Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

Efficacy Of CD154 Blockade With TNX - 1500 To Prevent Heart Allograft Immune Injury I. Ileka 1 , K. Kinoshita 1 , R. Chaban 1 , G. McGrath 1 , Z. Habibabady 1 , C. Miller 1 , J. O 1 , S. Landino 1 , J. Nawalaniec 1 ,S. Fogarty 2 , B. Daugherty 2 , S. Lederman 2 , J. C. Madsen 1 , R. N. Pierson III 1 1 Center for Transplantation Sciences, Massachusetts General Hospital, Boston MA 2 Tonix Pharmaceuticals Inc., Chatham, NJ, USA

Relevant Disclaimers • S. Fogarty, B. Daugherty, and S. Lederman are employees of Tonix Pharmaceuticals Inc. • R. Chaban is supported by the Benjamin Research Fellowship from the German Research Foundation (DFG)
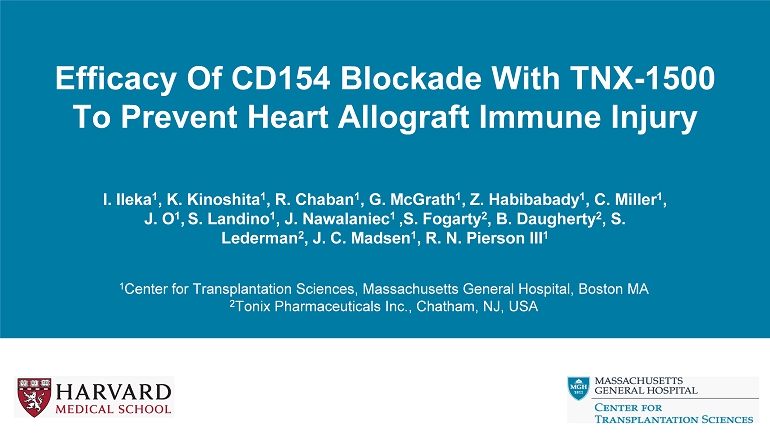
Background: Rationale α CD154 Co - stimulation Blockade Suppresses pathogenic alloimmunity Avoid CNI toxicity Zhang et al. Immunotherapy 2015 MHC + Ag TCR B7 - 1 CTLA - 4 B7 - 2 CD28 CD40 CD154 APC T cell
■ 1 st & 2 nd generations α CD154 mAb prolongs allograft in multiple NHP Txp models (Heart/ Kidney /Islet /Skin) 1 st Gen : Venous, arterial thrombotic events Ruplizumab, IDEC - 131 α CD154/sCD154 ICs activate plts via FcγRIIa 2 nd Gen : Reduced efficacy aglycosyl Ruplizumab Fc - silent domain antibody hu5c8 Background
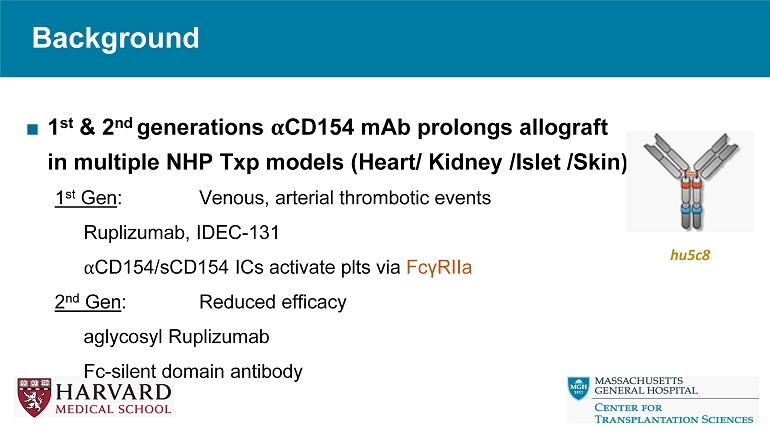
Fc - modified IgG4 FcγRIIa - binding region modified Avoid TE complications FcRN binding retained Ruplizumab Fab binding region Designed for preserved efficacy compared to Gen 2 TNX1500* hu5c8 Full Fab FcγRIIa - modulated Fc region Mutated FcγRIIa - binding region (prevent IC) FcRn - binding region (preserve efficacy) *TNX - 1500 is an investigational new biologic and has not been approved for any indication ■ 3 rd generation α CD154 mAb Background: TNX 1500
Study design 25 cyno heterotopic abdominal heart allografts – TNX monoRx (n=17) through EOS d120 (n=12) or d180 (n=5) 30mg/kg on days 0, 3, 7 and 14; then 20mg/kg/wk – TNX+additional Rx until EOS at d180 TNX+MMF (40mg/kg/d; n=4) TNX+Rapa (5 - 10ng/ml target trough; n=4)
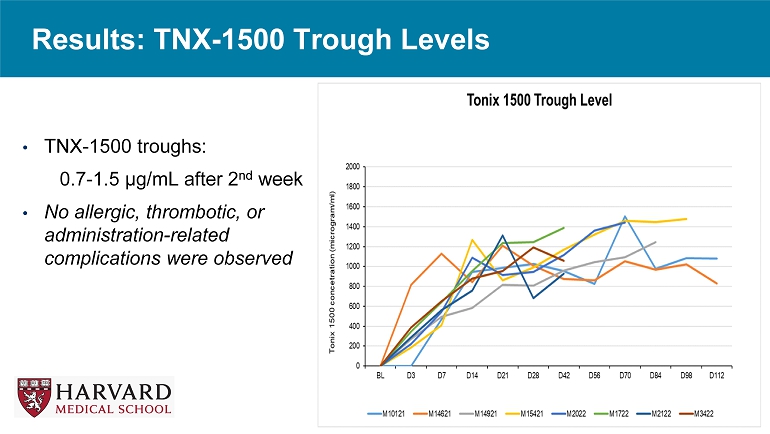
Results: TNX - 1500 Trough Levels • TNX - 1500 troughs: 0.7 - 1.5 µg/mL after 2 nd week • No allergic, thrombotic, or administration - related complications were observed
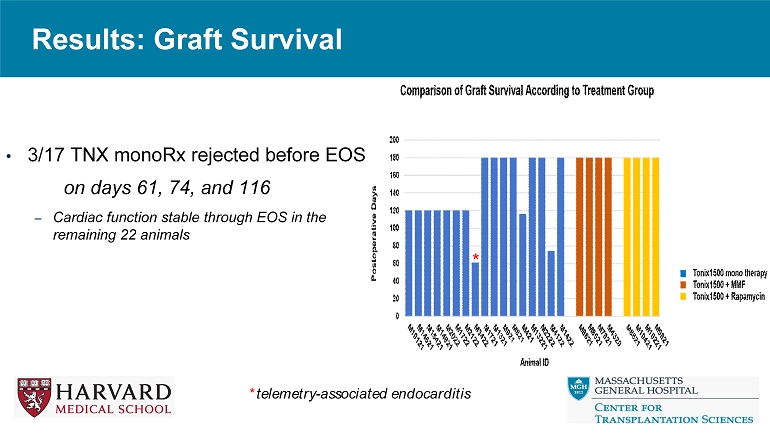
Results: Graft Survival • 3/17 TNX monoRx rejected before EOS on days 61, 74, and 116 – Cardiac function stable through EOS in the remaining 22 animals * telemetry - associated endocarditis *
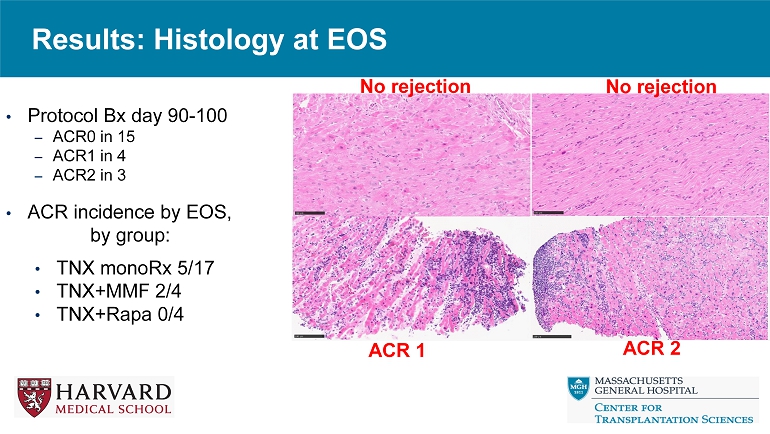
Results: Histology at EOS • Protocol Bx day 90 - 100 – ACR0 in 15 – ACR1 in 4 – ACR2 in 3 • ACR incidence by EOS, by group: • TNX monoRx 5/17 • TNX+MMF 2/4 • TNX+Rapa 0/4 ACR 1 ACR 2 No rejection No rejection
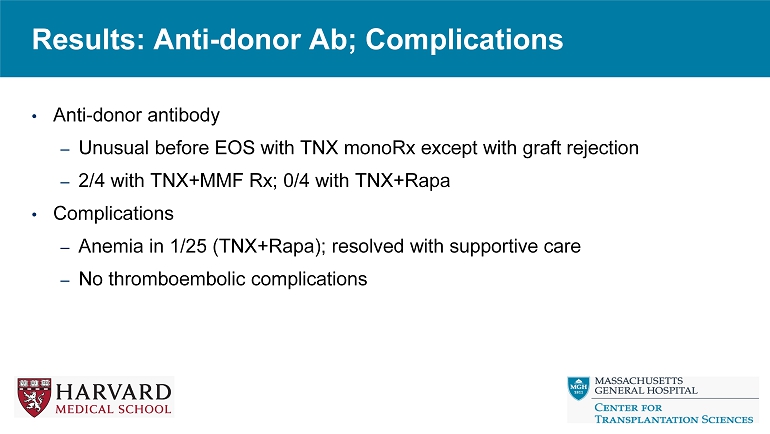
• Anti - donor antibody – Unusual before EOS with TNX monoRx except with graft rejection – 2/4 with TNX+MMF Rx; 0/4 with TNX+Rapa • Complications – Anemia in 1/25 (TNX+Rapa); resolved with supportive care – No thromboembolic complications Results: Anti - donor Ab; Complications
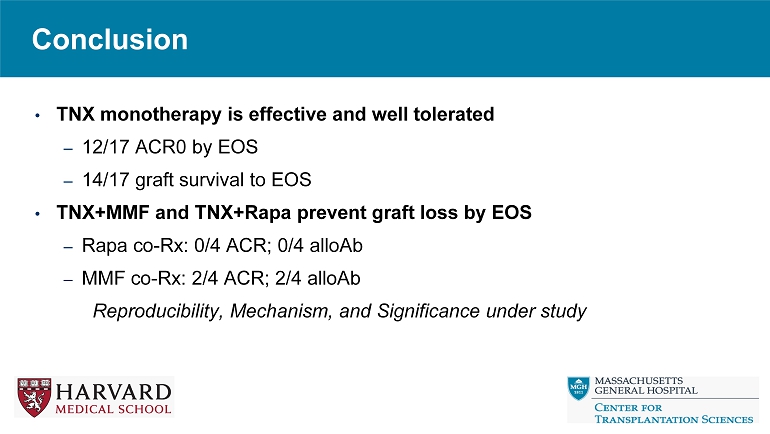
• TNX monotherapy is effective and well tolerated – 12/17 ACR0 by EOS – 14/17 graft survival to EOS • TNX+MMF and TNX+Rapa prevent graft loss by EOS – Rapa co - Rx: 0/4 ACR; 0/4 alloAb – MMF co - Rx: 2/4 ACR; 2/4 alloAb Reproducibility, Mechanism, and Significance under study Conclusion

Thank you