UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of report (date of earliest event reported):
(Exact name of registrant as specified in its charter)
|
(State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area
code:
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| The |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
On September 5, 2023, Tonix Pharmaceuticals Holding Corp. (the “Company”) announced topline results from the Phase 2 proof-of-concept PREVAIL study of its TNX-102 SL (cyclobenzaprine HCl sublingual tablet) 5.6 mg product candidate for the management of fibromyalgia-type Long COVID. A copy of the press release which discusses this matter is furnished hereto as Exhibit 99.01, and incorporated herein by reference.
The information in this Item 7.01 of this Current Report on Form 8-K, including Exhibit 99.01 attached hereto, shall not be deemed “filed” for purposes of Section 18 of the United States Securities Exchange Act of 1934 (the “Exchange Act”) or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the United States Securities Act of 1933 or the Exchange Act, except as shall be expressly set forth by specific reference in such a filing.
| Item 8.01. | Other Events. |
On September 5, 2023, the Company announced topline results from the Phase 2 proof-of-concept PREVAIL study of TNX-102 SL 5.6 mg for the management of fibromyalgia-type Long COVID. TNX-102 SL treatment showed a robust effect size (“ES”) in improving fatigue and showed consistent activity trending to improvements across the secondary endpoints of sleep quality, cognitive function, disability and patient global impression of change (“PGIC”). TNX-102 SL trended towards improvement but did not achieve the pre-specified primary endpoint of improving Long COVID pain intensity scores at Week 14.
The PREVAIL study was designed and conducted to guide the design of registrational studies of TNX-102 SL in fibromyalgia-type Long COVID. TNX-102 SL was generally well tolerated with an adverse event (“AE”) profile comparable to prior studies with TNX-102 SL. AE-related discontinuations were similar in drug and placebo arms. No new safety signals were observed.
The Company believes that the robust activity of TNX-102 SL on the PROMIS Fatigue scale (ES=0.5) is important because patients and experts view fatigue as the signature symptom of Long COVID, and it has been identified as the dominant symptom contributing to disability. In addition, TNX-102 SL showed consistent trends toward improvement in sleep quality, cognitive function, disability and the PGIC responder rate for TNX-102 SL compared to placebo at week 14 (34.4% vs. 16.1%, difference=18.2%). Together, these findings fulfill the objectives of this proof-of-concept study in supporting the decision to advance the program based on a proposed primary endpoint using the PROMIS Fatigue scale.
The Company intends to request an End-of-Phase 2 meeting with the U.S. Food and Drug Administration (“FDA”) to discuss a potential Phase 3 program based on a proposed primary outcome measure using the PROMIS Fatigue scale. The meeting is expected to take place in the first quarter of 2024. The Company believes that the PREVAIL trial results may guide the next phase of development for TNX-102 SL, supporting the design of a potential registrational trial for fibromyalgia-type Long COVID based on PROMIS fatigue as a primary endpoint, pending review and feedback from the FDA. In both prior Phase 3 studies of TNX-102 SL 5.6 mg in fibromyalgia, the Company observed numerical improvement in the PROMIS fatigue score (in RELIEF p=0.007 MMRM and in RALLY p=0.007 MMRM).”
Key Phase 2 PREVAIL Study Results
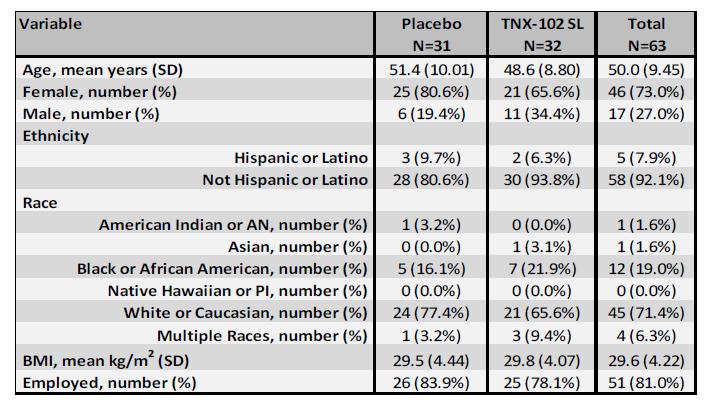
In the study, 63 subjects were enrolled and randomized 1:1 across approximately 30 U.S. sites to receive either TNX-102 SL or placebo daily at bedtime for 14 weeks. Subjects started with TNX-102 SL 2.8 mg tablet or one placebo tablet for the first 2 weeks and then increased to TNX-102 SL 5.6 mg (2 x 2.8 mg tablets) or two placebo tablets for the remaining 12 weeks of the treatment period. The percentage of subjects completing the study was 81.3% in the TNX-102 SL group and 80.6% in the placebo group. Demographics and baseline characteristics are shown in Table 1.
|
Table 1: Demographics and Baseline Characteristics |

|
Abbreviations: AN, Alaskan Native; BMI, body mass index; PI, Pacific Islander; SD, standard deviation |
Primary endpoint
Secondary endpoints
Safety profile
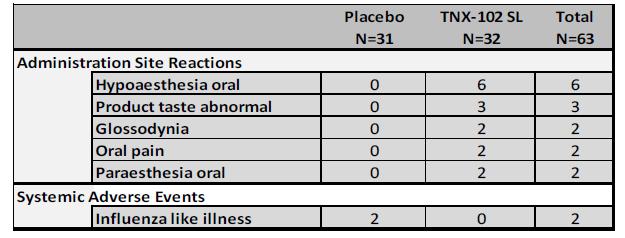
TNX-102 SL demonstrated a favorable safety and tolerability profile over 14 weeks of treatment with no new safety signals. The most common adverse events are shown in Table 2. Participants with at least one treatment-emergent adverse event (“TEAE”) were at a rate of 56.3% on TNX-102 SL and 38.7% on placebo. In the TNX-102 SL group, 6.3% discontinued due to TEAE compared to 9.7% on placebo. Only one TEAE in the study was rated as severe, gastritis in a participant in the TNX-102 SL group. There were no serious adverse events (“SAEs”) in the study.
|
Table 2: Adverse Events Occurring in ≥ 2 Participants in Either Treatment Group |
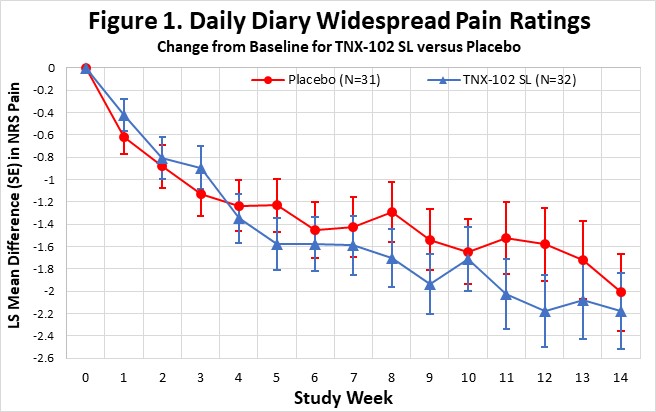
Abbreviations: LS, least squares; SE, standard error
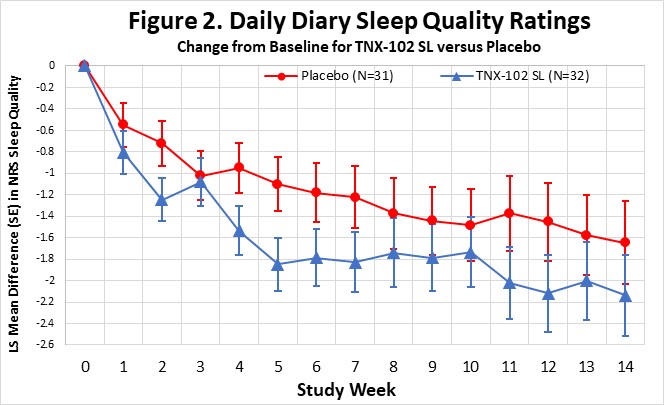
Abbreviations: LS, least squares; SE, standard error
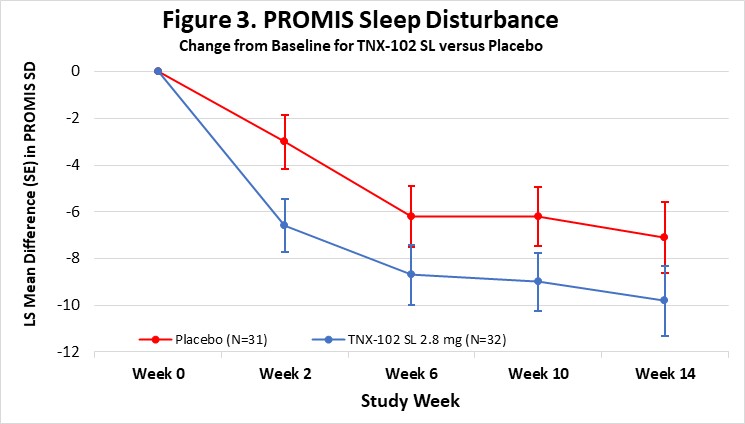
Abbreviations: LS, least squares; SE, standard error; SD, sleep disturbance
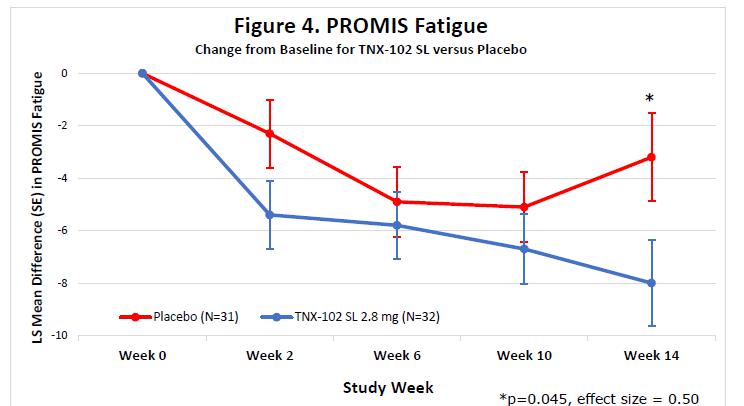
Abbreviations: LS, least squares; SE, standard error
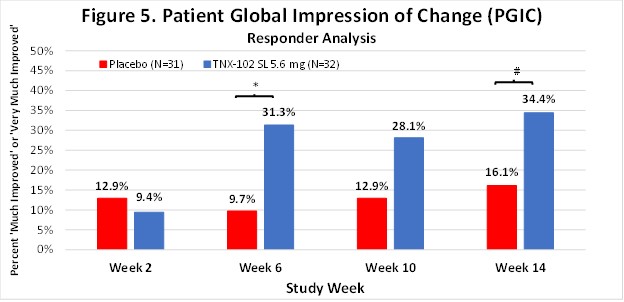
*p=0.034, #p=0.096
Forward- Looking Statements
This Current Report on Form 8-K contains certain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and Private Securities Litigation Reform Act, as amended, including those relating to the Company’s product development, clinical trials, clinical and regulatory timelines, market opportunity, competitive position, possible or assumed future results of operations, business strategies, potential growth opportunities and other statement that are predictive in nature. These forward-looking statements are based on current expectations, estimates, forecasts and projections about the industry and markets in which we operate and management’s current beliefs and assumptions.
These statements may be identified by the use of forward-looking expressions, including, but not limited to, “expect,” “anticipate,” “intend,” “plan,” “believe,” “estimate,” “potential,” “predict,” “project,” “should,” “would” and similar expressions and the negatives of those terms. These statements relate to future events or our financial performance and involve known and unknown risks, uncertainties, and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such factors include those set forth in the Company’s filings with the SEC. Prospective investors are cautioned not to place undue reliance on such forward-looking statements, which speak only as of the date of this press release. The Company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) |
Exhibit No. |
Description. | ||
|
104 |
Press Release of the Company, dated September 5, 2023 Cover Page Interactive Data File (embedded within the Inline XBRL document) |
SIGNATURE
Pursuant to the requirement of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| TONIX PHARMACEUTICALS HOLDING CORP. | |||
| Date: September 5, 2023 | By: | /s/ Bradley Saenger | |
| Bradley Saenger | |||
| Chief Financial Officer | |||