
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

© 2023 Tonix Pharmaceuticals Holding Corp. INVESTOR PRESENTATION NASDAQ: TNXP Version P0484 September 14, 2023 (Doc 1314)

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward-Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward-looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward-looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward-looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward-looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; risks related to the failure to successfully market any of our products; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward-looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward-looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10-K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward-looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 © 2023 Tonix Pharmaceuticals Holding Corp. Investment Highlights RICH PIPELINE OF THERAPEUTICS CANDIDATES IN DEVELOPMENT Tonix’s core focus is on central nervous system disorders, but we also target unmet needs across multiple therapeutic areas including immunology, infectious disease and rare disease. STRATEGIC PARTNERSHIPS Partnering strategically with other biotech companies, world-class academic and non-profit research organizations to bring innovative therapeutics to market faster. IN-HOUSE CAPABILITIES Internal capabilities in R&D and biologics process development and GMP manufacturing to accelerate development timelines. MARKETED PRODUCTS Tonix Medicines markets two FDA-approved products Zembrace ® SymTouch ® (sumatriptan injection) and Tosymra ® (sumatriptan nasal spray) for the treatment of acute migraine in adults with or without aura 1 ZembraceSymTouch[packageinsert].MapleGrove,MN:Upsher-SmithLaboratories,LLC:February2021-Formoreinformation,talktoyourproviderandreadthePatientInformationand InstructionsforUse.–ImportantSafetyInformationisprovidedintheappendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, LLC: Feb 2021 .For more information, talk to your provider and read the Patient Informationand Instructionsfor Use.– Important Safety Information is provided in the appendix Zembrace, SymTouchand Tosymraare registered trademarks of Tonix Medicines. Intravailis a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis, Inc.
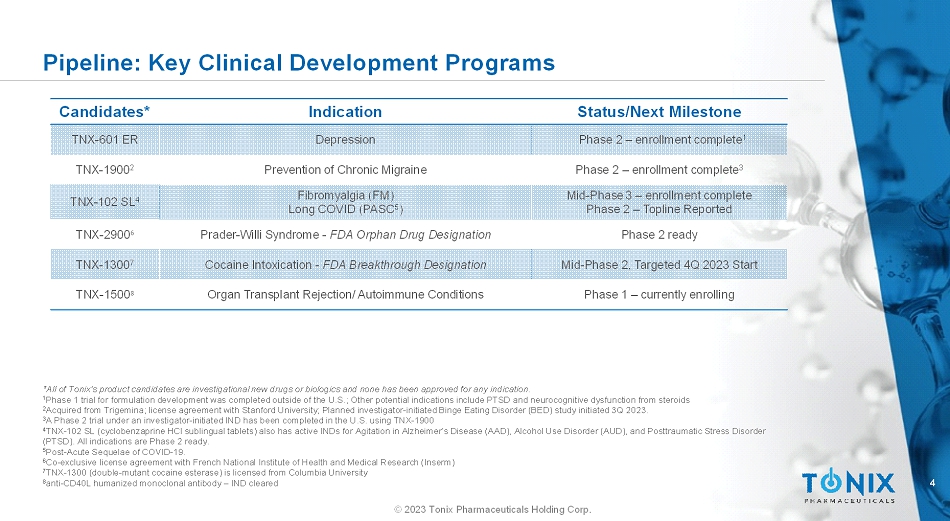
4 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Clinical Development Programs Status/Next MilestoneIndicationCandidates* Phase 2 –enrollment complete 1 DepressionTNX-601 ER Phase 2 –enrollment complete 3 Prevention of Chronic MigraineTNX-1900 2 Mid-Phase3 –enrollment complete Phase 2 –Topline Reported Fibromyalgia (FM) Long COVID (PASC 5 ) TNX-102 SL 4 Phase 2 readyPrader-Willi Syndrome -FDA Orphan Drug DesignationTNX-2900 6 Mid-Phase 2, Targeted 4Q 2023 StartCocaine Intoxication -FDA Breakthrough DesignationTNX-1300 7 Phase 1 –currently enrollingOrgan Transplant Rejection/ Autoimmune ConditionsTNX-1500 8 *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. 1 Phase 1 trial for formulation development was completed outside of the U.S.; Other potential indications include PTSD and neurocognitive dysfunction from steroids 2 Acquired from Trigemina; license agreement with Stanford University; Planned investigator-initiated Binge Eating Disorder (BED) study initiated 3Q 2023. 3 A Phase 2 trial under an investigator-initiated IND has been completed in the U.S. using TNX-1900 4 TNX-102 SL (cyclobenzaprine HCl sublingual tablets) also has active INDs for Agitation in Alzheimer’s Disease (AAD), Alcohol UseDisorder (AUD), and Posttraumatic Stress Disorder (PTSD). All indications are Phase 2 ready. 5 Post-Acute Sequelae of COVID-19. 6 Co-exclusive license agreement with French National Institute of Health and Medical Research (Inserm) 7 TNX-1300 (double-mutant cocaine esterase) is licensed from Columbia University 8 anti-CD40L humanized monoclonal antibody –IND cleared
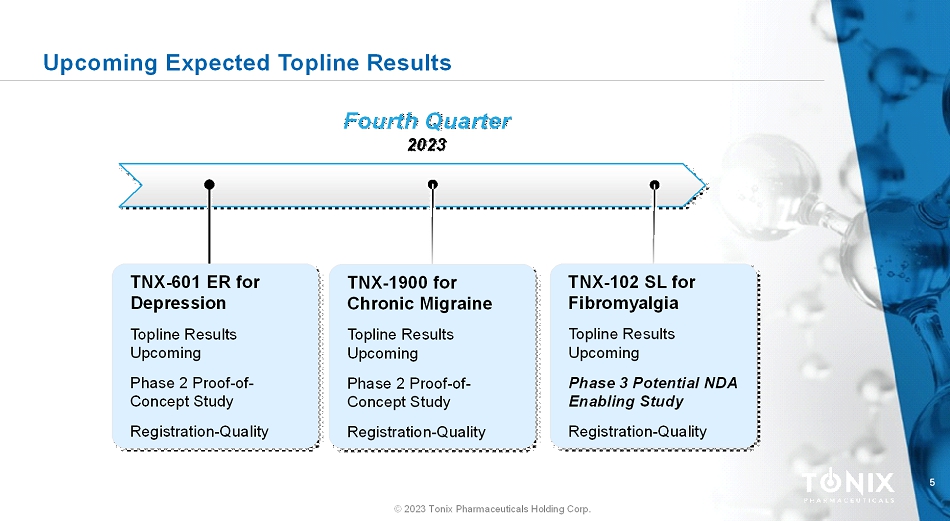
5 © 2023 Tonix Pharmaceuticals Holding Corp. Upcoming Expected Topline Results Fourth Quarter 2023 TNX-1900 for Chronic Migraine Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-601 ER for Depression Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-102 SL for Fibromyalgia ToplineResults Upcoming Phase 3 Potential NDA Enabling Study Registration-Quality

© 2023 Tonix Pharmaceuticals Holding Corp. TONIXMEDICINES: MARKETED PRODUCTS
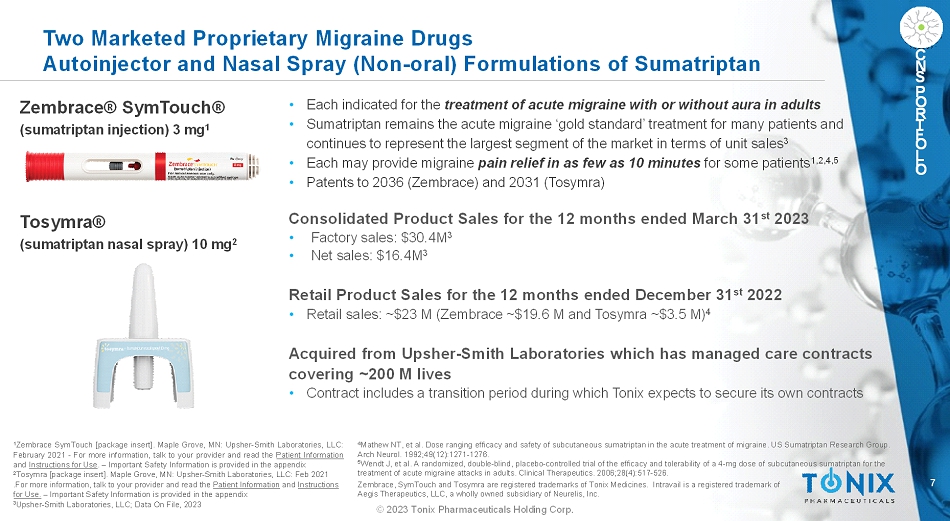
7 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Two Marketed Proprietary Migraine Drugs Autoinjector and Nasal Spray (Non-oral) Formulations of Sumatriptan • Each indicated for the treatment of acute migraine with or without aura in adults • Sumatriptan remains the acute migraine ‘gold standard’ treatment for many patients and continues to represent the largest segment of the market in terms of unit sales 3 • Each may provide migraine pain relief in as few as 10 minutes for some patients 1,2,4,5 • Patents to 2036 (Zembrace) and 2031 (Tosymra) 1 ZembraceSymTouch[packageinsert].MapleGrove,MN:Upsher-SmithLaboratories,LLC: February2021-Formoreinformation,talktoyourproviderandreadthePatientInformation andInstructionsforUse.–ImportantSafetyInformationisprovidedintheappendix 2 Tosymra [package insert]. Maple Grove, MN: Upsher-Smith Laboratories, LLC: Feb 2021 .For more information, talk to your provider and read the Patient Informationand Instructions for Use.–Important Safety Information is provided in the appendix 3 Upsher-Smith Laboratories, LLC; Data On File, 2023 Zembrace® SymTouch® (sumatriptan injection) 3 mg 1 Tosymra® (sumatriptan nasal spray) 10 mg 2 Consolidated Product Sales for the 12 months ended March 31 st 2023 • Factory sales: $30.4M 3 • Net sales: $16.4M 3 Retail Product Sales for the 12 months ended December 31 st 2022 • Retail sales: ~$23 M (Zembrace ~$19.6 M and Tosymra ~$3.5 M) 4 Acquired from Upsher-Smith Laboratories which has managed care contracts covering ~200 M lives • Contract includes a transition period during which Tonix expects to secure its own contracts 4 Mathew NT, et al. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. US Sumatriptan Research Group. Arch Neurol. 1992;49(12):1271-1276. 5 Wendt J, et al. A randomized, double-blind, placebo-controlled trial of the efficacy and tolerability of a 4-mg dose of subcutaneous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics. 2006;28(4):517-526. Zembrace, SymTouchand Tosymraare registered trademarks of Tonix Medicines. Intravailis a registered trademark of Aegis Therapeutics, LLC, a wholly owned subsidiary of Neurelis, Inc.
8 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Administration of Zembraceand TosymraBypass the GI Tract Bypassing the gastrointestinal (GI) tract is a potential advantage for treating acute migraine - Potential to provide a treatment option for migraines complicated by severe nausea and vomiting Need for acute non-oral treatments - GI absorption may be inconsistent in migraineurs due to gastric stasis (also called “gastroparesis”) 1-4 - Nausea and vomiting are symptoms of migraine 5 Existing intranasal products - Imitrex® nasal spray (sumatriptan) - Migranal® (dihydroergotamine) nasal spray –developed by Novartis, sold by Bausch Health New intranasal products bring attention to this non-oral route - Pfizer’s Zavzpret® (zavegepant), FDA approved in March, 2023 1 is the first intranasal gepant - Impel NeuroPharma’sTrudhesa® (dihydroergotamine) FDA approved 2021 2 - Precision Olfactory Delivery (POD) technology targets vascular-rich upper nasal space 1 Pfizer Press Release March 10, 2023. –https://www.pfizer.com/news/press-release/press-release-detail/pfizers-zavzprettm-zavegepant-migraine-nasal-spray 2 Impel Press Release September 3, 2021 -https://impelpharma.com/2021/09/03/impel-neuropharma-announces-u-s-fda-approval-of-trudhesa-dihydroergotamine-mesylate-nasal-spray-for- the-acute-treatment-of-migraine/

© 2023 Tonix Pharmaceuticals Holding Corp. CNS: KEY DEVELOPMENT CANDIDATES
10 © 2023 Tonix Pharmaceuticals Holding Corp. Upcoming Expected Topline Results Fourth Quarter 2023 TNX-1900 for Chronic Migraine Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-601 ER for Depression Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-102 SL for Fibromyalgia ToplineResults Upcoming Phase 3 Potential NDA Enabling Study Registration-Quality
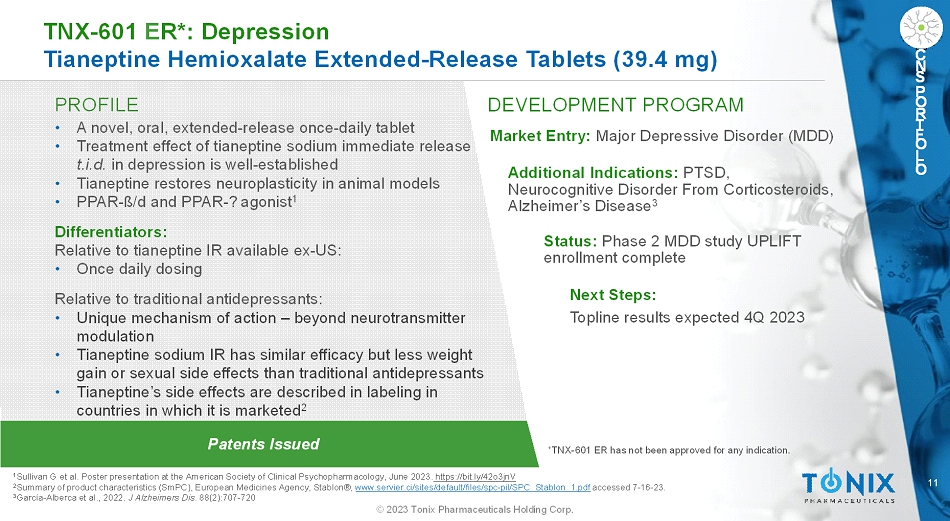
11 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PROFILE DEVELOPMENT PROGRAM Patents Issued TNX-601 ER*: Depression Tianeptine HemioxalateExtended-Release Tablets (39.4 mg) C N S P O R T F O L I O • A novel, oral, extended-release once-daily tablet • Treatment effect of tianeptine sodium immediate release t.i.d.in depression is well-established • Tianeptine restores neuroplasticity in animal models • PPAR-ß/dand PPAR-?agonist 1 Differentiators: Relative to tianeptine IR available ex-US: • Once daily dosing Relative to traditional antidepressants: • Unique mechanism of action –beyond neurotransmitter modulation • Tianeptine sodium IR has similar efficacy but less weight gain or sexual side effects than traditional antidepressants • Tianeptine’s side effects are described in labeling in countries in which it is marketed 2 Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 3 Status:Phase 2 MDD study UPLIFT enrollment complete Next Steps: Topline results expected 4Q 2023 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023.https://bit.ly/42o3jnV 2 Summary of product characteristics (SmPC), European Medicines Agency, Stablon®, www.servier.ci/sites/default/files/spc-pil/SPC_Stablon_1.pdfaccessed 7-16-23. 3 García-Alberca et al., 2022. J Alzheimers Dis. 88(2):707-720 *TNX-601 ER has not been approved for any indication.
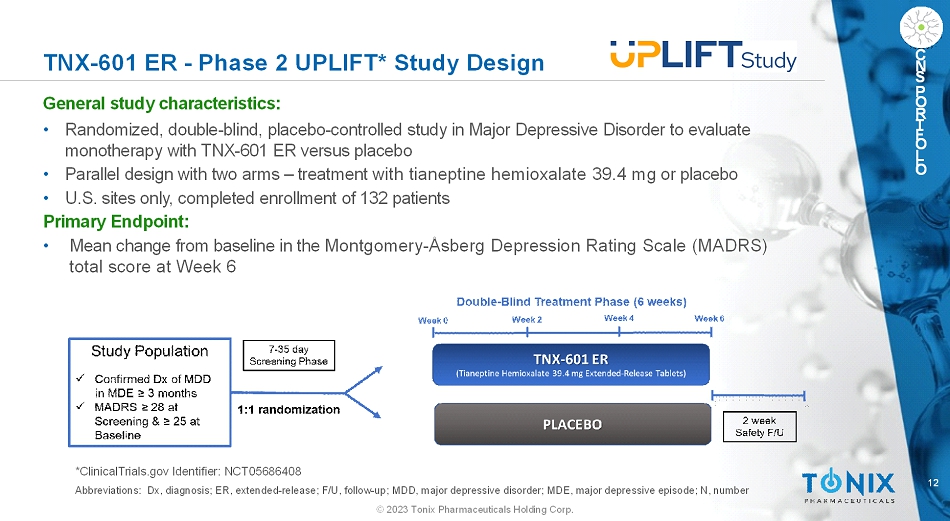
12 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O TNX-601 ER -Phase 2 UPLIFT* Study Design General study characteristics: • Randomized, double-blind, placebo-controlled study in Major Depressive Disorder to evaluate monotherapy with TNX-601 ER versus placebo • Parallel design with two arms –treatment with tianeptine hemioxalate 39.4 mgor placebo • U.S. sites only, completed enrollment of 132 patients Primary Endpoint: • Mean change from baseline in the Montgomery-ÅsbergDepression Rating Scale (MADRS) total score at Week 6 *ClinicalTrials.gov Identifier: NCT05686408 Abbreviations: Dx, diagnosis; ER, extended-release; F/U, follow-up; MDD, major depressive disorder; MDE, major depressive episode; N, number
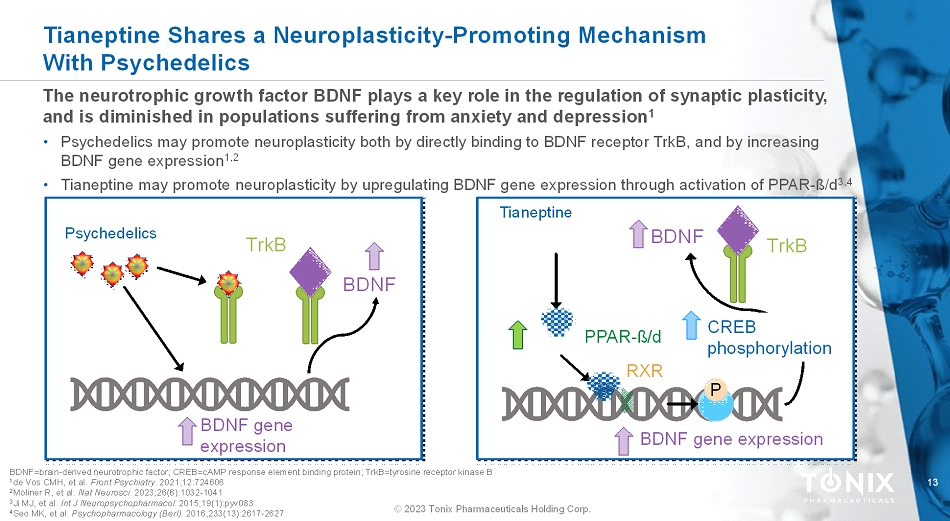
13 © 2023 Tonix Pharmaceuticals Holding Corp. The neurotrophicgrowth factor BDNF plays a key role in the regulation of synaptic plasticity, and is diminished in populations suffering from anxiety and depression 1 • Psychedelics may promote neuroplasticity both by directly binding to BDNF receptor TrkB, and by increasing BDNF gene expression 1,2 • Tianeptine may promote neuroplasticity by upregulating BDNF gene expression through activation of PPAR-ß/d 3,4 Tianeptine Shares a Neuroplasticity-Promoting Mechanism With Psychedelics BDNF=brain-derived neurotrophic factor; CREB=cAMP response element binding protein; TrkB=tyrosine receptor kinase B 1 de Vos CMH, et al. Front Psychiatry. 2021;12:724606 2 Moliner R, et al. Nat Neurosci. 2023;26(6):1032-1041 3 Ji MJ, et al. Int J Neuropsychopharmacol. 2015;19(1):pyv083 4 Seo MK, et al. Psychopharmacology (Berl). 2016;233(13):2617-2627 Tianeptine PPAR-ß/d RXR P BDNF BDNF gene expression CREB phosphorylation TrkB Psychedelics BDNF BDNF gene expression TrkB
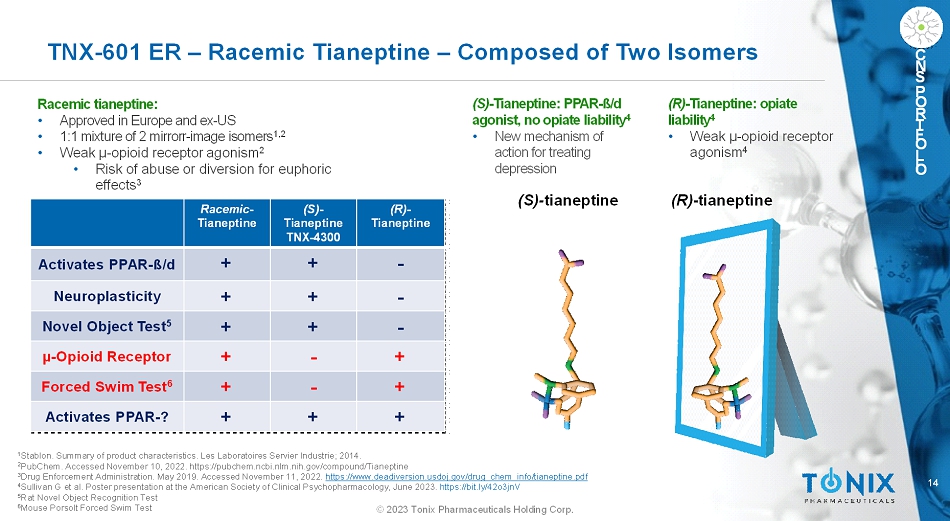
14 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O TNX-601 ER –Racemic Tianeptine –Composed of Two Isomers Racemic tianeptine: • Approved in Europe and ex-US • 1:1 mixture of 2 mirrorr-image isomers 1,2 • Weak µ-opioid receptor agonism 2 • Risk of abuse or diversion for euphoric effects 3 (S)-tianeptine (R)-tianeptine 1 Stablon. Summary of product characteristics. Les LaboratoiresServierIndustrie; 2014. 2 PubChem. Accessed November 10, 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Tianeptine 3 Drug Enforcement Administration. May 2019. Accessed November 11, 2022. https://www.deadiversion.usdoj.gov/drug_chem_info/tianeptine.pdf 4 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023.https://bit.ly/42o3jnV 5 Rat Novel Object Recognition Test 6 Mouse PorsoltForced Swim Test (R)- Tianeptine (S)- Tianeptine TNX-4300 Racemic- Tianeptine -++ Activates PPAR-ß/d -++ Neuroplasticity -++ Novel Object Test 5 +-+ µ-Opioid Receptor +-+ Forced Swim Test 6 +++ Activates PPAR-? (S)-Tianeptine: PPAR-ß/d agonist, no opiate liability 4 • New mechanism of action for treating depression (R)-Tianeptine: opiate liability 4 • Weak µ-opioid receptor agonism 4
15 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PROFILE DEVELOPMENT PROGRAM Patents Issued TNX-4300*: Depression, Alzheimer’s & Parkinson’s diseases Estianeptine(Single (S)-isomer of Tianeptine) C N S P O R T F O L I O • Single isomer, oral treatment • Proposed mechanism of action from lab studies indicates estianeptineis the active ingredient of TNX-601 ER 1 • PPAR-ß/dand PPAR-?agonist • Free of µ-opioid receptor activity • Estianeptinerestores neuroplasticity in tissue culture Differentiators: Relative to racemic tianeptine IR or TNX-601 ER: • Lack of opioid liability Relative to traditional antidepressants: • Unique mechanism of action–beyond neurotransmitter modulation • Racemic tianeptine sodium IR has similar efficacy but fewer side effects than traditional antidepressants Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 2 Status:Pre-clinical Next Steps:Expect IND can be supported by pre-clinical and clinical data from TNX-601 (racemic tianeptine) development 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023.https://bit.ly/42o3jnV 2 García-Alberca et al., 2022. J Alzheimers Dis. 88(2):707-720 *TNX-4300 is in the pre-IND stage of development and has not been approved for any indication
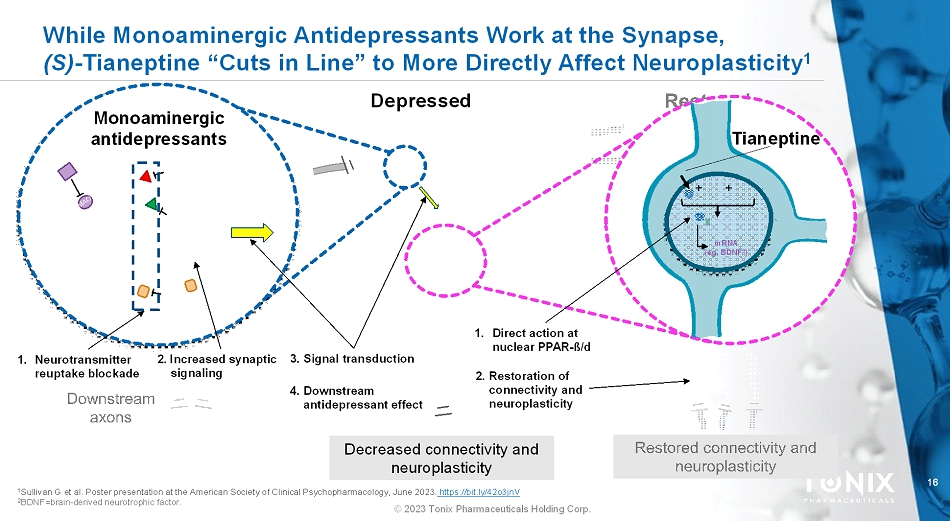
16 © 2023 Tonix Pharmaceuticals Holding Corp. While Monoaminergic Antidepressants Work at the Synapse, (S)-Tianeptine “Cuts in Line” to More Directly Affect Neuroplasticity 1 Depressed Decreased connectivity and neuroplasticity Tianeptine + + mRNA (eg, BDNF 2 ) 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023.https://bit.ly/42o3jnV 2 BDNF=brain-derived neurotrophic factor. Monoaminergic antidepressants 1. Neurotransmitter reuptake blockade 1. Direct action at nuclear PPAR-ß/d 2. Increased synaptic signaling 3. Signal transduction 4. Downstream antidepressant effect 2. Restoration of connectivity and neuroplasticity
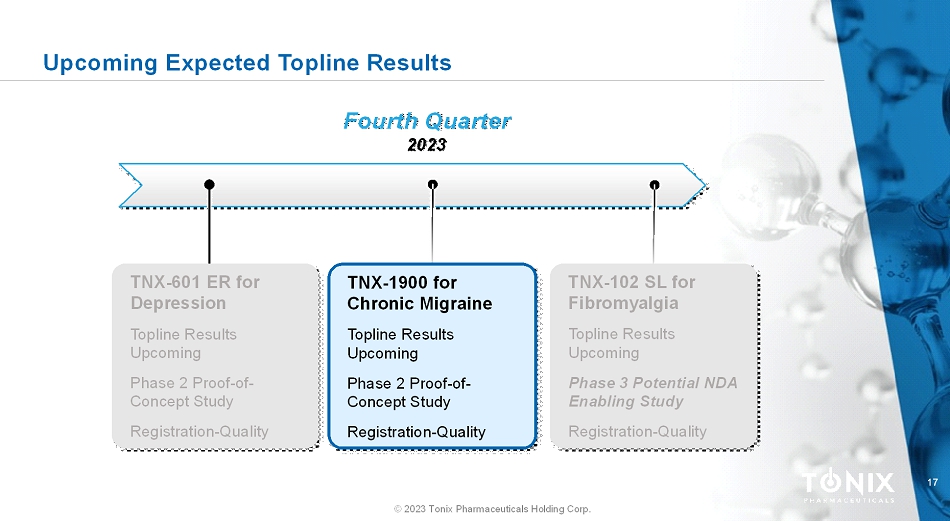
17 © 2023 Tonix Pharmaceuticals Holding Corp. Upcoming Expected Topline Results Fourth Quarter 2023 TNX-1900 for Chronic Migraine Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-601 ER for Depression Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-102 SL for Fibromyalgia ToplineResults Upcoming Phase 3 Potential NDA Enabling Study Registration-Quality
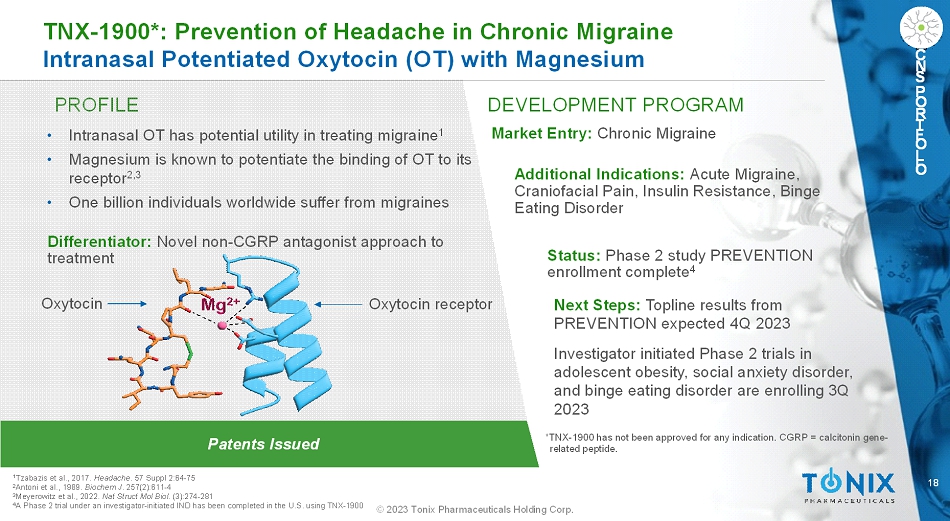
18 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PROFILE DEVELOPMENT PROGRAM Patents Issued TNX-1900*: Prevention of Headache in Chronic Migraine Intranasal Potentiated Oxytocin (OT) with Magnesium C N S P O R T F O L I O • Intranasal OT has potential utility in treating migraine 1 • Magnesium is known to potentiate the binding of OT to its receptor 2,3 • One billion individuals worldwide suffer from migraines Differentiator: Novel non-CGRP antagonist approach to treatment Market Entry:Chronic Migraine Additional Indications:Acute Migraine, Craniofacial Pain, Insulin Resistance, Binge Eating Disorder Status:Phase 2 study PREVENTION enrollment complete 4 Next Steps:Toplineresults from PREVENTION expected 4Q 2023 Investigator initiated Phase 2 trials in adolescent obesity, social anxiety disorder, and binge eating disorder are enrolling 3Q 2023 1 Tzabazis et al., 2017. Headache. 57 Suppl 2:64-75 2 Antoni et al., 1989. BiochemJ. 257(2):611-4 3 Meyerowitzet al., 2022.Nat Struct Mol Biol. (3):274-281 4 A Phase 2 trial under an investigator-initiated IND has been completed in the U.S. using TNX-1900 *TNX-1900 has not been approved for any indication. CGRP = calcitonin gene- related peptide. Oxytocin receptor Oxytocin
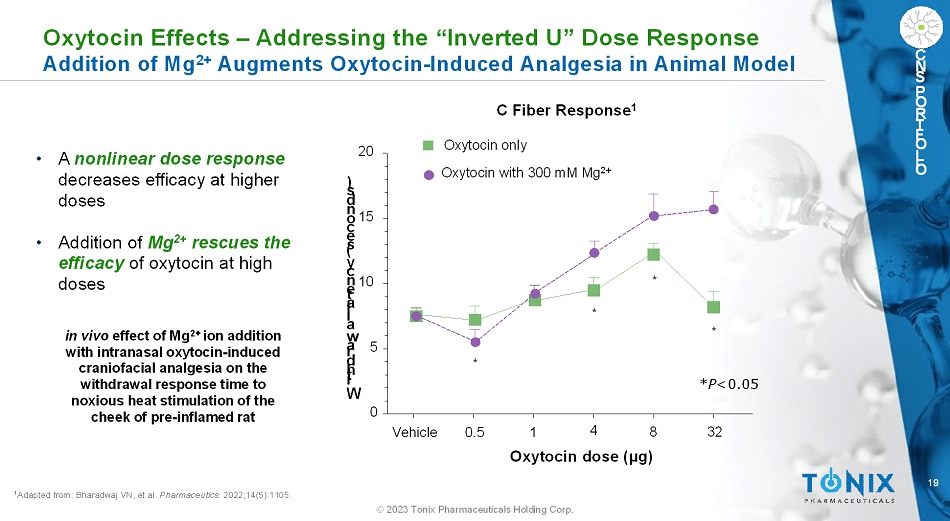
19 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O 1 Adapted from: Bharadwaj VN, et al. Pharmaceutics. 2022;14(5):1105. in vivoeffect of Mg 2+ ion addition with intranasal oxytocin-induced craniofacial analgesia on the withdrawal response time to noxious heat stimulation of the cheek of pre-inflamed rat Vehicle 0.5 1 4 8 32 0 5 10 15 20 Oxytocin only Oxytocin with 300 mM Mg 2+ * * * * Oxytocin dose (µg) W i t h d r a w a l l a t e n c y ( s e c o n d s ) C Fiber Response 1 *P<0.05 Oxytocin Effects –Addressing the “Inverted U” Dose Response Addition of Mg 2+ Augments Oxytocin-Induced Analgesia in Animal Model • A nonlinear dose response decreases efficacy at higher doses • Addition of Mg 2+ rescues the efficacy of oxytocin at high doses
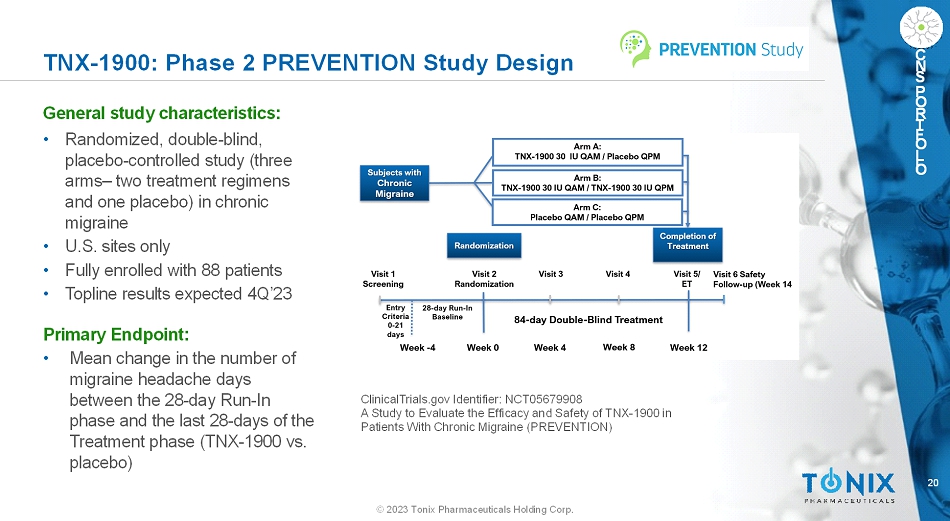
20 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O TNX-1900: Phase 2 PREVENTION Study Design General study characteristics: • Randomized, double-blind, placebo-controlled study (three arms–two treatment regimens and one placebo) in chronic migraine • U.S. sites only • Fully enrolled with 88 patients • Topline results expected 4Q’23 Primary Endpoint: • Meanchange in the number of migraine headache days between the 28-day Run-In phase and the last 28-days of the Treatment phase (TNX-1900 vs. placebo) ClinicalTrials.gov Identifier: NCT05679908 A Study to Evaluate the Efficacy and Safety of TNX-1900 in Patients With ChronicMigraine(PREVENTION) N = 50 N = 50 N = 50
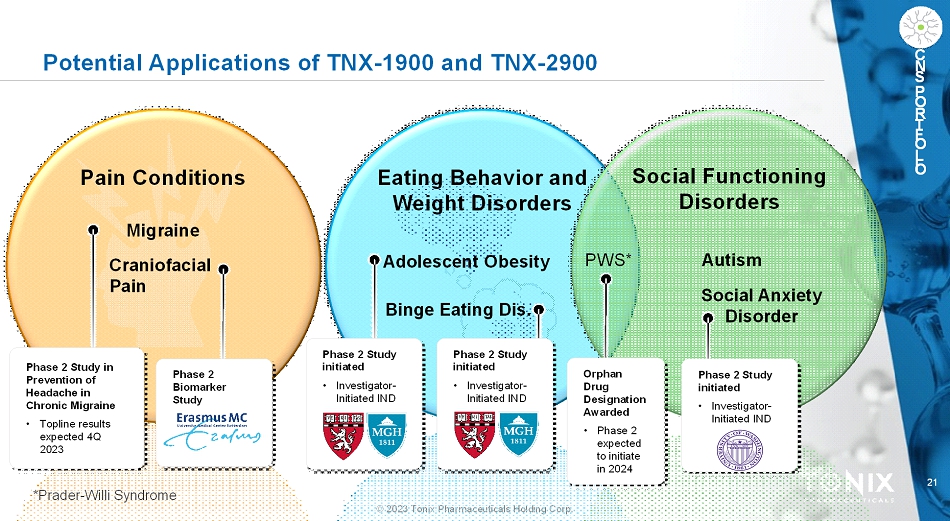
21 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Potential Applications of TNX-1900 and TNX-2900 Phase 2 Study in Prevention of Headache in Chronic Migraine • Topline results expected 4Q 2023 Phase 2 Biomarker Study PWS* *Prader-Willi Syndrome Pain Conditions Migraine Phase 2 Study initiated • Investigator- Initiated IND Phase 2 Study initiated • Investigator- Initiated IND Craniofacial Pain Adolescent Obesity Binge Eating Dis. Eating Behavior and Weight Disorders Autism Social Functioning Disorders Orphan Drug Designation Awarded • Phase 2 expected to initiate in 2024 Phase 2 Study initiated • Investigator- Initiated IND Social Anxiety Disorder
22 © 2023 Tonix Pharmaceuticals Holding Corp. Upcoming Expected Topline Results Fourth Quarter 2023 TNX-1900 for Chronic Migraine Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-601 ER for Depression Topline Results Upcoming Phase 2 Proof-of- Concept Study Registration-Quality TNX-102 SL for Fibromyalgia ToplineResults Upcoming Phase 3 Potential NDA Enabling Study Registration-Quality
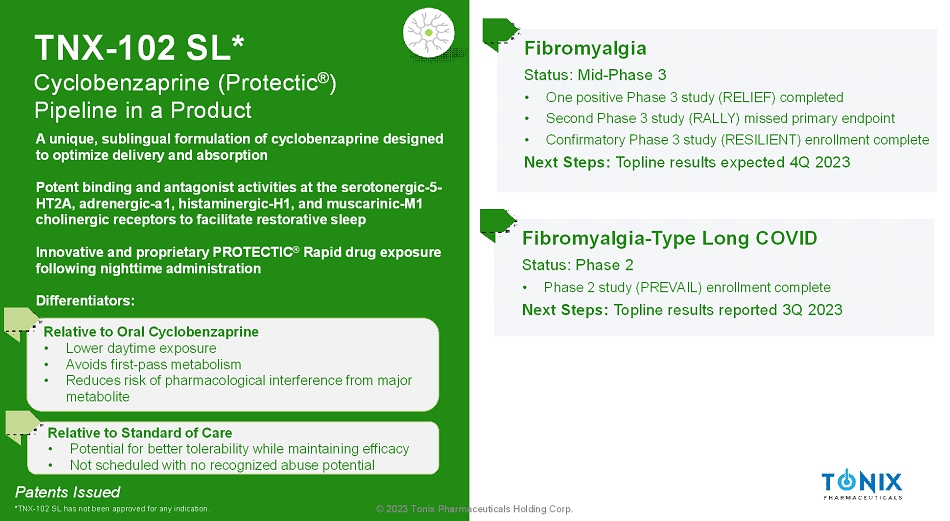
© 2023 Tonix Pharmaceuticals Holding Corp. TNX-102 SL* Cyclobenzaprine (Protectic ® ) Pipeline in a Product Fibromyalgia Status: Mid-Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) enrollment complete Next Steps: Topline results expected 4Q 2023 Fibromyalgia-Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) enrollment complete Next Steps: Topline results reported 3Q 2023 Patents Issued *TNX-102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption Potent binding and antagonist activities at the serotonergic-5- HT2A, adrenergic-a1, histaminergic-H1, and muscarinic-M1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following nighttime administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first-pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled with no recognized abuse potential

24 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PROFILE DEVELOPMENT PROGRAM Patents Issued TNX-102 SL*: Fibromyalgia Cyclobenzaprine Protectic ® Sublingual Tablets C N S P O R T F O L I O Fibromyalgia (FM) is a chronic pain disorder resulting from amplified sensory and pain signaling within the CNS • Afflictsan estimated 6-12 million adults in the U.S., approximately 90% of whom are women 1 • Symptoms include chronic widespread pain, nonrestorative sleep, fatigue, and cognitive dysfunction • Patients struggle with daily activities, have impaired quality of life, and frequently are disabled • Physicians and patients report common dissatisfaction with currently marketed products Market Entry:Fibromyalgia Additional Indications:Long COVID, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder Status:One Positive Phase 3 study RELIEF completed 2 Second Phase 3 study RALLY missed primary endpoint Confirmatory Phase 3 study RESILIENT enrollment complete Next Steps:Toplineresults expected 4Q 2023 *TNX-102 SL has not been approved for any indication. 1 American Chronic Pain Association (www.theacpa.org, 2019) 2 Lederman et al., (2023) Arthritis Care & Research "Efficacy and Safety of TNX-102 SL (Sublingual Cyclobenzaprine) for the Treatment of Fibromyalgia: Results From the RELIEF Trial",doi: 10.1002/acr.25142. Epubahead of print. PMID: 37165930. When the check engine light malfunctions, the light is on even though the car is not malfunctioning
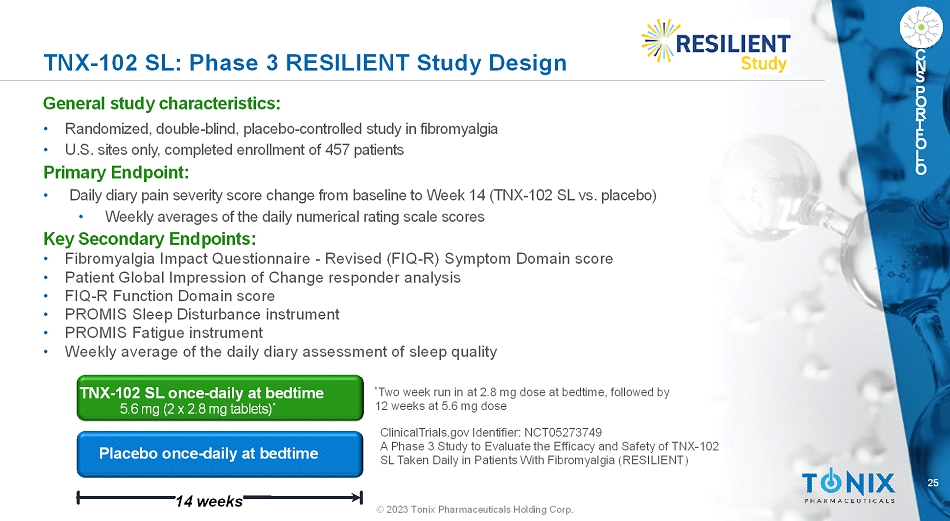
25 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O TNX-102 SL: Phase 3 RESILIENTStudy Design General study characteristics: • Randomized, double-blind, placebo-controlled study in fibromyalgia • U.S. sites only, completed enrollment of 457 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX-102 SL vs. placebo) • Weekly averages of the daily numerical rating scale scores Key Secondary Endpoints: • Fibromyalgia Impact Questionnaire -Revised (FIQ-R) Symptom Domain score • Patient Global Impression of Change responder analysis • FIQ-R Function Domain score • PROMIS Sleep Disturbance instrument • PROMIS Fatigue instrument • Weekly average of the daily diary assessment of sleep quality Placebo once-daily at bedtime 14 weeks TNX-102 SL once-daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05273749 A Phase 3 Study to Evaluate the Efficacy and Safety of TNX-102 SL Taken Daily in Patients WithFibromyalgia(RESILIENT)
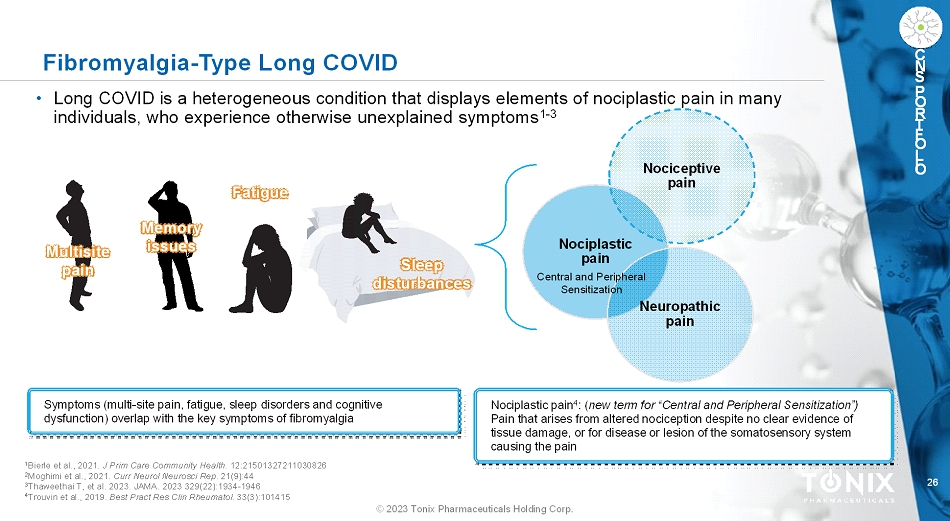
26 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Fibromyalgia-Type Long COVID • Long COVID is a heterogeneous condition that displays elements of nociplastic pain in many individuals, who experience otherwise unexplained symptoms 1-3 Symptoms (multi-site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia Multisite pain Memory issues Fatigue Sleep disturbances 1 Bierle et al., 2021. J Prim Care Community Health. 12:21501327211030826 2 Moghimi et al., 2021. CurrNeurol NeurosciRep. 21(9):44 3 ThaweethaiT, et al. 2023. JAMA. 2023 329(22):1934-1946 4 Trouvin et al., 2019. Best PractRes Clin Rheumatol. 33(3):101415 Nociceptive pain Nociplastic pain Neuropathic pain Nociplasticpain 4 : (new term for “Central and Peripheral Sensitization”) Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Central and Peripheral Sensitization
27 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PROFILE DEVELOPMENT PROGRAM Patents Issued TNX-102 SL*: Fibromyalgia-Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 19% of recovered COVID-19 patients 1 • As many as 40% of Long COVID patients experience multi-site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi-site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and ME/CFS Market Entry: Fibromyalgia-TypeLong COVID (PASC) Status:Phase 2 study PREVAIL topline reported Next Steps: End of Phase 2 Meeting with FDA expected 1 st Quarter 2024 1 June 22, 2022-CDC -https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics *TNX-102 SL has not been approved for any indication. C N S P O R T F O L I O Additional Indications:Fibromyalgia, PTSD, Agitation in Alzheimer’s, Alcohol Use Disorder 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Long COVID.
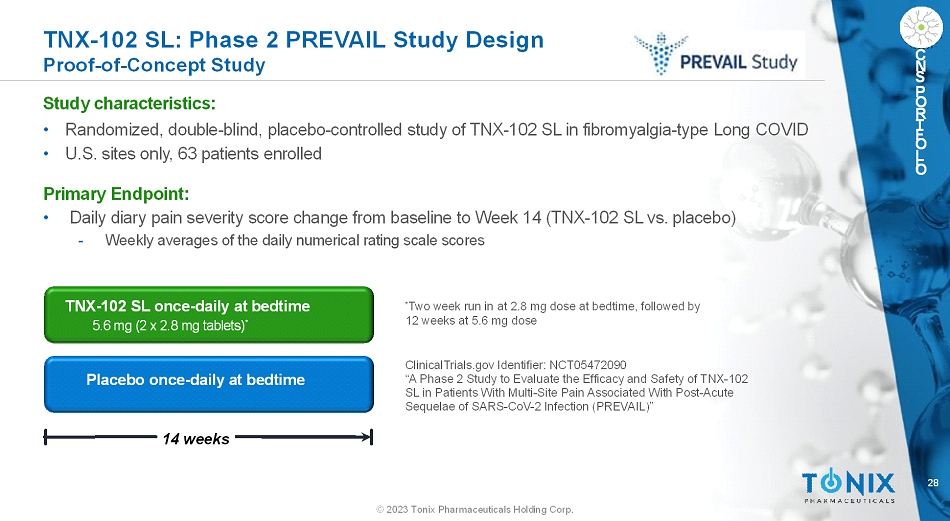
28 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O TNX-102 SL: Phase 2 PREVAIL Study Design Proof-of-Concept Study Study characteristics: • Randomized, double-blind, placebo-controlled study of TNX-102 SL in fibromyalgia-type Long COVID • U.S. sites only, 63 patients enrolled Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX-102 SL vs. placebo) - Weekly averages of the daily numerical rating scale scores Placebo once-daily at bedtime 14 weeks TNX-102 SL once-daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two week run in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 “A Phase 2 Study to Evaluate the Efficacy and Safety ofTNX-102 SLin Patients With Multi-Site Pain Associated With Post-Acute Sequelae of SARS-CoV-2 Infection (PREVAIL)”

29 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PREVAIL Topline Results 1 TNX-102 SL showed a robust effect size of 0.5 in improving fatigue and showed consistent activity across secondary measures of sleep quality, cognitive function, disability and Patient Global Impression of Change, but did not meet the primary endpoint of multi-site pain reduction at week 14 - There is currently no drug approved to treat Long COVID TNX-102 SL was generally well tolerated with an adverse event (AE) profile comparable to prior studies with TNX-102 SL. - AE-related discontinuations were similar in drug and placebo arms. - No new safety signals were observed Findings fulfill the objectives of this proof-of-concept study, supporting the decision to advance the program based on a proposed primary endpoint using the PROMIS Fatigue scale - Fatigue is the signature symptom of Long COVID and it has been identified as the dominant symptom contributing to disability 2 - In both of our prior Phase 3 studies of TNX-102 SL 5.6 mg in fibromyalgia, we observed numerical improvement in the PROMIS fatigue score (in RELIEF p=0.007 MMRM and in RALLY p=0.007 MMRM) - Although the validity of PROMIS Fatigue is not yet established in Long COVID, we believe the results of PREVAIL, together with extensive data from studies in other chronic conditions 3-5 –including Tonix’sstudies in fibromyalgia –make PROMIS Fatigue a solid candidate for the primary endpoint of future Long COVID registrational studies. 1 Tonix Press Release, September 5, 2023 -https://bit.ly/3Z6FQHQ 2 Walker S, et al. BMJ Open 2023;13:e069217. doi:10.1136/ bmjopen-2022-069217 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology, 73, 89-102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology, 73, 128–134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation, 92(10 Supplement), S20-S27.

30 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O PREVAIL Next Steps Tonix plans to meet with FDA to discuss a path to registration - Expected date of End of Phase 2 meeting is 1 st Quarter 2024 Fatigue is the principal symptom overlapping with chronic fatigue syndrome/myalgicencephalomyelitis (CFS/ME) and fibromyalgia syndromes - Expected date of fibromyalgia topline is 4 th Quarter 2023

© 2023 Tonix Pharmaceuticals Holding Corp. IMMUNOLOGY: KEY CANDIDATES
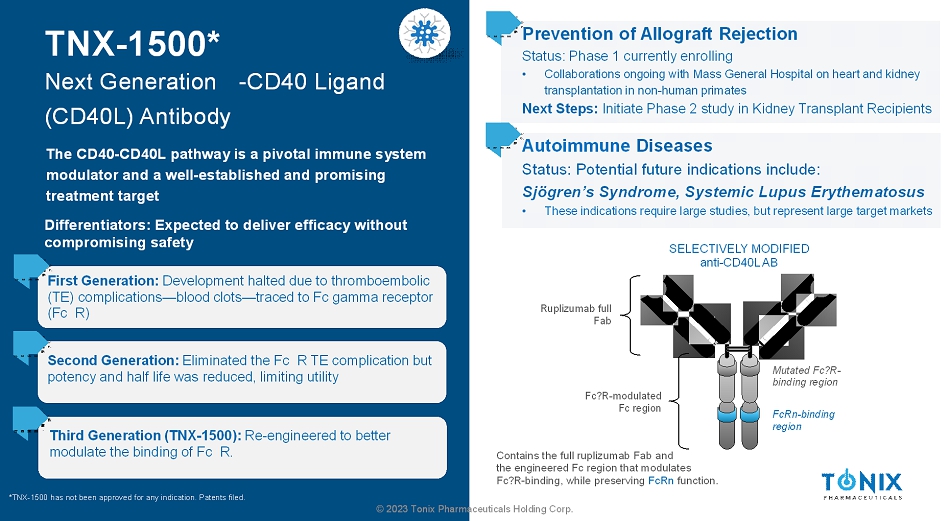
© 2023 Tonix Pharmaceuticals Holding Corp. TNX-1500* Next Generation -CD40 Ligand (CD40L) Antibody The CD40-CD40L pathway is a pivotal immune system modulator and a well-established and promising treatment target First Generation: Development halted due to thromboembolic (TE) complications—blood clots—traced to Fc gamma receptor (Fc R) Prevention of Allograft Rejection Status: Phase 1 currently enrolling • Collaborations ongoing with Mass General Hospital on heart and kidney transplantation in non-human primates Next Steps: Initiate Phase 2 study in Kidney Transplant Recipients SELECTIVELY MODIFIED anti-CD40L AB Ruplizumab full Fab Contains the full ruplizumab Fab and the engineered Fc region that modulates Fc?R-binding, while preserving FcRnfunction. Mutated Fc?R- binding region FcRn-binding region Fc?R-modulated Fc region Second Generation: Eliminated the Fc R TE complication but potency and half life was reduced, limiting utility Third Generation (TNX-1500): Re-engineered to better modulate the binding of Fc R. *TNX-1500 has not been approved for any indication. Patents filed. Differentiators: Expected to deliver efficacy without compromising safety Autoimmune Diseases Status: Potential future indications include: Sjögren’sSyndrome, Systemic Lupus Erythematosus • These indications require large studies, but represent large target markets
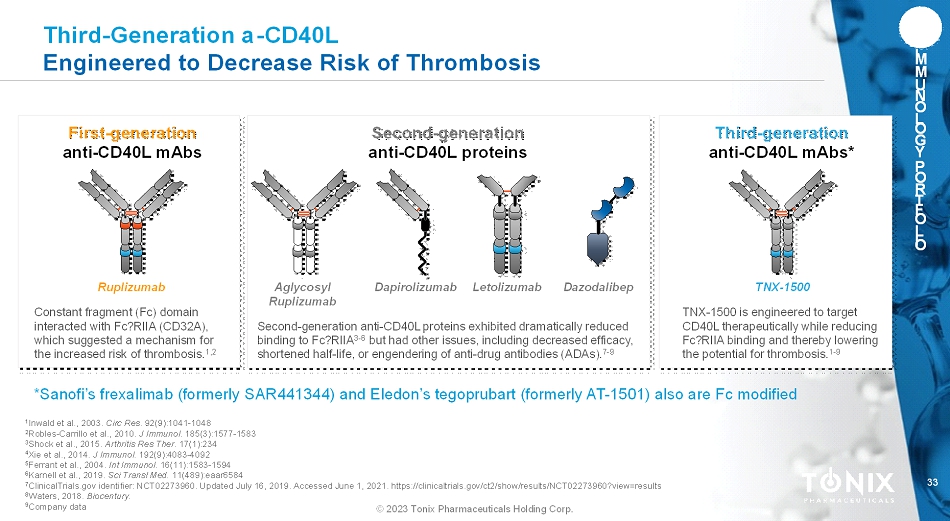
33 © 2023 Tonix Pharmaceuticals Holding Corp. I M M U N O L O G Y P O R T F O L I O Third-Generation a-CD40L Engineered to Decrease Risk of Thrombosis First-generation anti-CD40L mAbs Constant fragment (Fc) domain interacted with Fc?RIIA(CD32A), which suggested a mechanism for the increased risk of thrombosis. 1,2 Ruplizumab Second-generation anti-CD40L proteins Second-generation anti-CD40L proteins exhibited dramatically reduced binding to Fc?RIIA 3-6 but had other issues, including decreased efficacy, shortened half-life, or engendering of anti-drug antibodies (ADAs). 7-9 Dapirolizumab LetolizumabAglycosyl Ruplizumab Third-generation anti-CD40L mAbs* TNX-1500 is engineered to target CD40L therapeutically while reducing Fc?RIIAbinding and thereby lowering the potential for thrombosis. 1-9 TNX-1500 *Sanofi’s frexalimab(formerly SAR441344) and Eledon’stegoprubart(formerly AT-1501) also are Fc modified 1 Inwald et al., 2003. Circ Res. 92(9):1041-1048 2 Robles-Carrillo et al., 2010. J Immunol. 185(3):1577-1583 3 Shock et al., 2015. Arthritis Res Ther. 17(1):234 4 Xie et al., 2014. J Immunol. 192(9):4083-4092 5 Ferrant et al., 2004. Int Immunol. 16(11):1583-1594 6 Karnell et al., 2019. Sci TranslMed.11(489):eaar6584 7 ClinicalTrials.gov identifier: NCT02273960. Updated July 16, 2019. Accessed June 1, 2021. https://clinicaltrials.gov/ct2/show/results/NCT02273960?view=results 8 Waters, 2018. Biocentury. 9 Companydata Dazodalibep
34 © 2023 Tonix Pharmaceuticals Holding Corp. I M M U N O L O G Y P O R T F O L I O TNX-1500 anti-CD40L Monoclonal Antibody 1 Lassiter,G.,etal.(2023).TNX-1500,acrystallizablefragment–modifiedanti-CD154antibody,prolongsnon-humanprimaterenalallograftsurvival.AmericanJournalofTransplantation.April3, 2023.https://doi.org/10.1016/j.ajt.2023.03.022 2 Miura,S.,etal.(2023)TNX-1500,acrystallizablefragment–modifiedanti-CD154antibody,prolongsnon-humanprimatecardiacallograftsurvival.AmericanJournalofTransplantation.April6, 2023.https://doi.org/10.1016/j.ajt.2023.03.025 Proposed indication -prevention of rejection in kidney transplant: • Supported by pre-clinical studies Phase 1 study initiated: • A Phase 1 study of TNX-1500 was initiated in the third quarter of 2023. Peer reviewed articles: • Two articles have recently published in the American Journal of Transplantationthat demonstrate TNX-1500 prolongs non-human primate renal and heart allograft survival. 1,2

35 © 2023 Tonix Pharmaceuticals Holding Corp. I M M U N O L O G Y P O R T F O L I O Otheranti-CD40L Monoclonal Antibodies in Development Sanofi –Sjögren's Syndrome (SjS), Multiple Sclerosis (MS), Systemic Lupus Erythematosus (SLE) • Phase 2 Trial Currently Enrolling in SjS(NCT04572841) and SLE (NCT05039840) • Active Phase 2 Trial in Relapsing MS (NCT04879628) –positive results reported 1,2 • Frexalimab, f.k.a.SAR441344 (Fc-modified) Eledon–Kidney Transplant • Phase 2 Trial Completed in ALS (NCT04322149) • Phase 1/2 Trial Currently Enrolling in Kidney Transplant (NCT05027906) • Tegoprubart, f.k.a. AT-1501 (Fc-modified) 1 SanofipressreleaseMay31,2023“PressRelease:PositivePhase2dataofnovelinvestigationalanti-CD40Lantibodyfrexalimabshowsignificantlyreduceddiseaseactivityinrelapsingmultiple sclerosis”:www.sanofi.com/en/media-room/press-releases/2023/2023-05-31-05-00-00-2678991(accessedAugust112023) 2 Carvalho,T.NatureMedicine(News)(2023).29:1882 3 HorizonpressreleaseSeptember12,2022“HorizonTherapeuticsplcAnnouncesPhase2TrialEvaluatingDazodalibepfortheTreatmentofSjögren’sSyndromeMeetsPrimaryEndpoint” https://ir.horizontherapeutics.com/news-releases/news-release-details/horizon-therapeutics-plc-announces-phase-2-trial-evaluating(accessedAugust112023) 4 HorizonPressReleaseJanuary18,2023“HorizonTherapeuticsplcAnnouncesPhase2TrialEvaluatingDazodalibepfortheTreatmentofSjögren’sSyndromeMeetsPrimaryEndpointinthe SecondStudyPopulation;OnlyPhase2TrialtoMeetPrimaryEndpointinBothPatientPopulations 5 https://www.ucb.com/our-science/pipeline UCB (Co-developed with Biogen) –Systemic Lupus Erythematosus (SLE) • Phase 3 Trial Currently Enrolling (NCT04294667) - Topline results expected 1H 2024 5 • Dapirolizumab pegol (pegylated Fab) Horizon (being acquired by Amgen) –Sjögren's Syndrome (SjS) • Two Positive Phase 2 studies reported 3,4 • Dazodalibep (tn03 fusion protein)
36 © 2023 Tonix Pharmaceuticals Holding Corp. I M M U N O L O G Y P O R T F O L I O Patents Filed TNX-1700*: Gastric and Colorectal Cancers Recombinant Trefoil Factor 2 (rTFF2-HSA) Fusion Protein Potential New Cancer Treatment • TNX-1700 (rTFF2) has effects on cancer by altering the tumor micro-environment • Mechanism of action: suppresses myeloid-derived suppressor cells and activates anti-cancer CD8+ T cells • Potential synergy with anti-PD-1 or anti-PD-L1 monoclonal antibodies (mAbs) Preclinical Evidence for Inhibiting Growth of Cancer Cells • Data showed that mTFF2-CTP augmented the efficacy of mAbanti-PD-1 therapy. Anti-PD-1 in combination with TFF2-CTP showed greater anti-tumor activity in PD-L1- overexpressing mice • mTNX-1700 (mTFF2-MSA fusion protein) and anti-PD-1 monotherapy each was able to evoke anti-tumor immunity in the MC38 model of colorectal cancer 1 • mTNX-1700 augmented the anti-tumor efficacy of anti-PD- 1 therapy in both the MC38 and the CT26.wt models 1 Market Entry: Immuno-oncology, combination therapy with PD1 blockers for gastric and colorectal cancer Status: Preclinical Next Steps: Animal studies ongoing *TNX-1700 is in the pre-IND stage of development and has not been approved for any indication. Differentiator: No product yet identified consistently augments PD1 effects on cold tumors Licensed from Columbia University • Developing in partnership under sponsored research agreement 1 Daugherty, B. et al. March 6, 2023 Keystone Poster; www.tonixpharma.com/wp-content/uploads/2023/03/mTFF2-MSA_mTNX-1700_Suppresses-Tumor-Growth-and-Increases-Survival-in-an-Anti-PD-1-Treated-MC38-Colorectal-Cancer-Model-by- Targeting-MDSCs.pdf

© 2023 Tonix Pharmaceuticals Holding Corp. INFECTIOUS DISEASE: KEY CANDIDATES
© 2023 Tonix Pharmaceuticals Holding Corp. TNX-801* Recombinant Pox Vaccine (RPV) PlatformUsing Live Virus Technology Mpoxand Smallpox Vaccine Status: Preclinical • TNX-801 is a cloned version of horsepox 1 (without any DNA insert) purified from cell culture Milestone:Successful completion of pre-IND meeting Next Steps:Preparation of IND submission Vaccine for Future Emerging Infectious Diseases Example: TNX-1850 for COVID-19 Status: Model System *TNX-801 is in the pre-IND stage of development and has not been approved for any indication. Patents filed. • Live virus vaccines are the most established vaccine technology - Starting with Edward Jenner’s smallpox vaccine, the first vaccine, which eradicated smallpox - Prevents forward transmission - Effective in eliciting durable or long-term immunity • Economical to manufacture at scale - Low dose because replication amplifies dose in vivo - Single shot administration • Standard refrigeration required for shipping and storage 1 Noyce et al., 2018. PLoSOne. 13(1):e0188453. Differentiators: TNX-801* scHPXV (Horsepox) 212,811 bp

39 © 2023 Tonix Pharmaceuticals Holding Corp. I N F E C T I O U S D I S E A S E P O R T F O L I O Internal Development & Manufacturing Capabilities R&D Center (RDC) –Frederick, MD • Functions: - Research advancing CNS and immunology drugs - Accelerated development of vaccines and antiviral drugs against COVID-19, its variants and other infectious diseases • Description: ~48,000 square feet, BSL-2 with some areas designated BSL-3 • Status: Operational Advanced Development Center (ADC) –North Dartmouth, MA • Function: Development and clinical scale manufacturing of biologics • Description: ~45,000 square feet, BSL-2 • Status: Operational

40 © 2023 Tonix Pharmaceuticals Holding Corp. I N F E C T I O U S D I S E A S E P O R T F O L I O Broad Spectrum Antivirals New DoD approach 1 raises importance of broad-spectrum medical counter measures • Need to improve medical readiness of the warfighter in biological threat environments • Beyond “one bug, one drug” approach • Issued Dec 2022 FOR OFFICIAL USE ONLY 0 Chemical and Biological Defense Program Approach for Research, Development, and Acquisition of Medical Countermeasure and Test Products 2022 UNCLASSIFIED UNCLASSIFIED CLEARED For Open Publication Department of Defense OFFICE OF PREPUBLICATION AND SECURITY REVIEW Jun 15, 2022 FOR OFFICIAL USE ONLY 0 Chemical and Biological Defense Program Approach for Research, Development, and Acquisition of Medical Countermeasure and Test Products 2022 UNCLASSIFIED UNCLASSIFIED CLEARED For Open Publication Department of Defense OFFICE OF PREPUBLICATION AND SECURITY REVIEW Jun 15, 2022 1 Vergun, D. DOD News. January 10, 2023. DoD aims to shield warfighters from novel biological agents. https://www.defense.gov/News/News-Stories/Article/Article/3261095/dod-aims-to-shield- warfighters-from-novel-biological-agents/

41 © 2023 Tonix Pharmaceuticals Holding Corp. I N F E C T I O U S D I S E A S E P O R T F O L I O DTRA RFP for Broad Spectrum Antivirals Through Medical CBRN DTRA Through Medical CBRN Defense Consortium: New DoD approach raises importance of broad-spectrum medical counter measures • MCDC issued a request for project proposals in the Fall of November, 2021 on behalf of DTRA for, “Novel Biologics as Medical Countermeasures(MCM) against Biological Threats of Concern.” RFP = request for proposals DTRA = Defense Threat Reduction Agency CBRN = Chemical, Biological, Radiological and Nuclear

42 © 2023 Tonix Pharmaceuticals Holding Corp. I N F E C T I O U S D I S E A S E P O R T F O L I O Broad-Spectrum Antiviral Discovery Programs Host-directed antiviral discovery programs CD45 targeted therapeutics • Small molecule therapeutics that reduce endogenous levels of CD45, a protein tyrosine phosphatase • Reduction in CD45 protects against many viruses including the Ebola virus Cathepsin inhibitors Small molecule therapeutics that inhibit essential cathepsins which are required by viruses such as coronaviruses and filoviruses to infect cells Activity as monotherapy and in combination with other antivirals Virus-directed antivirals discovery program Viral glycan-targeted engineered biologics • Bindto viral densely branched high-mannose (DBH) glycans • Neutralize circulating virusand stop the entry of the progeny virus into cells • Antiviral activity against a broad range of RNA viruses • Activity as monotherapy and in combination with other antivirals

© 2023 Tonix Pharmaceuticals Holding Corp. TEAM, NETWORK, & UPCOMING MILESTONES
44 © 2023 Tonix Pharmaceuticals Holding Corp. TNX-1300: COCAINE INTOXICATION TNX-1700: GASTRIC AND COLORECTAL CANCERS Key Development Partners TNX-1500: ALLOGRAFT REJECTION TNX-1900: MIGRAINE & OTHER INDICATIONS TNX-801: SMALLPOX AND MONKEYPOX VACCINE TNX-1850: COVID-19 VACCINE TNX-2900: PRADER-WILLI SYNDROME TNX-3700: COVID-19 VACCINE (ZINC NANOPARTICLE mRNA TECHNOLOGY) TNX-2300: BOVINE PARAINFLUEZNA VIRUS
45 © 2023 Tonix Pharmaceuticals Holding Corp. Upcoming: Expected Topline Clinical Data and Trial Initiations 2023 3 rd Quarter Clinical Trial Initiations • Phase 1 study of TNX-1500 for prevention of allograft rejection -started 4 th Quarter Clinical Trial Initiations • Phase 2 study of TNX-1300 for the treatment of cocaine intoxication -expected 4 th Quarter • Phase 2 PREVENTION study of TNX-1900 for chronic migraine - Affects approximately 3-7 M adults in the U.S 1 • Phase 2 UPLIFT study of TNX-601 ER for major depressive disorder - Affects approximately 47 M adults in the U.S (18.4% of population) 2 • Phase 3 RESILIENT study of TNX-102 SL for fibromyalgia - Affects approximately 6-12 M adults in the U.S 3 1 Natoliet al., Global prevalence of chronic migraine: a systematic review, Cephalagia, 2010, 30:599-609 2 CDC -https://www.cdc.gov/mmwr/volumes/72/wr/mm7224a1.htm?s_cid=mm7224a1_w 3 American Chronic Pain Association (www.theacpa.org, 2019)

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU

47 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Zembrace® IMPORTANT SAFETY INFORMATION(1 of 2) ZembraceSymTouch(Zembrace) can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop use and get emergency help if you have any signs of a heart attack: Discomfort in the center of your chest that lasts for more than a few minutes or goes away and comes back; severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw; pain or discomfort in your arms, back, neck, jaw or stomach; shortness of breath with or without chest discomfort; breaking out in a cold sweat; nausea or vomiting; feeling lightheaded Zembraceis not for people with risk factors for heart disease (high blood pressure or cholesterol, smoking, overweight, diabetes, family history of heart disease) unless a heart exam shows no problem. Do not use Zembraceif you have: History of heart problems; narrowing of blood vessels to your legs, arms, stomach, or kidney (peripheral vascular disease); uncontrolled high blood pressure; hemiplegic or basilar migraines. If you are not sure if you have these, ask your provider. Had a stroke, transient ischemic attacks (TIAs), or problems with blood circulation; severe liver problems; taken any of the following medicines in the last 24 hours: almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, ergotamines, dihydroergotamine; are taking certain antidepressants, known as monoamine oxidase (MAO)-A inhibitors or it has been 2 weeks or less since you stopped taking a MAO-A inhibitor. Ask your provider for a list of these medicines if you are not sure. An allergy to sumatriptan or any of the components of Zembrace Tell your provider about all of your medical conditions and medicines you take, including vitamins and supplements. Zembracecan cause dizziness, weakness, or drowsiness. If so, do not drive a car, use machinery, or do anything where you need to be alert.

48 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Zembrace® IMPORTANT SAFETY INFORMATION(2 of 2) Zembracemay cause serious side effects including: • Changes in color or sensation in your fingers and toes; sudden or severe stomach pain, stomach pain after meals, weight loss, nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; cramping and pain in your legs or hips; feeling of heaviness or tightness in your leg muscles; burning or aching pain in your feet or toes while resting; numbness, tingling, or weakness in your legs; cold feeling or color changes in one or both legs or feet; increased blood pressure including a sudden severe increase even if you have no history of high blood pressure; medication overuse headaches from using migraine medicine for 10 or more days each month. If your headaches get worse, call your provider. • Serotonin syndrome, a rare but serious problem that can happen in people using Zembrace, especially when used with anti-depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not there (hallucinations), agitation, or coma; fast heartbeat; changes in blood pressure; high body temperature; tight muscles; or trouble walking. • Hives (itchy bumps); swelling of your tongue, mouth, or throat • Seizures even in people who have never had seizures before The most common side effects of Zembraceinclude: pain and redness at injection site; tingling or numbness in your fingers or toes; dizziness; warm, hot, burning feeling to your face (flushing); discomfort or stiffness in your neck; feeling weak, drowsy, or tired. Tell your provider if you have any side effect that bothers you or does not go away. These are not all the possible side effectsof Zembrace. For more information, ask your provider. This is the most important information to know about Zembracebut is not comprehensive. For more information, talk to your provider and read the Patient Informationand Instructions for Use. You can also visit www.upsher-smith.comor call 1-888-650-3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6e5b104f-2b9e-416e-92fb-ef1bdaea867d You are encouraged to report adverse effects of prescription drugs to the FDA. Visit www.fda.gov/medwatchor call 1-800-FDA-1088. Zembraceis a prescription medicine used to treat acute migraine headaches with or without aura in adults who have been diagnosed with migraine. Zembraceis not used to prevent migraines. It is not known if it is safe and effective in children under 18 years of age.

49 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Tosymra®IMPORTANT SAFETY INFORMATION(1 of 2) Tosymra® can cause serious side effects, including heart attack and other heart problems, which may lead to death. Stop Tosymra and get emergency medical help if you have any signs of heart attack: • Discomfortinthecenterofyourchestthatlastsformorethanafewminutes orgoes away and comes back; severe tightness,pain,pressure,orheavinessinyourchest,throat,neck,orjaw; painordiscomfortinyourarms,back, neck,jaw, orstomach; shortnessofbreathwithorwithoutchestdiscomfort; breakingout inacoldsweat; nauseaorvomiting; feeling lightheaded Tosymraisnotforpeoplewithriskfactorsforheartdisease(highbloodpressureor cholesterol,smoking,overweight, diabetes,familyhistoryofheartdisease)unlessa heart exam is done and shows no problem. DonotuseTosymraifyouhave: • Historyof heartproblems; narrowingofbloodvesselstoyourlegs,arms,stomach,orkidney(peripheral vascular disease); uncontrolledhighblood pressure; severeliverproblems; hemiplegicorbasilarmigraines.Ifyouarenotsureifyouhavethese, askyourhealthcare provider. • Hadastroke,transientischemicattacks(TIAs),orproblemswithbloodcirculation; takenanyofthefollowingmedicinesinthe last24hours:almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, ergotamines, ordihydroergotamine. Askyourprovider ifyouarenotsureifyourmedicineis listedabove • aretakingcertainantidepressants,knownasmonoamineoxidase(MAO)-Ainhibitorsor it has been 2 weeks or less since you stopped taking a MAO-Ainhibitor. Ask your provider for a list of these medicines if you are notsure • AnallergytosumatriptanoranyingredientinTosymra Tellyourprovideraboutallofyourmedicalconditions andmedicinesyoutake,including vitamins and supplements. Tosymracan causedizziness,weakness,ordrowsiness. Ifso,donotdriveacar,use machinery, or do anything where you need to be alert.

50 © 2023 Tonix Pharmaceuticals Holding Corp. C N S P O R T F O L I O Tosymramaycauseserioussideeffectsincluding: • Changesincolororsensationin yourfingersandtoes; suddenor severestomachpain,stomachpainaftermeals,weightloss,nausea or vomiting, constipation or diarrhea, bloody diarrhea, fever; crampingandpaininyourlegsorhips,feelingof heavinessortightnessin yourleg muscles,burningorachingpainin yourfeetortoeswhileresting,numbness,tingling,or weakness in your legs, cold feeling or color changes in one or both legs or feet; increasedbloodpressureincludingasuddensevereincreaseevenif youhavenohistory of high blood pressure; medicationoveruseheadachesfromusingmigrainemedicinefor10ormoredayseach month. If your headaches get worse, call your provider. • Serotoninsyndrome,ararebutseriousproblemthatcanhappeninpeopleusingTosymra, especially when used with anti-depressant medicines called SSRIs or SNRIs. Call your provider right away if you have: mental changes such as seeing things that are not there(hallucinations),agitation,orcoma;fastheartbeat;changesinbloodpressure;high body temperature; tight muscles; or trouble walking. • Seizureseveninpeople whohaveneverhadseizuresbefore The most common side effects of Tosymra include: tingling, dizziness, feeling warm or hot, burning feeling, feeling of heaviness, feeling of pressure, flushing, feeling of tightness,numbness,applicationsite(nasal)reactions,abnormaltaste,andthroatirritation. Tellyourproviderifyouhaveanysideeffectthatbothersyouordoesnotgoaway.These are not all the possible side effects of Tosymra. For more information, ask your provider. ThisisthemostimportantinformationtoknowaboutTosymrabutisnotcomprehensive.For more information, talk to your provider and read the Patient Informationand Instructionsfor Use.You can also visit www.upsher-smith.comor call 1-888-650-3789. For full Prescribing Information, visit: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=015a5cf9-f246-48bc-b91e-cd730a53d8aa YouareencouragedtoreportnegativesideeffectsofprescriptiondrugstotheFDA.Visit www.fda.gov/medwatch,or call 1-800-FDA-1088. Tosymraisaprescriptionmedicineusedtotreatacutemigraineheadacheswithorwithout aura in adults. Tosymraisnotusedtotreatothertypesofheadachessuchashemiplegicorbasilar migraines or cluster headaches. Tosymraisnotusedtopreventmigraines.Itisnot knownifTosymraissafeand effective in children under 18 years ofage. Tosymra®IMPORTANT SAFETY INFORMATION(2 of 2)