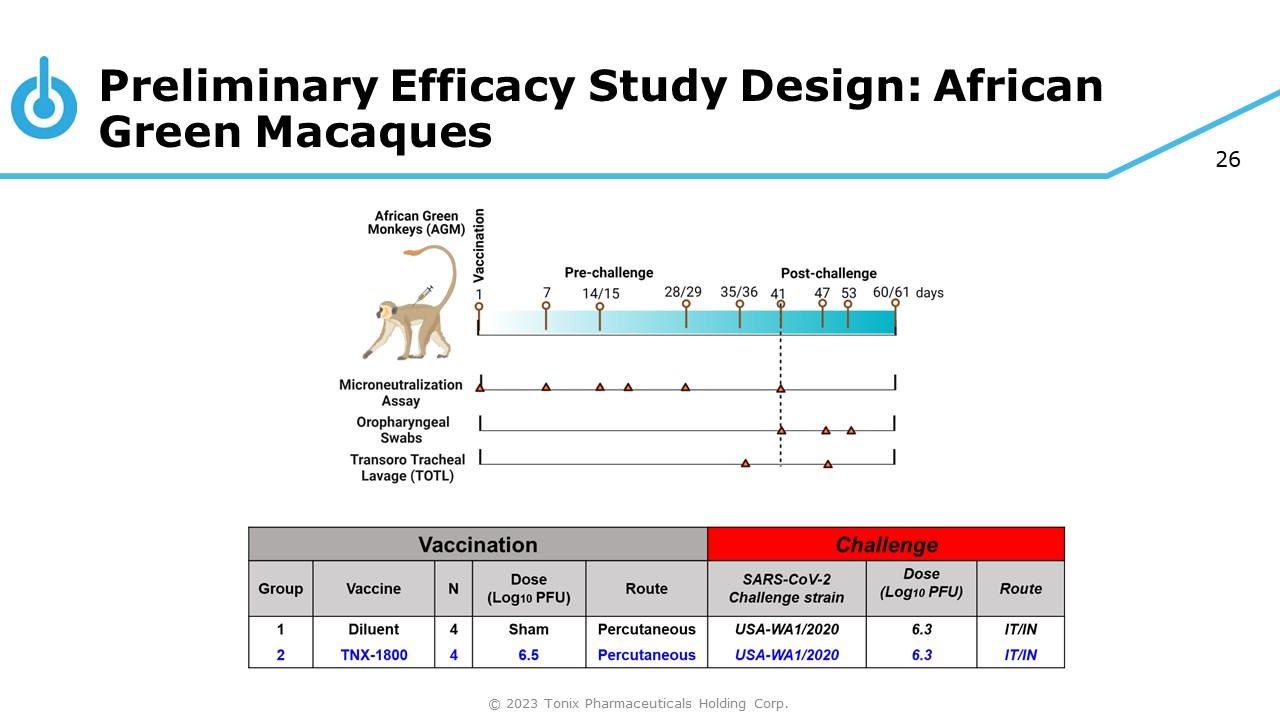
26 Preliminary Efficacy Study Design: African Green Macaques © 2023 Tonix Pharmaceuticals Holding Corp.
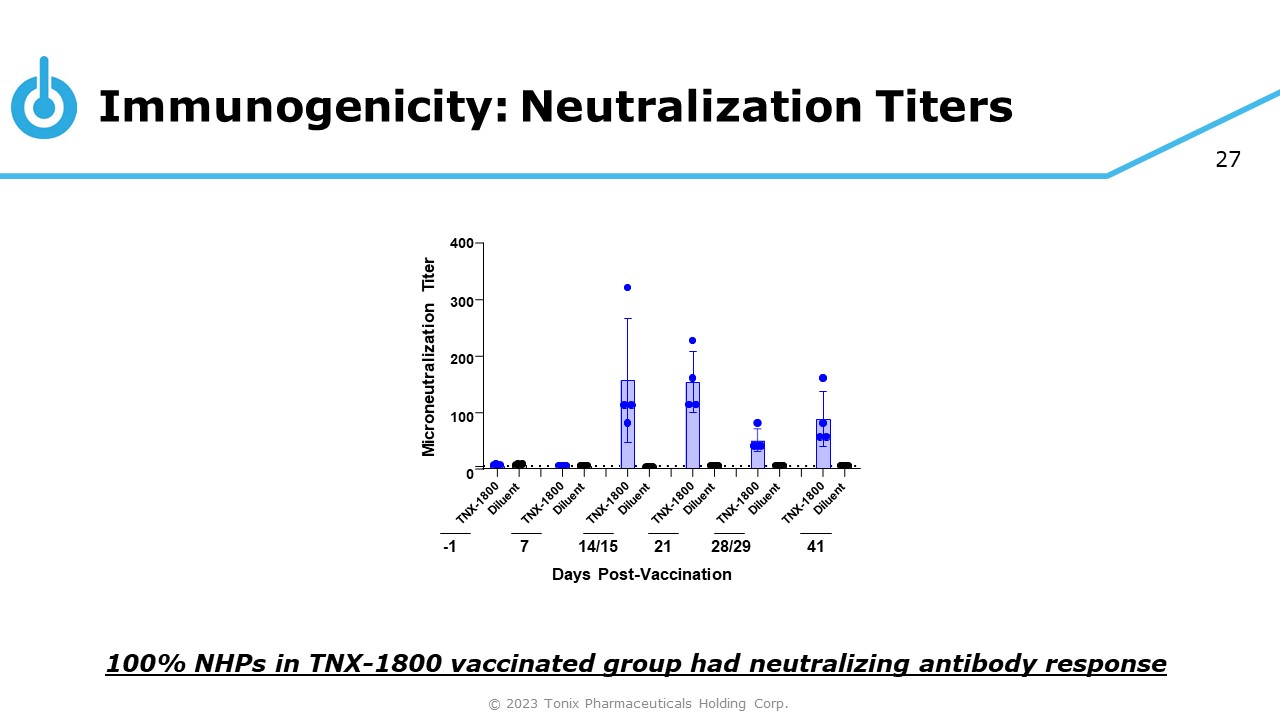
27 400 300 200 100 0 Microneutralization Titer - 1 7 14/15 21 28/29 Days Post - Vaccination 41 Immunogenicity: Neutralization Titers © 2023 Tonix Pharmaceuticals Holding Corp. 100% NHPs in TNX - 1800 vaccinated group had neutralizing antibody response
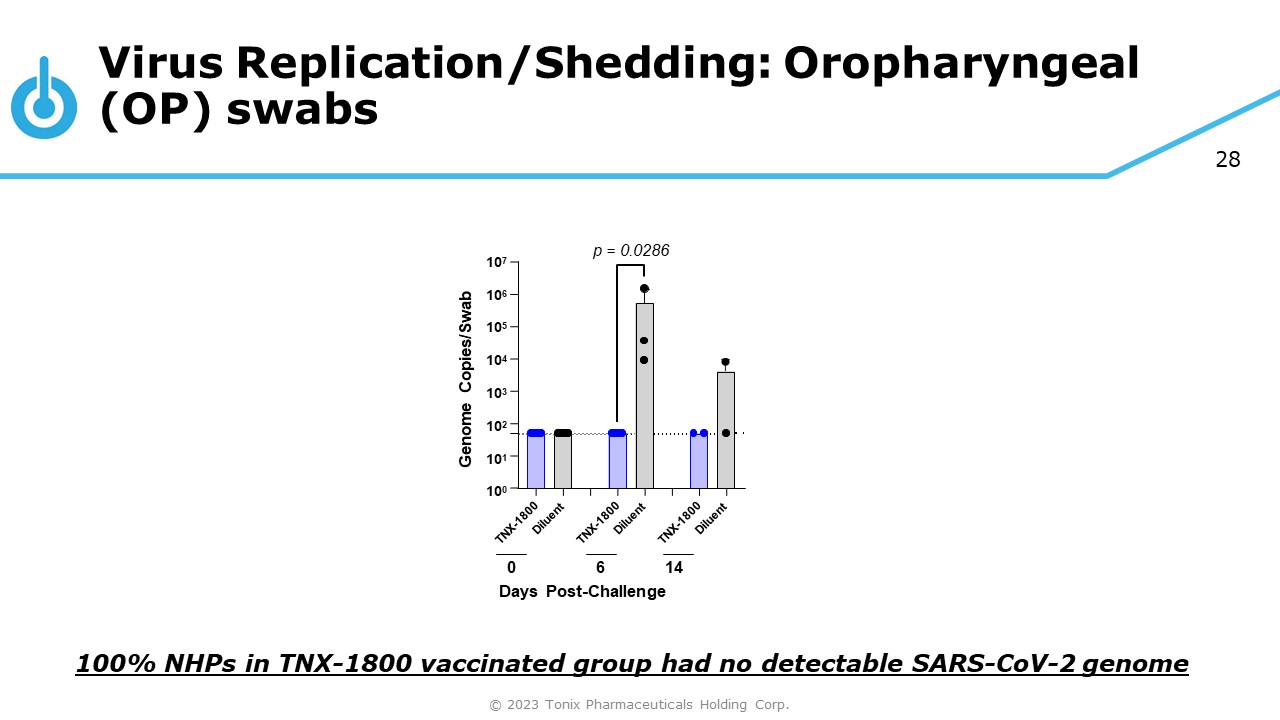
28 Virus Replication/Shedding: Oropharyngeal (OP) swabs 10 6 10 5 10 4 10 3 10 2 10 1 10 0 10 7 Genome Copies/Swab 0 6 14 Days Post - Challenge p = 0.0286 100% NHPs in TNX - 1800 vaccinated group had no detectable SARS - CoV - 2 genome © 2023 Tonix Pharmaceuticals Holding Corp.
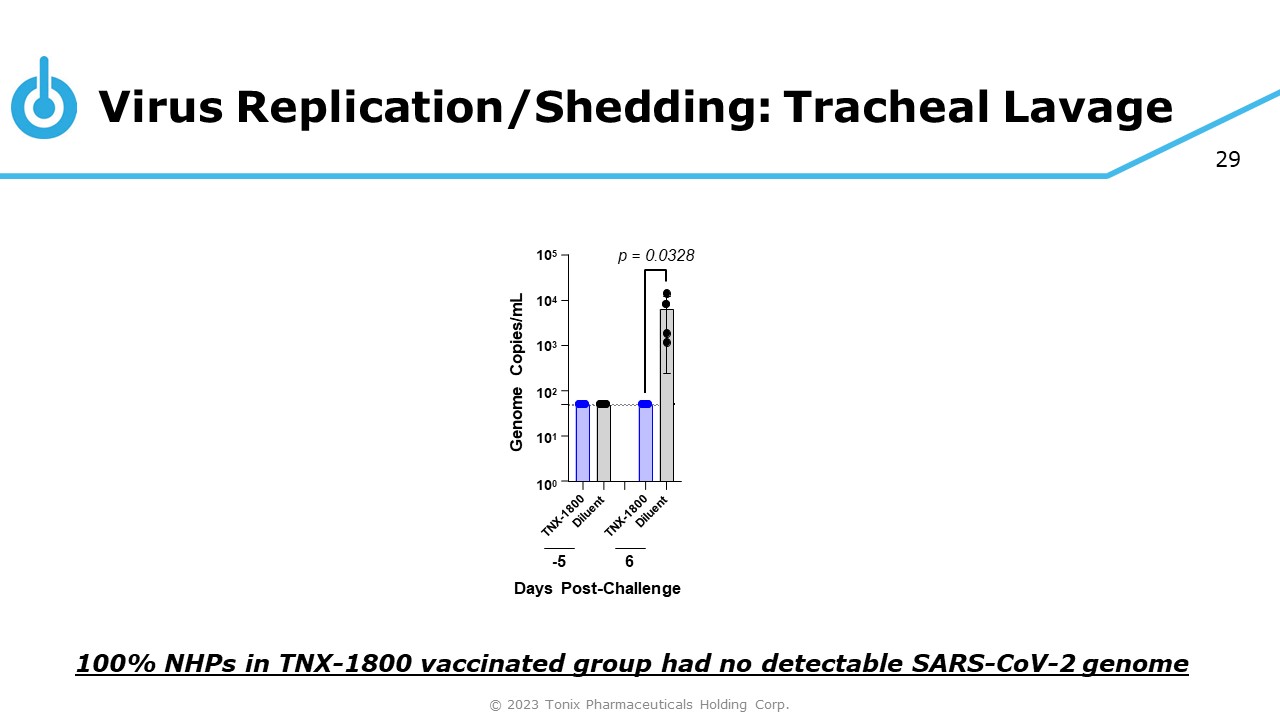
29 Virus Replication/Shedding: Tracheal Lavage 10 4 10 3 10 2 10 1 10 0 10 5 Genome Copies/mL - 5 6 Days Post - Challenge p = 0.0328 100% NHPs in TNX - 1800 vaccinated group had no detectable SARS - CoV - 2 genome © 2023 Tonix Pharmaceuticals Holding Corp.
30 Preliminary Efficacy Study Design: Cynomolgus Macaques © 2023 Tonix Pharmaceuticals Holding Corp.
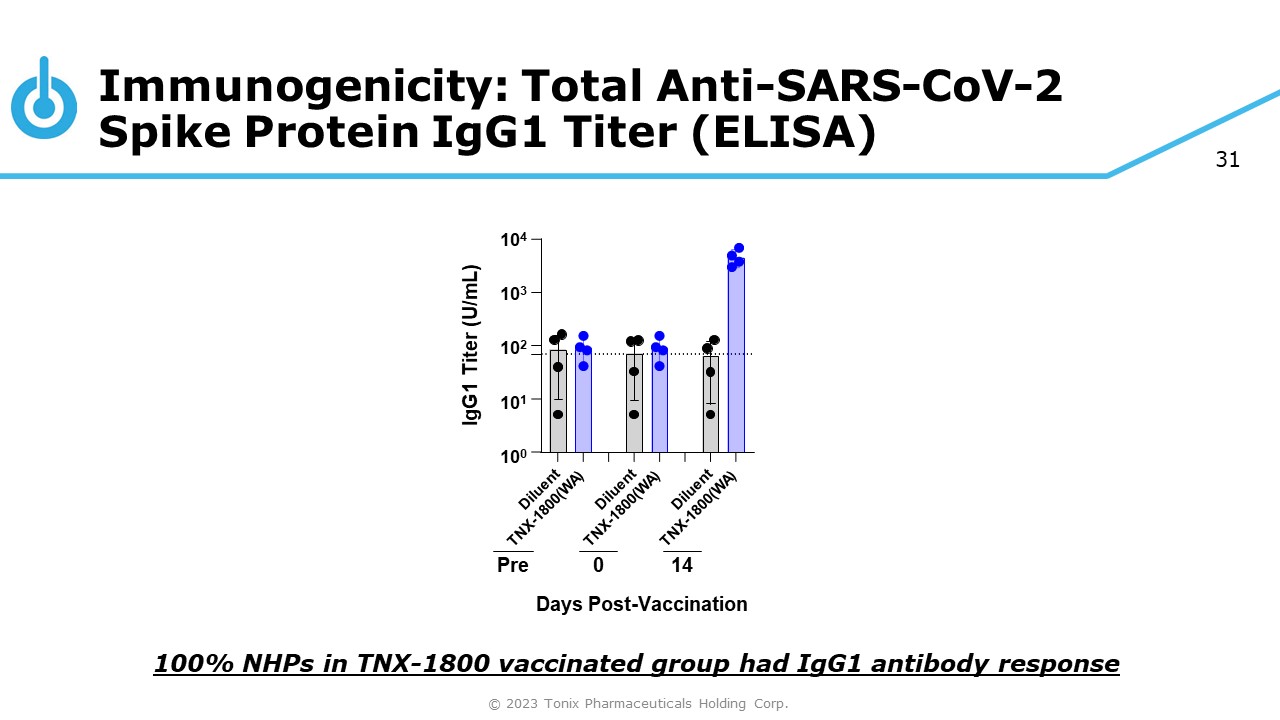
31 Immunogenicity: Total Anti - SARS - CoV - 2 Spike Protein IgG1 Titer (ELISA) 100% NHPs in TNX - 1800 vaccinated group had IgG1 antibody response 10 4 10 3 10 2 10 1 10 0 IgG1 Titer (U/mL) 0 14 Days Post - Vaccination Pre © 2023 Tonix Pharmaceuticals Holding Corp.
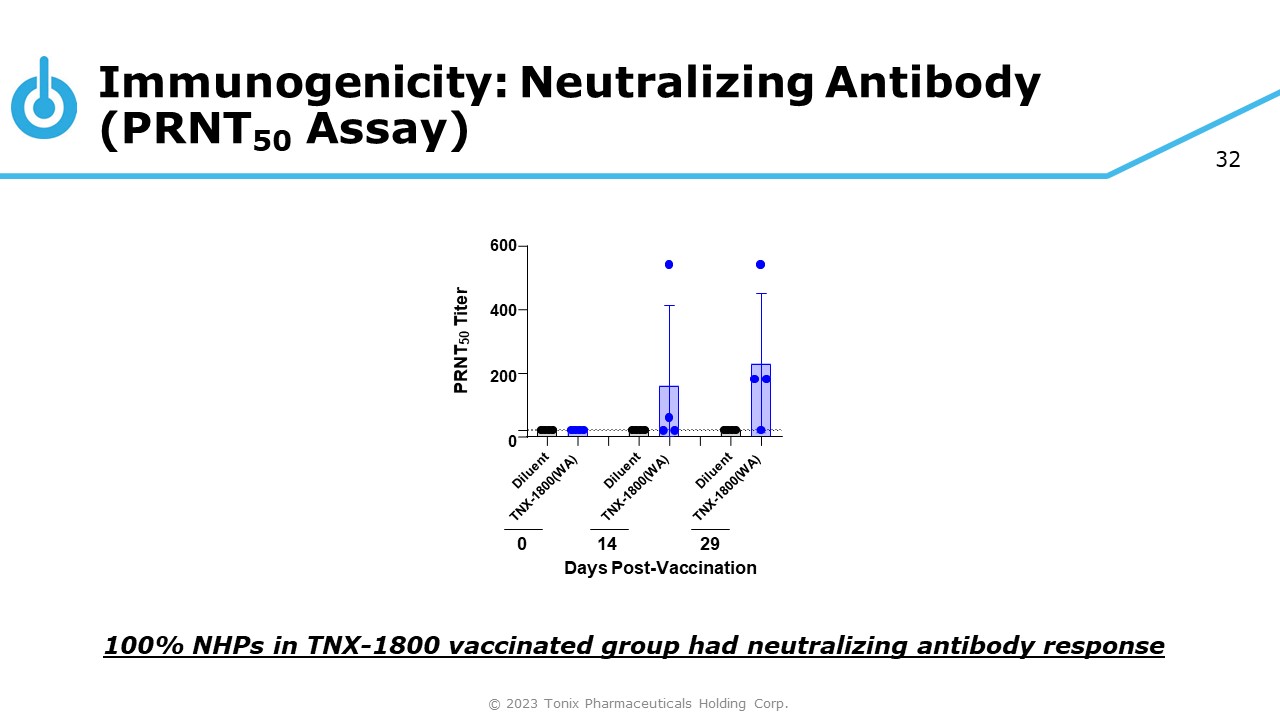
32 Immunogenicity: Neutralizing Antibody (PRNT 50 Assay) 100% NHPs in TNX - 1800 vaccinated group had neutralizing antibody response 600 400 200 0 PRNT 50 Titer 0 14 29 Days Post - Vaccination © 2023 Tonix Pharmaceuticals Holding Corp.
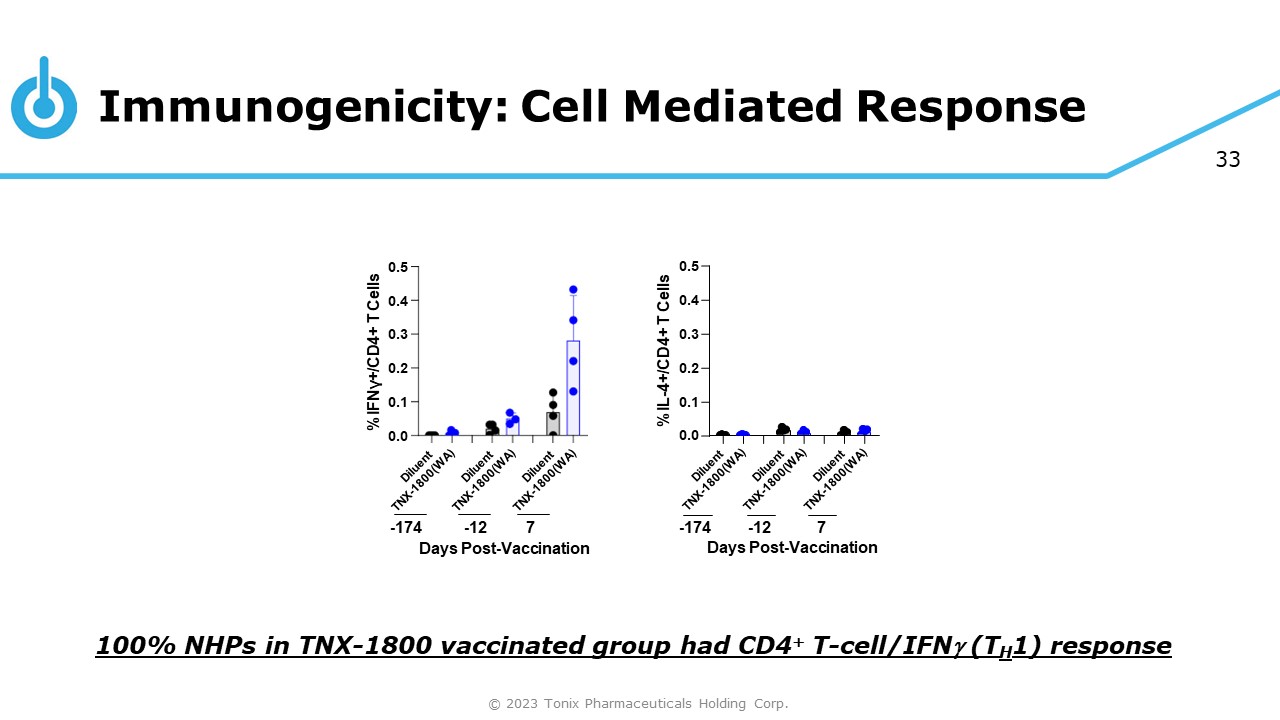
33 0.0 0.1 0.2 0.3 0.4 0.5 %IFNγ+/CD4+ T Cells - 174 - 12 7 Days Post - Vaccination 0.0 0.1 0.2 0.3 0.4 0.5 %IL - 4+/CD4+ T Cells - 174 - 12 7 Days Post - Vaccination Immunogenicity: Cell Mediated Response 100% NHPs in TNX - 1800 vaccinated group had CD4 + T - cell/IFN (T H 1) response © 2023 Tonix Pharmaceuticals Holding Corp.
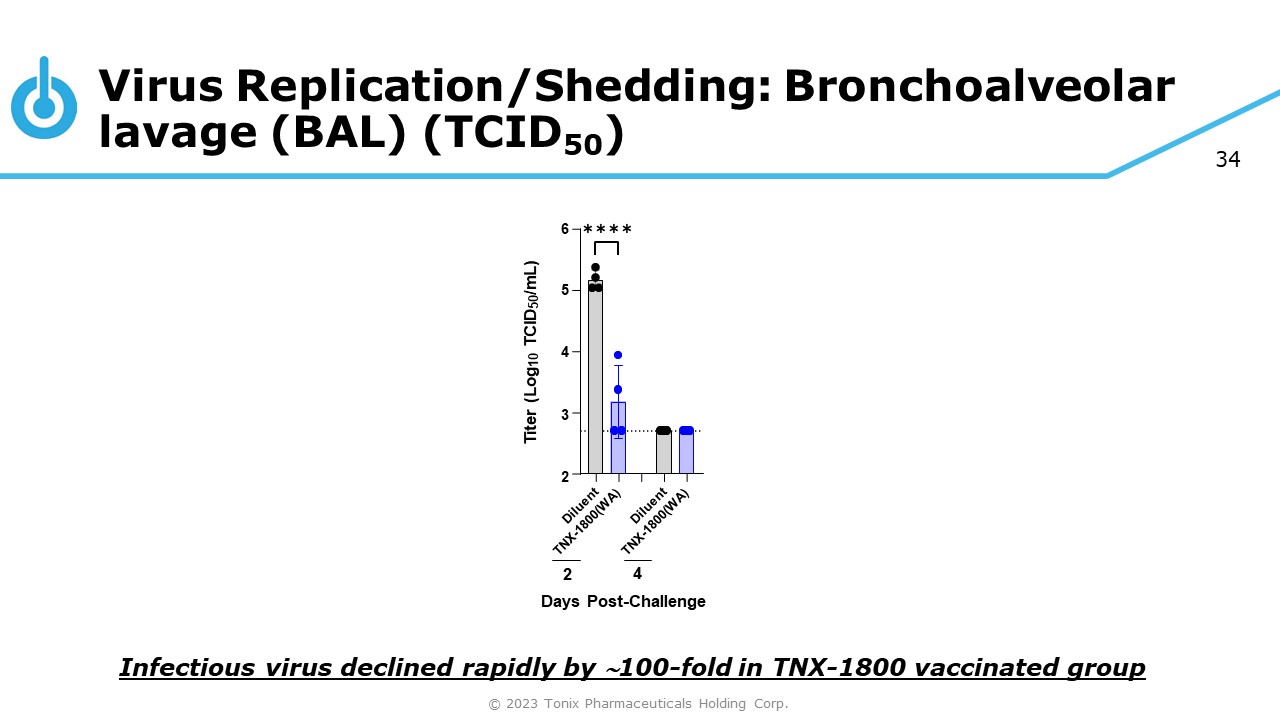
34 Virus Replication/Shedding: Bronchoalveolar lavage (BAL) (TCID 50 ) 5 4 3 2 6 Titer (Log 10 TCID 50 /mL) 2 4 Days Post - Challenge Infectious virus declined rapidly by 100 - fold in TNX - 1800 vaccinated group ٓٓٓٓ © 2023 Tonix Pharmaceuticals Holding Corp.
35 Conclusions © 2023 Tonix Pharmaceuticals Holding Corp. » TNX - 1800 engineered to expressed heterologous antigen ▪ “Proof of concept” ▪ SARS - CoV - 2 WA - 2020 Spike protein » 2 preliminary immunogenicity and 2 efficacy studies ▪ Animal models: Hamsters, Rabbits, Cynomolgus and African green macaques » A single dose of TNX - 1800 vaccination was well tolerated ▪ No severe adverse events following vaccination ▪ Did not produce disseminated infection in any animal model
Conclusions © 2023 Tonix Pharmaceuticals Holding Corp. 36 » TNX - 1800 vaccination via route percutaneous was immunogenic ▪ 100% response in all 4 animal models ▪ Rapid generation of antibody response (Total IgG and/or neutralizing antibody) ▪ Induced CD4 + T - cell response ▪ Responses were skewed to T H 1 » Efficacy studies in cynomolgus and African green macaques ▪ Challenged with SARS - CoV - 2 WA - 2020 ▪ Virus shedding/replication was reduced by 10 to 1,000 - fold
Conclusions © 2023 Tonix Pharmaceuticals Holding Corp. 37 » No longer continuing with clinical development of SARS - CoV - 2 vaccine program 1) New variants (e.g., XBB) appear to be boosting pre - existing immunity resulting in “herd immunity” 2) Challenging regulatory hurdles for clinical evidence » Additional vector development for heterologous genes from other pathogens underway: 1) Additional insertion sites for stable expression 2) Multivalency for additional heterologous antigens 3) Additional routes of vaccination
38 » Tonix Pharmaceuticals ▪ Siobhan Fogarty ▪ Helen Stillwell ▪ Bruce Daugherty ▪ Seth Lederman Acknowledgements © 2023 Tonix Pharmaceuticals Holding Corp. » University of Alberta ▪ Ryan Noyce ▪ David Evans » Southern Research » BIOQUAL