Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

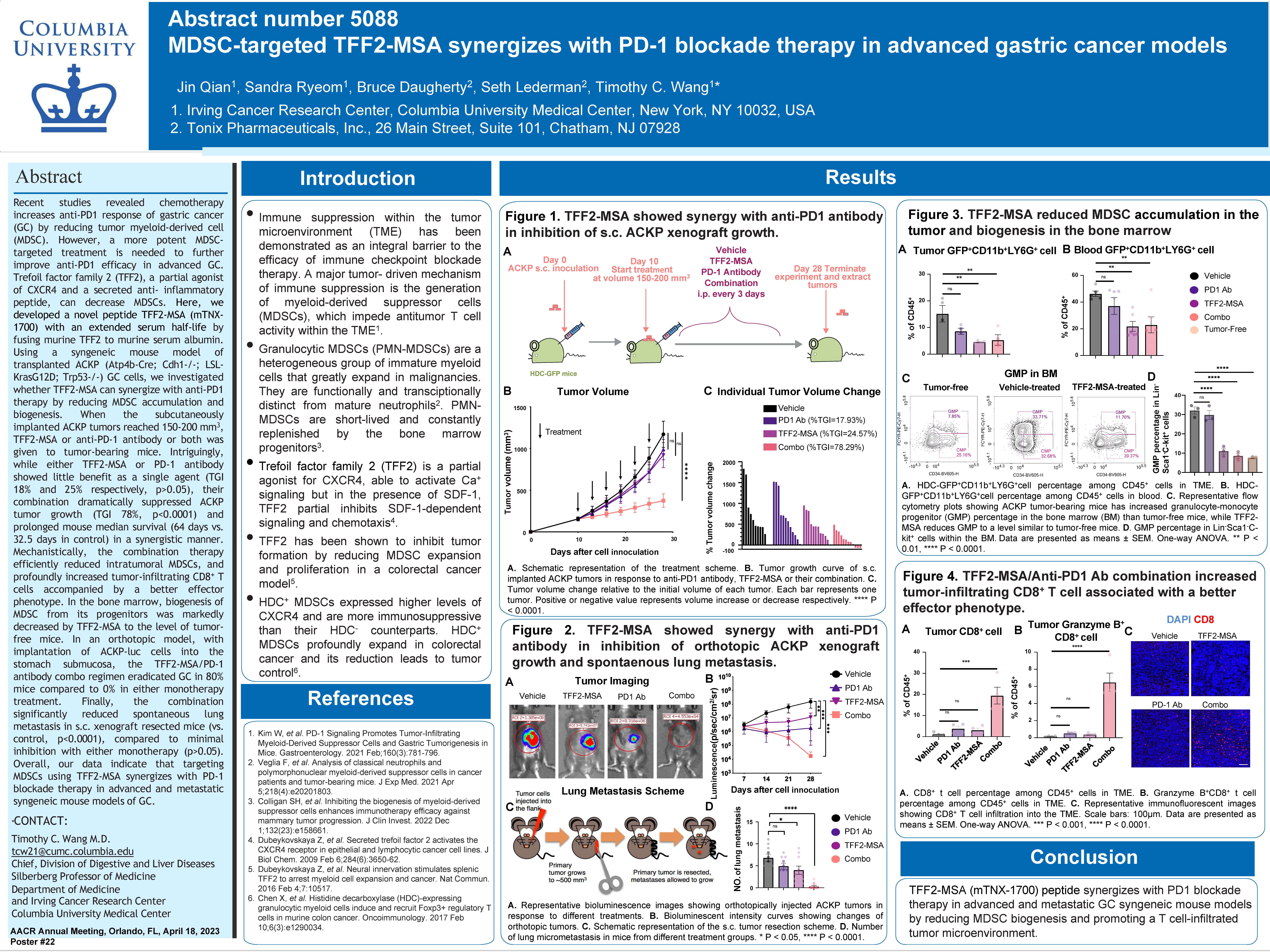
Abstract number 5088MDSC-targetedTFF2-MSAsynergizeswithPD-1blockade therapy inadvancedgastriccancermodels JinQian1,SandraRyeom1,BruceDaugherty2,SethLederman2,TimothyC.Wang1* Abstract Figure1.TFF2-MSAshowedsynergywithanti-PD1 antibodyininhibitionofs.c.ACKP xenograftgrowth. Figure2.TFF2-MSAshowedsynergywithanti-PD1antibodyininhibitionoforthotopicACKPxenograftgrowthandspontaenouslungmetastasis. Figure3.TFF2-MSAreducedMDSCaccumulationinthetumorand biogenesis in the bone marrow Figure4.TFF2-MSA/Anti-PD1Abcombinationincreasedtumor-infiltratingCD8+Tcellassociatedwithabettereffector phenotype. 1. Irving Cancer Research Center, Columbia University Medical Center, New York, NY 10032, USA 2. TonixPharmaceuticals, Inc., 26 Main Street, Suite 101, Chatham, NJ 07928 • Immunesuppressionwithinthetumormicroenvironment(TME)hasbeendemonstratedasanintegralbarriertotheefficacyofimmunecheckpointblockadetherapy.Amajortumor-drivenmechanismofimmunesuppressionisthegenerationofmyeloid-derivedsuppressorcells(MDSCs),whichimpedeantitumorTcellactivitywithintheTME1. • GranulocyticMDSCs(PMN-MDSCs)areaheterogeneousgroupofimmaturemyeloidcellsthatgreatlyexpandinmalignancies.Theyarefunctionallyandtransciptionallydistinctfrommatureneutrophils2.PMN-MDSCsareshort-livedandconstantlyreplenishedbythebonemarrowprogenitors3. • Trefoilfactorfamily2(TFF2)isapartialagonistforCXCR4,abletoactivateCa+signalingbutinthepresenceofSDF-1,TFF2partialinhibitsSDF-1-dependentsignalingandchemotaxis4. • TFF2hasbeenshowntoinhibittumorformationbyreducingMDSCexpansionandproliferationinacolorectalcancermodel5. • HDC+MDSCsexpressedhigherlevelsofCXCR4andaremoreimmunosuppressivethantheirHDC-counterparts.HDC+MDSCsprofoundlyexpandincolorectalcanceranditsreductionleadstotumorcontrol6. Recentstudiesrevealedchemotherapyincreasesanti-PD1responseofgastriccancer(GC)byreducingtumormyeloid-derivedcell(MDSC).However,amorepotentMDSC-targetedtreatmentisneededtofurtherimproveanti-PD1efficacyinadvancedGC.Trefoilfactorfamily2(TFF2),apartialagonistofCXCR4andasecretedanti-inflammatorypeptide,candecreaseMDSCs.Here,wedevelopedanovelpeptideTFF2-MSA(mTNX-1700)withanextendedserumhalf-lifebyfusingmurineTFF2tomurineserumalbumin.UsingasyngeneicmousemodeloftransplantedACKP(Atp4b-Cre;Cdh1-/-;LSL-KrasG12D;Trp53-/-)GCcells,weinvestigatedwhetherTFF2-MSAcansynergizewithanti-PD1therapybyreducingMDSCaccumulationandbiogenesis.WhenthesubcutaneouslyimplantedACKPtumorsreached150-200mm3,TFF2-MSAoranti-PD-1antibodyorbothwasgiventotumor-bearingmice.Intriguingly,whileeitherTFF2-MSAorPD-1antibodyshowedlittlebenefitasasingleagent(TGI18%and25%respectively,p>0.05),theircombinationdramaticallysuppressedACKPtumorgrowth(TGI78%,p<0.0001)andprolongedmousemediansurvival(64daysvs.32.5daysincontrol)inasynergisticmanner.Mechanistically,thecombinationtherapyefficientlyreducedintratumoralMDSCs,andprofoundlyincreasedtumor-infiltratingCD8+Tcellsaccompaniedbyabettereffectorphenotype.Inthebonemarrow,biogenesisofMDSCfromitsprogenitorswasmarkedlydecreasedbyTFF2-MSAtotheleveloftumor-freemice.Inanorthotopicmodel,withimplantationofACKP-luccellsintothestomachsubmucosa,theTFF2-MSA/PD-1antibodycomboregimeneradicatedGCin80%micecomparedto0%ineithermonotherapytreatment.Finally,thecombinationsignificantlyreducedspontaneouslungmetastasisins.c.xenograftresectedmice(vs.control,p<0.0001),comparedtominimalinhibitionwitheithermonotherapy(p>0.05).Overall,ourdataindicatethattargetingMDSCsusingTFF2-MSAsynergizeswithPD-1blockadetherapyinadvancedandmetastaticsyngeneicmousemodelsofGC. Day0 ACKPs.c.inoculation Day10 Starttreatment Day28Terminateexperimentand extract tumors Vehicle TFF2-MSA PD-1 Antibody Combination i.p.every 3 days atvolume150-200mm3 *CONTACT: TimothyC.WangM.D. tcw21@cumc.columbia.edu Chief,DivisionofDigestiveandLiverDiseases SilberbergProfessorofMedicine DepartmentofMedicine andIrvingCancerResearchCenter ColumbiaUniversityMedicalCenter TFF2-MSA(mTNX-1700) peptidesynergizeswithPD1blockadetherapyin advancedandmetastaticGCsyngeneicmousemodelsbyreducing MDSCbiogenesisandpromotingaTcell-infiltratedtumor microenvironment. Tumor Volume Individual Tumor Volume Change Introduction Results 500 1000 1500 2000 %Tumor volume change Vehicle PD1Ab (%TGI=17.93%) TFF2-MSA(%TGI=24.57%) Combo(%TGI=78.29%) 0 30 0 500 1000 1500 Daysaftercellinnoculation Tumorvolume(mm3) Treatment ns ns ✱ ✱ ✱ ✱ A B C Tumor Imaging Vehicle PD1Ab TFF2-MSA Combo A B 0 -100 Vehicle PD1 Ab TFF2-MSA Combo Daysaftercellinnoculation 20 10 Luminescence(p/sec/cm2/sr) C D 0 5 10 15 NO.oflungmetastasis ✱ ns ✱✱✱✱ References Conclusion 0 10 20 30 %ofCD45+ Tumor GFP+CD11b+LY6G+cell ns ✱✱ ✱✱ A HDC-GFP mice B C 0 20 40 60 ns ✱✱ Blood GFP+CD11b+LY6G+cell ✱✱ %ofCD45+ Tumor-free Vehicle-treated TFF2-MSA-treated Vehicle PD1 Ab TFF2-MSA Combo Vehicle PD1 Ab TFF2-MSA Combo Tumor-Free GMP in BM 0 10 20 30 40 ns ns ✱✱✱ A Tumor CD8+cell 0 2 4 6 8 10 ns ns ✱✱✱✱ Tumor Granzyme B+ CD8+cell B C Vehicle TFF2-MSA PD-1 Ab Combo DAPICD8 %ofCD45+ %ofCD45+ GMP percentage in Lin-Sca1-C-kit+cells A.Schematicrepresentationofthetreatmentscheme.B.Tumorgrowthcurveofs.c.implantedACKPtumorsinresponsetoanti-PD1antibody,TFF2-MSAortheircombination.C.Tumorvolumechangerelativetotheinitialvolumeofeachtumor.Eachbarrepresentsonetumor.Positiveornegativevaluerepresentsvolumeincreaseordecreaserespectively.****P<0.0001. A.RepresentativebioluminescenceimagesshowingorthotopicallyinjectedACKPtumorsinresponsetodifferenttreatments.B.Bioluminescentintensitycurvesshowingchangesoforthotopictumors.C.Schematicrepresentationofthes.c.tumorresectionscheme.D.Numberoflungmicrometastasisinmicefromdifferenttreatmentgroups.*P<0.05,****P<0.0001. A.CD8+tcellpercentageamongCD45+cellsinTME.B.GranzymeB+CD8+tcellpercentageamongCD45+cellsinTME.C.RepresentativeimmunofluorescentimagesshowingCD8+TcellinfiltrationintotheTME.Scalebars:100μm.Dataarepresentedasmeans±SEM.One-wayANOVA.***P<0.001,****P<0.0001. A.HDC-GFP+CD11b+LY6G+cellpercentageamongCD45+cellsinTME.B.HDC-GFP+CD11b+LY6G+cellpercentageamongCD45+cellsinblood.C.RepresentativeflowcytometryplotsshowingACKPtumor-bearingmicehasincreasedgranulocyte-monocyteprogenitor(GMP)percentageinthebonemarrow(BM)thantumor-freemice,whileTFF2-MSAreducesGMPtoalevelsimilartotumor-freemice.D.GMPpercentageinLin-Sca1-C-kit+cellswithintheBM.Dataarepresentedasmeans±SEM.One-wayANOVA.**P<0.01,****P<0.0001. D 1. Kim W, et al. PD-1 Signaling Promotes Tumor-Infiltrating Myeloid-Derived Suppressor Cells and Gastric Tumorigenesis in Mice. Gastroenterology. 2021 Feb;160(3):781-796. 2. VegliaF, et al. Analysis of classical neutrophils and polymorphonuclear myeloid-derived suppressor cells in cancer patients and tumor-bearing mice. J Exp Med. 2021 Apr 5;218(4):e20201803. 3. Colligan SH, et al. Inhibiting the biogenesis of myeloid-derived suppressor cells enhances immunotherapy efficacy against mammary tumor progression. J Clin Invest. 2022 Dec 1;132(23):e158661. 4. DubeykovskayaZ, et al. Secreted trefoil factor 2 activates the CXCR4 receptor in epithelial and lymphocytic cancer cell lines. J Biol Chem. 2009 Feb 6;284(6):3650-62. 5. DubeykovskayaZ, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016 Feb 4;7:10517. 6. Chen X, et al. Histidine decarboxylase (HDC)-expressing granulocytic myeloid cells induce and recruit Foxp3+regulatory T cells in murine colon cancer. Oncoimmunology. 2017 Feb 10;6(3):e1290034. Lung Metastasis Scheme AACR Annual Meeting, Orlando, FL, April 18, 2023 Poster #22