
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02


Fc - Modified Anti - CD154 Mab Induced Long Term Renal Allograft Survival without Thromboembolic Complications Ryo Otsuka, Grace Lassiter, Takayuki Hirose, Ahmad Karadagi , Toshihide Tomosugi , Ivy Rosales, Tatsuo Kawai Center for Transplantation Sciences Massachusetts General Hospital, Boston, MA, USA

Ryo Otsuka, PhD Research Fellow Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA, USA I have no financial relationships with commercial interests to disclose. AND My presentation does not include discussion of off - label or investigational use.
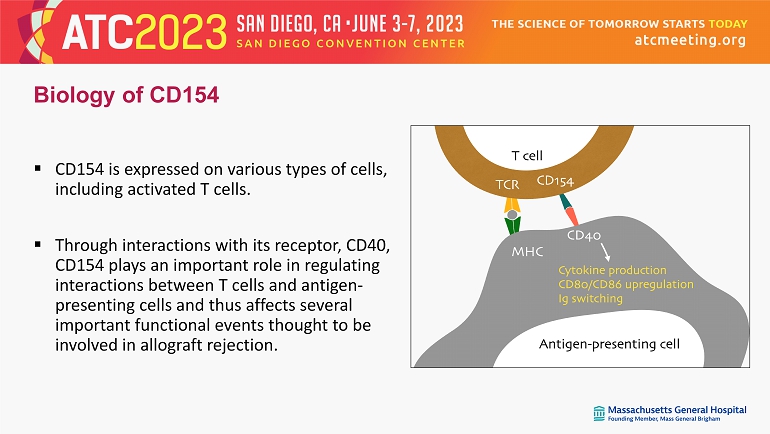
Biology of CD154 ▪ CD154 is expressed on various types of cells, including activated T cells. ▪ Through interactions with its receptor, CD40, CD154 plays an important role in regulating interactions between T cells and antigen - presenting cells and thus affects several important functional events thought to be involved in allograft rejection.
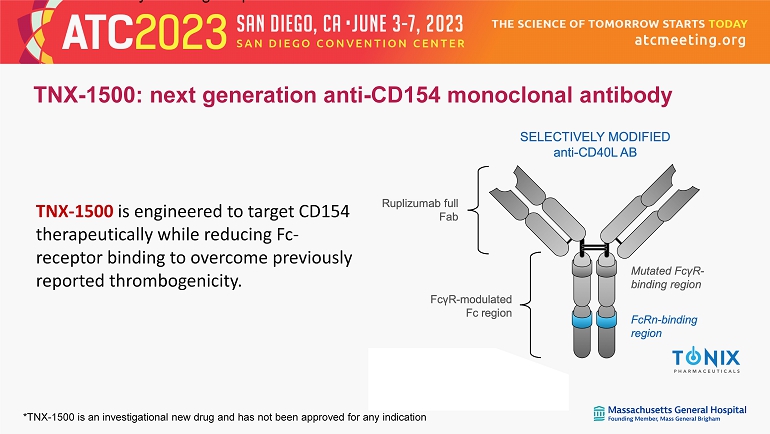
*TNX - 1500 is an investigational new drug and has not been approved for any indication thereby lowering the potential for thrombosis. TNX - 1500: next generation anti - CD154 monoclonal antibody TNX - 1500 is engineered to target CD154 therapeutically while reducing Fc - receptor binding to overcome previously reported thrombogenicity.
Aims of the study In this study, we compared TNX - 1500 with conventional anti - CD154 antibodies in terms of platelet activation in vitro and evaluated the efficacy of TNX - 1500 to prevent kidney allograft rejection in an NHP kidney transplantation model.
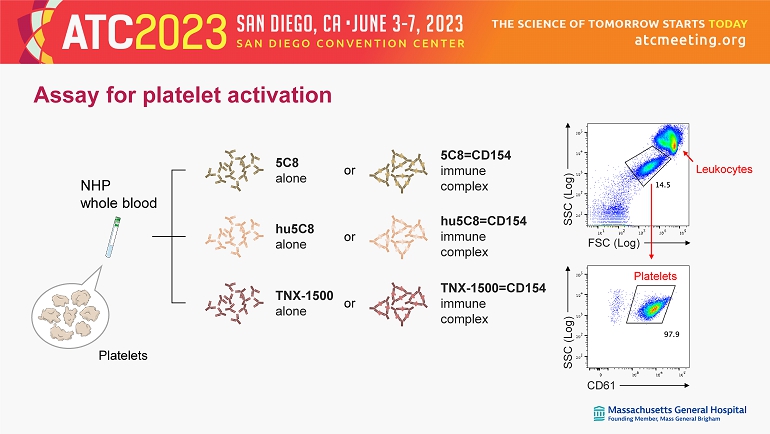
FSC (Log) SSC (Log) CD61 Platelets Leukocytes SSC (Log) 5C8=CD154 immune complex hu5C8=CD154 immune complex TNX - 1500=CD154 immune complex or 5C8 alone hu5C8 alone TNX - 1500 alone or or Assay for platelet activation Platelets NHP whole blood
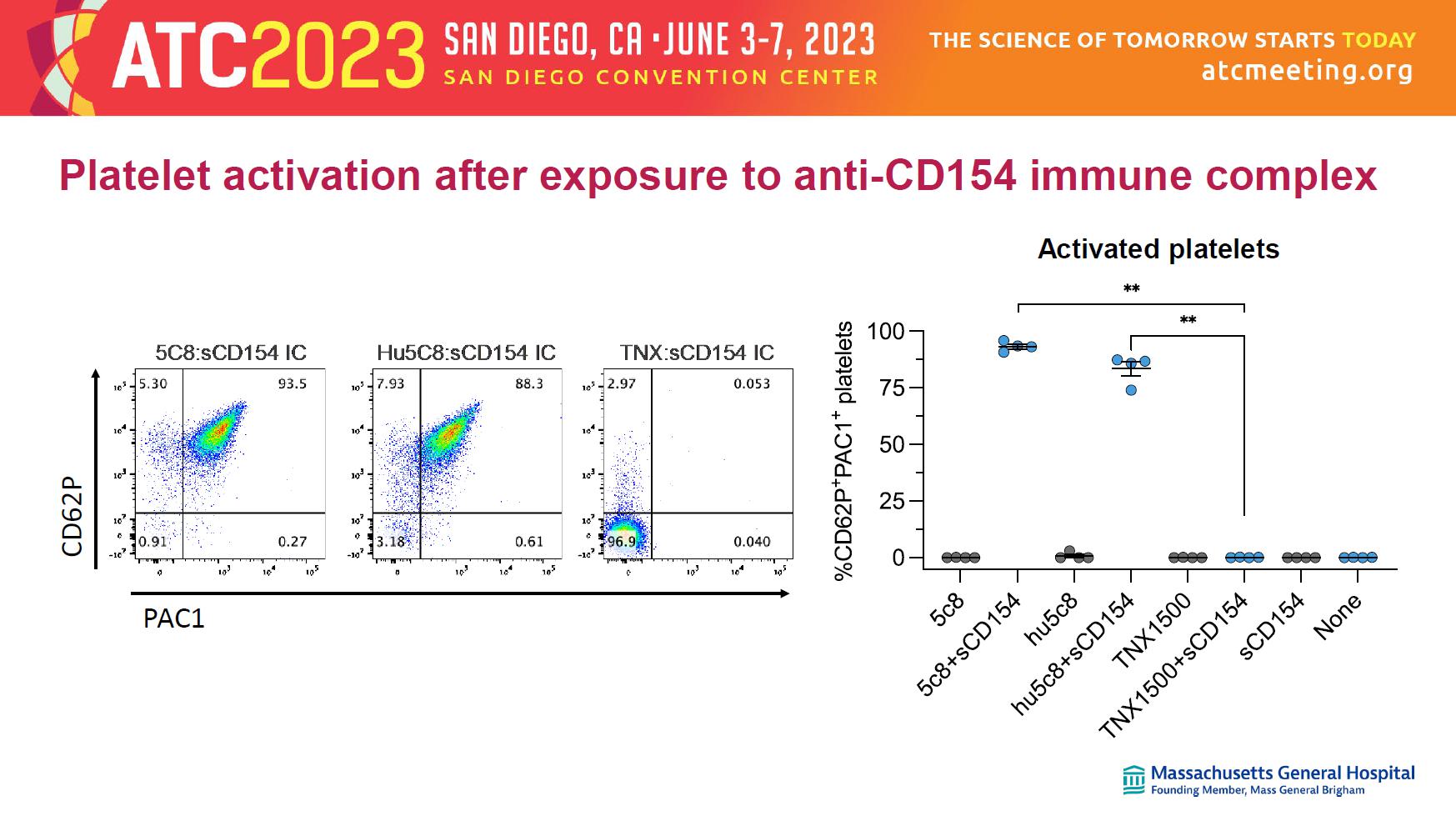
Activated platelets Platelet activation after exposure to anti - CD154 immune complex C D 6 2 P 5C8:sCD154 IC TNX:sCD154 IC PAC-1 Hu5C8:sCD154 IC FSC (Log) S S C ( L o g ) CD61 5C8 TNXHu5C8 PAC1 CD62P
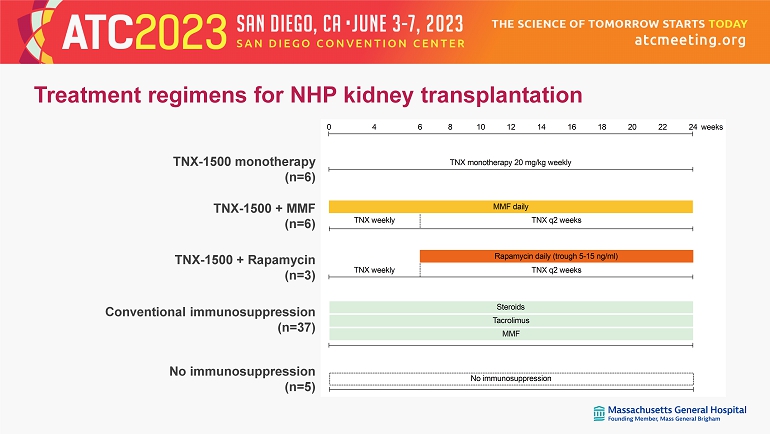
TNX monotherapy 20 mg/kg weekly TNX weekly TNX q2 weeks MMF daily 0 24126 184 108 1614 2220 TNX weekly TNX q2 weeks Rapamycin daily (trough 5-15 ng/ml) weeks Steroids Tacrolimus MMF No immunosuppression TNX - 1500 monotherapy (n=6) TNX - 1500 + MMF (n=6) TNX - 1500 + Rapamycin (n=3) Conventional immunosuppression (n=37) No immunosuppression (n=5) Treatment regimens for NHP kidney transplantation
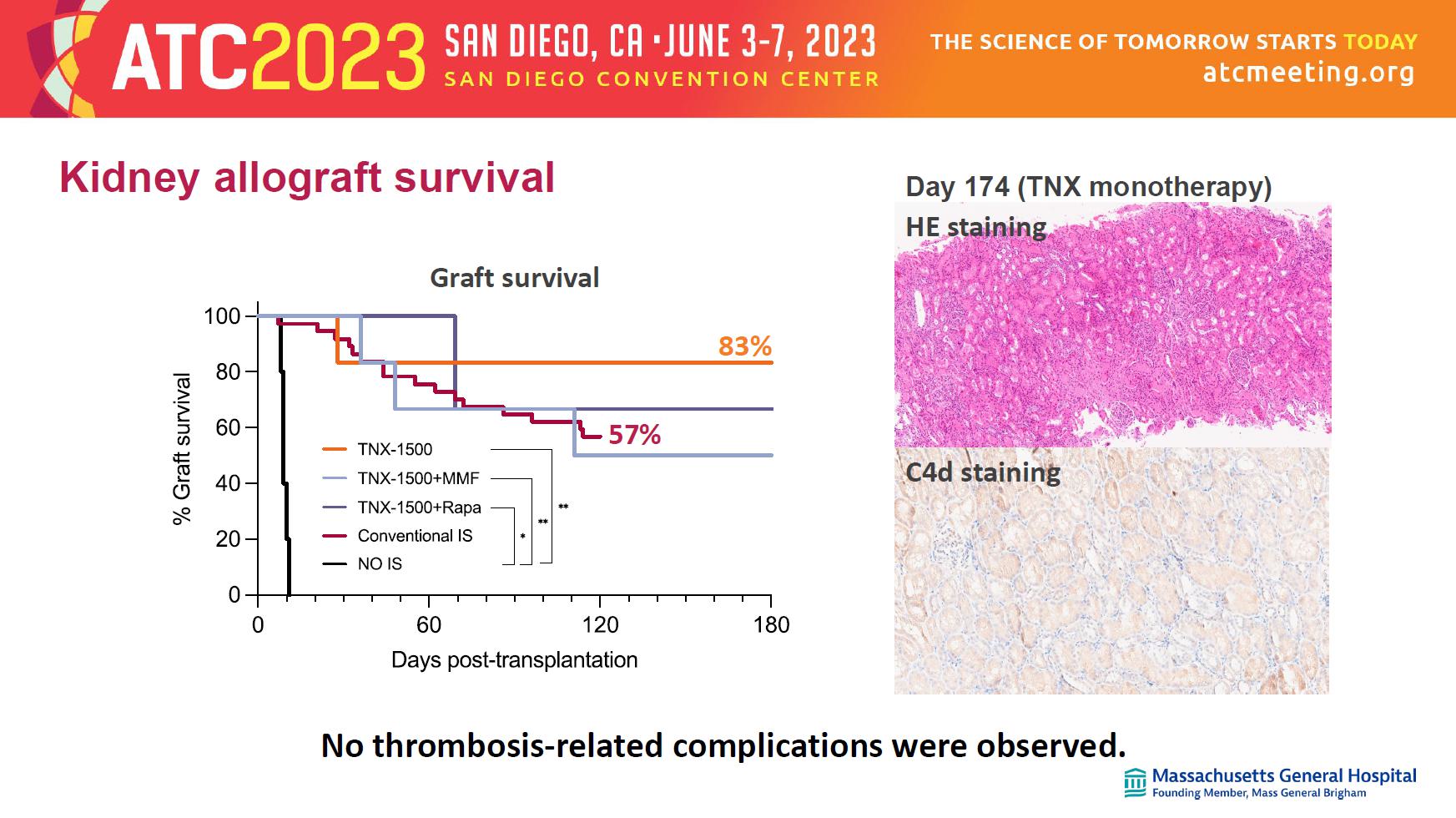
Kidney allograft survival No thrombosis - related complications were observed. Graft survival 83% 57% HE staining C4d staining Day 174 (TNX monotherapy)
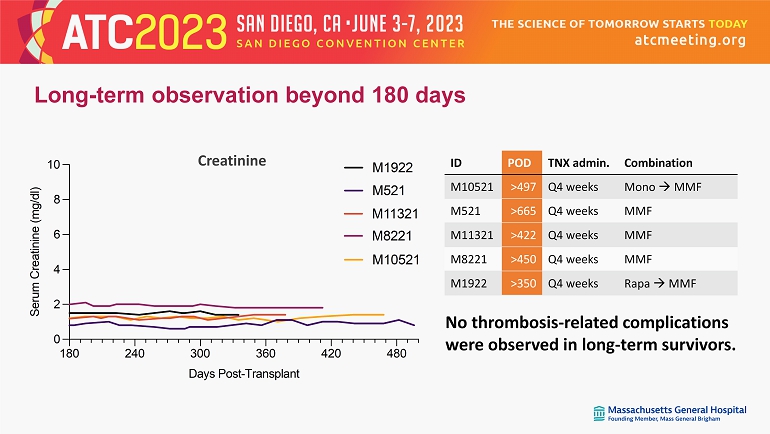
Long - term observation beyond 180 days Combination TNX admin. POD ID Mono MMF Q4 weeks >497 M10521 MMF Q4 weeks >665 M521 MMF Q4 weeks >422 M11321 MMF Q4 weeks >450 M8221 Rapa MMF Q4 weeks >350 M1922 180 240 300 360 420 480 0 2 4 6 8 10 Days Post-Transplant S e r u m C r e a t i n i n e ( m g / d l ) M1922 M521 M8221 M11321 M10521 180 240 300 360 420 480 0 2 4 6 8 10 Days Post-Transplant S e r u m C r e a t i n i n e ( m g / d l ) M1922 M521 M8221 M11321 M10521 No thrombosis - related complications were observed in long - term survivors. Creatinine

Conclusion • TNX - 1500 can be an effective alternative to conventional immunosuppression therapy in kidney transplantation • TNX - 1500 inhibited renal allograft rejection without thromboembolism in NHPs • Optimal dosage for clinical application remains to be clarified • Fc - modification effectively prevented platelet activation

Center for Transplantation Sciences Tatsuo Kawai Grace Lassiter Takayuki Hirose Ahmad Karadagi Toshihide Tomosugi Ashley D’Attilio Andrea Yanulevich Richard Pierson Kohei Kinoshita Abbas Dehnadi Cindy Miller Jane O Samantha Landino James Nawalaniec Dylan Muldoon Jayne Marie Muoio Knight Surgery Research Laboratory Michael Duggan Jessica Burke Anet Calisir Nelson Marquez Carvajal Elijah Smith Erin Marx Carolyn Betty Wike Immunopathology Research Laboratory Robert Colvin Ivy Rosales TONIX Pharmaceuticals Seth Lederman Bruce Daugherty Siobhan Fogarty Center for Comparative Medicine Joanne Morris Diane Chen Jibing Yang CCM Husbandry staff Acknowledgment