
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

© 2023 Tonix Pharmaceuticals Holding Corp. TNX - 102 SL FOR LONG COVID RESULTS OF THE TNX - CY - PA201 ‘PREVAIL’ TRIAL BRIEFING DECK Version P0487 September 20, 2023 (Doc 1319 )

2 © 2023 Tonix Pharmaceuticals Holding Corp. Pipeline: Key Clinical Development Programs Status/Next Milestone Indication Candidates* Mid - Phase 3 – enrollment complete Phase 2 – Topline Data Announced 5 Sept 2023 Fibromyalgia (FM) Long COVID (PASC 2 ) TNX - 102 SL 1 Mid - Phase 2, Targeted 3Q 2023 Start Cocaine Intoxication - FDA Breakthrough Designation TNX - 1300 3 Phase 2 – enrollment complete 5 Prevention of Chronic Migraine TNX - 1900 4 Phase 2 – enrollment complete 6 Major Depressive Disorder TNX - 601 ER Phase 2 ready Prader - Willi Syndrome - FDA Orphan Drug Designation TNX - 2900 7 Phase 1 – currently enrolling Organ Transplant Rejection/ Autoimmune Conditions TNX - 1500 8 *All of Tonix’s product candidates are investigational new drugs or biologics and none has been approved for any indication. 1 TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) also has active INDs for Agitation in Alzheimer’s Disease (AAD), Alcohol Use Disorder (AUD), and Posttraumatic Stress Disorder (PTSD). These 3 indications are Phase 2 ready 2 Post - Acute Sequelae of COVID - 19. Completed Phase 2 Proof of Concept Study 3 TNX - 1300 (double - mutant cocaine esterase) is licensed from Columbia University 4 Acquired from Trigemina ; license agreement with Stanford University; Planned investigator - initiated Binge Eating Disorder (BED) study initiated 3 Q 2023 5 A Phase 2 trial under an investigator - initiated IND has been completed in the U.S. using TNX - 1900 6 Phase 2 trial for formulation development of TNX - 601 ER (tianeptine hemioxalate extended - release tablets) has completed enrollme nt – topline data in Q4 2023 ; Other potential indications include PTSD and neurocognitive dysfunction from steroids 7 Co - exclusive license agreement with French National Institute of Health and Medical Research ( Inserm ) 8 anti - CD40L humanized monoclonal antibody – IND cleared and Phase 1 PK/PD trial in healthy volunteers is currently ongoing
© 2023 Tonix Pharmaceuticals Holding Corp. INVESTIGATIONAL PRODUCT TNX - 102 SL* Cyclobenzaprine HCl ( Protectic ® ) Fibromyalgia Status: Mid - Phase 3 • One positive Phase 3 study (RELIEF) completed • Second Phase 3 study (RALLY) missed primary endpoint • Confirmatory Phase 3 study (RESILIENT) enrollment complete Next Steps: Topline results expected 4Q 2023 Fibromyalgia - Type Long COVID Status: Phase 2 • Phase 2 study (PREVAIL) enrollment complete Next Steps: Topline results reported 3Q 2023 Patents Issued *TNX - 102 SL has not been approved for any indication. A unique, sublingual formulation of cyclobenzaprine designed to optimize delivery and absorption, bypassing 1 st pass metabolism Potent binding and antagonist activities at the serotonergic - 5 - HT 2A , adrenergic - α 1 , histaminergic - H 1 , and muscarinic - M 1 cholinergic receptors to facilitate restorative sleep Innovative and proprietary PROTECTIC ® Rapid drug exposure following once nightly sublingual administration Differentiators: Relative to Oral Cyclobenzaprine • Lower daytime exposure • Avoids first - pass metabolism • Reduces risk of pharmacological interference from major metabolite Relative to Standard of Care • Potential for better tolerability while maintaining efficacy • Not scheduled, without recognized abuse potential Indications Most Recently Pursued
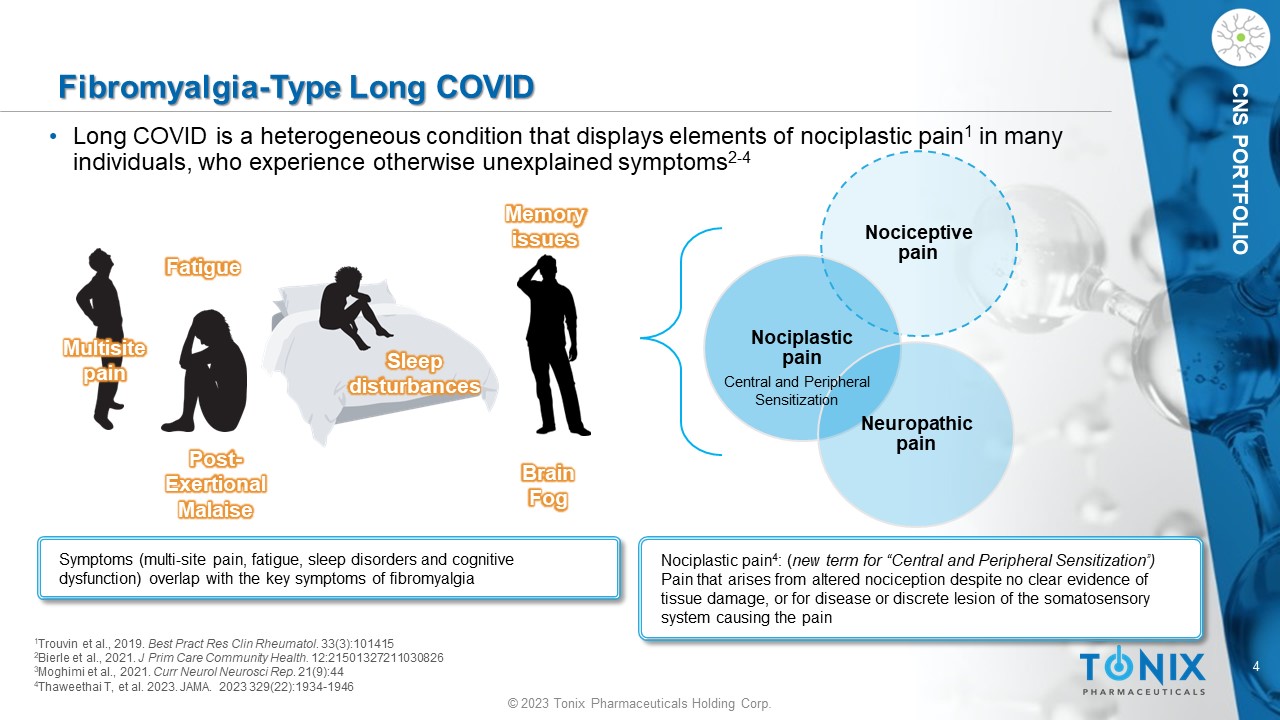
4 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Fibromyalgia - Type Long COVID • Long COVID is a heterogeneous condition that displays elements of nociplastic pain 1 in many individuals, who experience otherwise unexplained symptoms 2 - 4 Symptoms (multi - site pain, fatigue, sleep disorders and cognitive dysfunction) overlap with the key symptoms of fibromyalgia Multisite pain Memory issues Fatigue Sleep disturbances 1 Trouvin et al., 2019. Best Pract Res Clin Rheumatol . 33(3):101415 2 Bierle et al., 2021. J Prim Care Community Health. 12:21501327211030826 3 Moghimi et al., 2021. Curr Neurol Neurosci Rep . 21(9):44 4 Thaweethai T, et al. 2023. JAMA. 2023 329(22):1934 - 1946 Nociceptive pain Nociplastic pain Neuropathic pain Nociplastic pain 4 : ( new term for “Central and Peripheral Sensitization”) Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or discrete lesion of the somatosensory system causing the pain Central and Peripheral Sensitization Post - Exertional Malaise Brain Fog
5 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 102 SL*: Fibromyalgia - Type Long COVID (PASC) Cyclobenzaprine Protectic ® Sublingual Tablets • Occurs in approximately 19% of recovered COVID - 19 patients 1 • As many as 40% of Long COVID patients experience multi - site pain, a hallmark of fibromyalgia 2,3 • Symptoms of Long COVID, like multi - site pain, fatigue, brain fog, and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) • In August 2022, the HHS released the National Research Action Plan on Long COVID 4 which endorses the connection between Long COVID and ME/CFS Market Entry : Fibromyalgia - Type Long COVID (PASC) Status: Phase 2 study PREVAIL completed Results : Topline data reported 5 Sept 2023 1 J une 22, 2022 - CDC - https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2022/20220622.htm 2 Harris, H, et al. Tonix data on file. 2022 3 TriNetX Analytics *TNX - 102 SL has not been approved for any indication. CNS PORTFOLIO 4 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Long COVID. Next Steps: End of Phase 2 Meeting with FDA expected 1 st Quarter 2024

6 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 102 SL: Phase 2 Study Design Study c haracteristics: • Randomized, double - blind, placebo - controlle d study of TNX - 102 SL in fibromyalgia - type Long COVID • U.S. sites only, completed enrollment of 63 patients Primary Endpoint: • Daily diary pain severity score change from baseline to Week 14 (TNX - 102 SL vs. placebo) − Weekly averages of the daily numerical rating scale scores Secondary Endpoints: • Change from Baseline in the weekly average of the daily diary NRS assessment of sleep quality at the Week 14 endpoint • Change from Baseline in the PROMIS Fatigue score at the Week 14 endpoint • Change from Baseline in the PROMIS Cognitive Function – Abilities score at the Week 14 endpoint • Change from Baseline in the PROMIS Sleep Dis turbance score at the Week 14 endpoint • Proportion of patients with a Patient Global Impression of Change (PGIC) rating of “much improved” or “very much improved” at th e Week 14 endpoint • Change from Baseline to Week 14 in the Sheehan Disability Scale (SDS) total score Placebo once - daily at bedtime 14 weeks TNX - 102 SL once - daily at bedtime 5.6 mg (2 x 2.8 mg tablets) * * Two - week run - in at 2.8 mg dose at bedtime, followed by 12 weeks at 5.6 mg dose ClinicalTrials.gov Identifier: NCT05472090 (2 x placebo tablets) * Study Characteristics and Select Inclusion Criteria: • Approximately 30 clinical trial sites across the US • Visits: Screening, Baseline, Week 2, Week 6, Week 10, Week 14, Safety F/U Weeks 16 & 18 • Inclusion: age 18 - 65, female or male, confirmed hx SARS - CoV - 2 ≥ 3 months prior to enrollment ( documented PCR, nucleic acid tests, or rapid antigen tests) • New onset or worsening of pain that coincides with index COVID - 19 illness • Multi - site pain as defined by modified Michigan Body Map following COVID - 19 illness • O n - site 7 - day recall NRS average daily Long COVID - r elated pain intensity score ≥ 4 and ≤ 9 • Post - COVID - 19 Functional Status (PCFS) scale score of ≥ at Screening & Baseline • Willing and able to withdraw from: approved FM drugs, opioids, tramadol, tapentadol, tricyclics, trazodone, orexin receptor antagonists, benzodiazepines
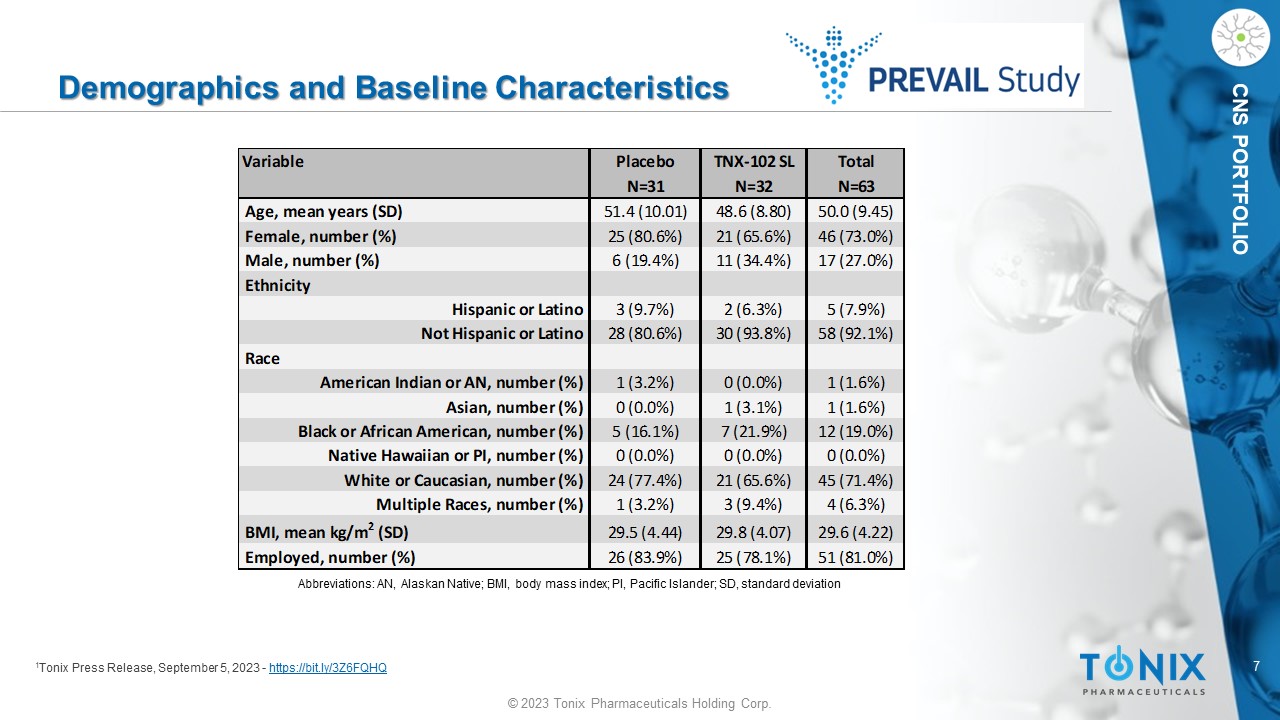
7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Demographics and Baseline Characteristics 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ Variable Placebo TNX-102 SL Total N=31 N=32 N=63 Age, mean years (SD) 51.4 (10.01) 48.6 (8.80) 50.0 (9.45) Female, number (%) 25 (80.6%) 21 (65.6%) 46 (73.0%) Male, number (%) 6 (19.4%) 11 (34.4%) 17 (27.0%) Ethnicity Hispanic or Latino 3 (9.7%) 2 (6.3%) 5 (7.9%) Not Hispanic or Latino 28 (80.6%) 30 (93.8%) 58 (92.1%) Race American Indian or AN, number (%) 1 (3.2%) 0 (0.0%) 1 (1.6%) Asian, number (%) 0 (0.0%) 1 (3.1%) 1 (1.6%) Black or African American, number (%) 5 (16.1%) 7 (21.9%) 12 (19.0%) Native Hawaiian or PI, number (%) 0 (0.0%) 0 (0.0%) 0 (0.0%) White or Caucasian, number (%) 24 (77.4%) 21 (65.6%) 45 (71.4%) Multiple Races, number (%) 1 (3.2%) 3 (9.4%) 4 (6.3%) BMI, mean kg/m 2 (SD) 29.5 (4.44) 29.8 (4.07) 29.6 (4.22) Employed, number (%) 26 (83.9%) 25 (78.1%) 51 (81.0%) Abbreviations: AN, Alaskan Native; BMI, body mass index; PI, Pacific Islander; SD, standard deviation
8 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Topline Results 1 TNX - 102 SL showed a robust effect size of 0.5 in improving fatigue and showed consistent activity across secondary measures of sleep quality, cognitive function, disability and Patient Global Impression of Change, but did not meet the primary endpoint of multi - site pain reduction at Week 14 ‒ There is currently no drug approved to treat Long COVID TNX - 102 SL was generally well tolerated with an adverse event (AE) profile comparable to prior studies with TNX - 102 SL. ‒ AE - related discontinuations were similar in drug and placebo arms. ‒ No new safety signals were observed Findings fulfill the objectives of this proof - of - concept study, supporting the decision to advance the program based on a proposed primary endpoint using the PROMIS Fatigue scale ‒ Fatigue is the signature symptom of Long COVID and it has been identified as the dominant symptom contributing to disability 2 ‒ In both of our prior Phase 3 studies of TNX - 102 SL 5.6 mg in fibromyalgia, we observed numerical improvement in the PROMIS fatig ue score (in RELIEF p= 0.007 MMRM and in RALLY p= 0.007 MMRM) ‒ Although the validity of PROMIS Fatigue is not yet established in Long COVID, we believe the results of PREVAIL, toge ther with extensive data from studies in other chronic conditions 3 - 5 – including Tonix’s studies in fibromyalgia – make PROMIS Fatigue a solid candidate for the primary endpoint of future Long COVID registrational studies. 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Walker S, et al . BMJ Open 2023;13:e069217. doi:10.1136/ bmjopen - 2022 - 069217 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology , 73, 89 - 102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology , 73, 128 – 134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation , 92(10 Supplement), S20 - S27.
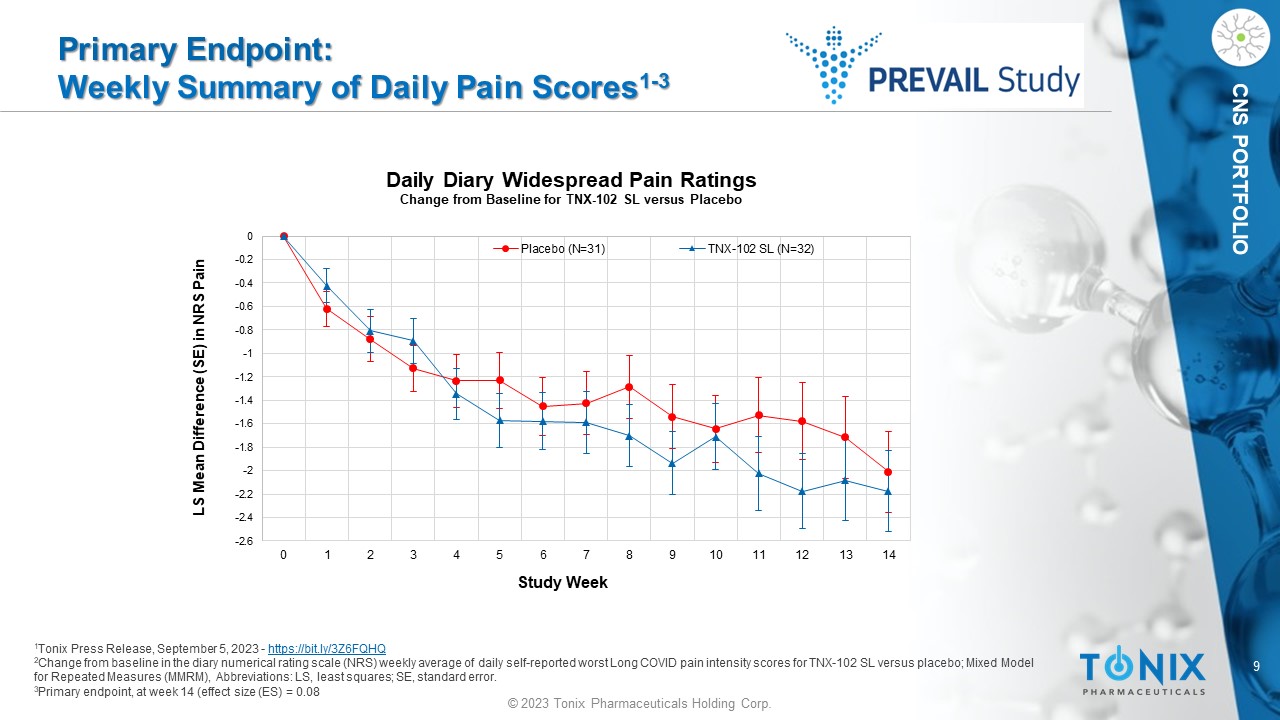
9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Primary Endpoint: Weekly Summary of Daily Pain Scores 1 - 3 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Change from baseline in the diary numerical rating scale (NRS) weekly average of daily self - reported worst Long COVID pain inten sity scores for TNX - 102 SL versus placebo; M ixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error. 3 Primary endpoint, at week 14 (effect size (ES) = 0.08 -2.6 -2.4 -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean Difference (SE) in NRS Pain Study Week Daily Diary Widespread Pain Ratings Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL (N=32)
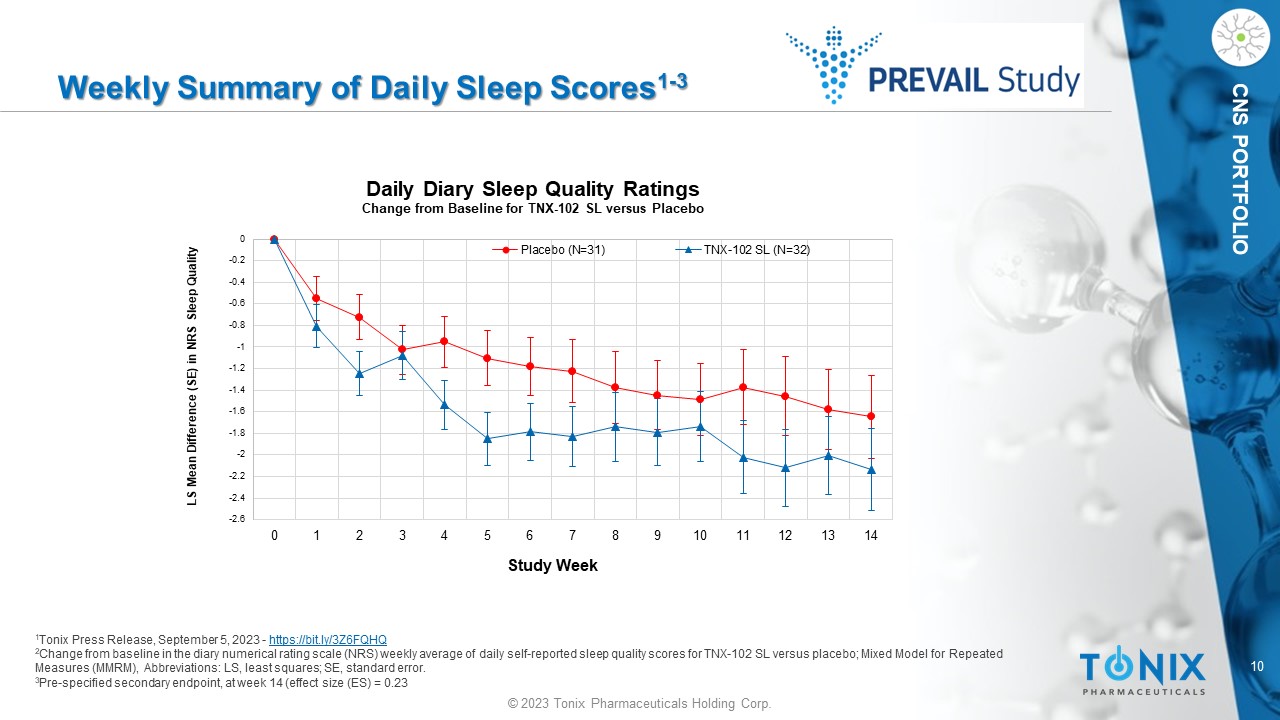
10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Weekly Summary of Daily Sleep Scores 1 - 3 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Change from baseline in the diary numerical rating scale (NRS) weekly average of daily self - reported sleep quality scores for TN X - 102 SL versus placebo; M ixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error. 3 Pre - specified secondary endpoint, at week 14 (effect size (ES) = 0.23 -2.6 -2.4 -2.2 -2 -1.8 -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 LS Mean Difference (SE) in NRS Sleep Quality Study Week Daily Diary Sleep Quality Ratings Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL (N=32)
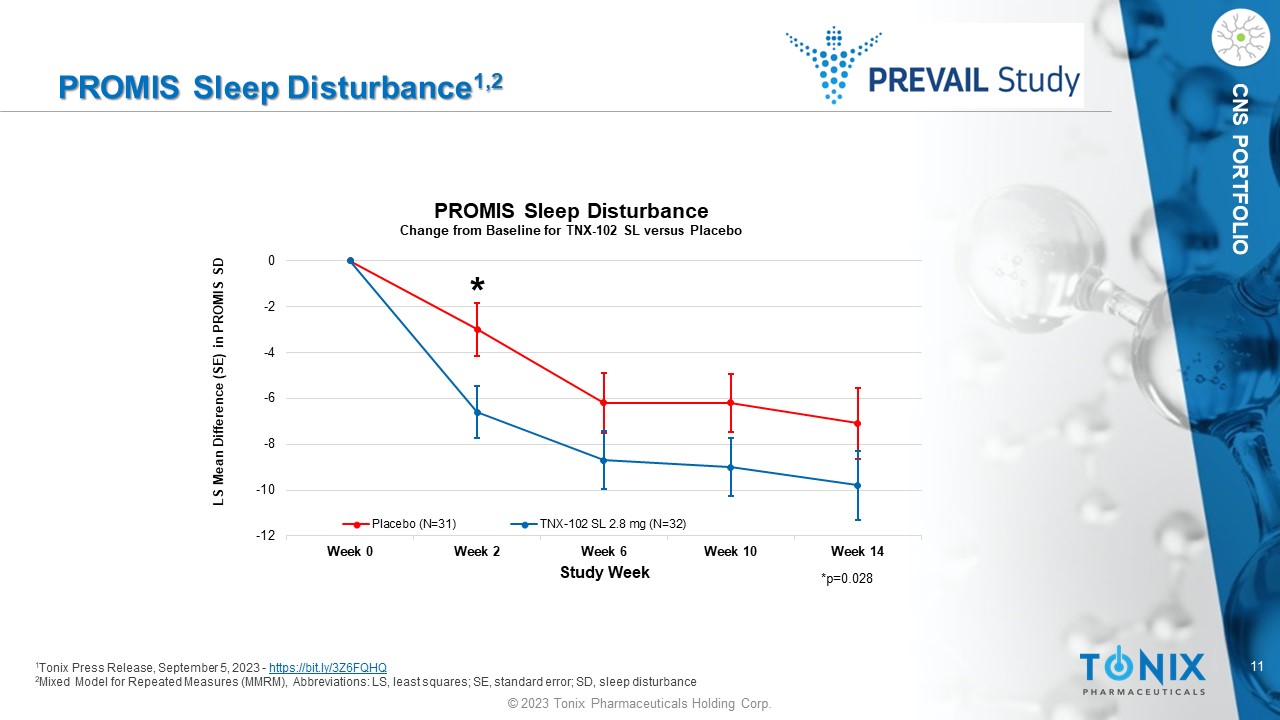
11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROMIS Sleep Disturbance 1,2 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Mixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error; SD, sleep disturbance -12 -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean Difference (SE) in PROMIS SD Study Week PROMIS Sleep Disturbance Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL 2.8 mg (N=32) *p=0.028 *
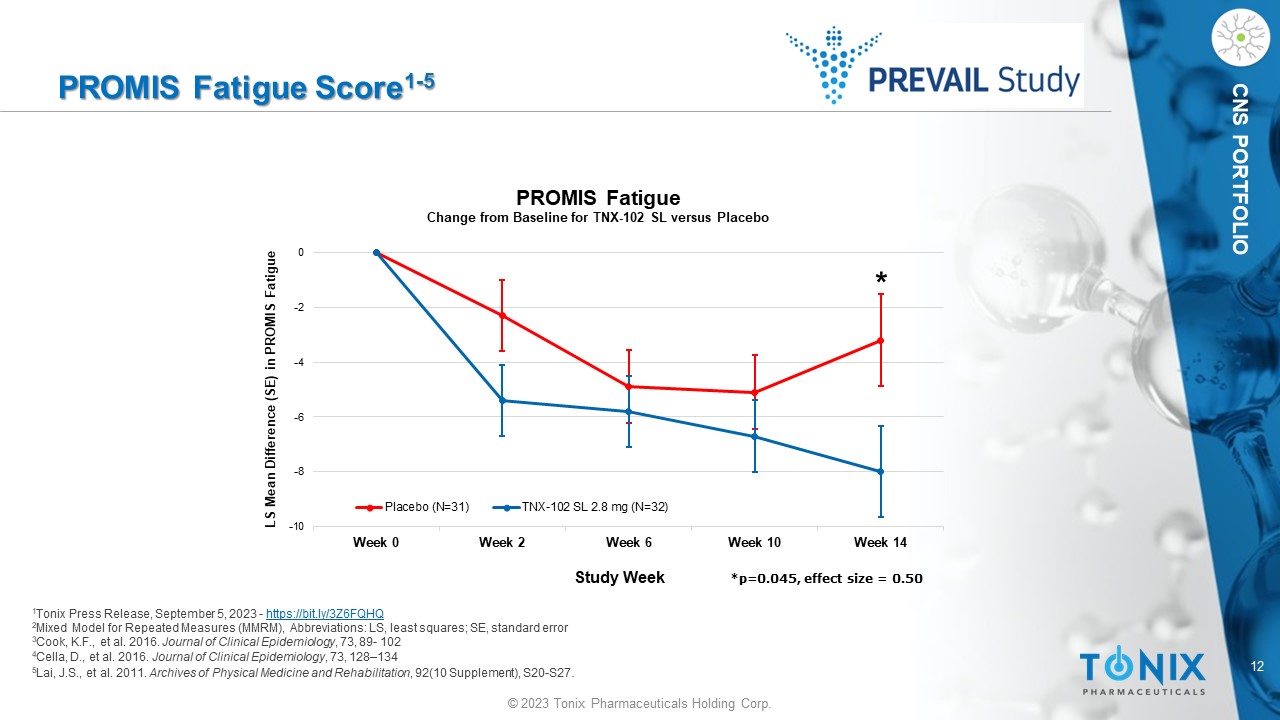
12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROMIS Fatigue Score 1 - 5 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 2 Mixed Model for Repeated Measures (MMRM), Abbreviations: LS, least squares; SE, standard error 3 Cook, K.F., et al. 2016. Journal of Clinical Epidemiology , 73, 89 - 102 4 Cella, D., et al. 2016. Journal of Clinical Epidemiology , 73, 128 – 134 5 Lai, J.S., et al. 2011. Archives of Physical Medicine and Rehabilitation , 92(10 Supplement), S20 - S27 . -10 -8 -6 -4 -2 0 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean Difference (SE) in PROMIS Fatigue Study Week PROMIS Fatigue Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL 2.8 mg (N=32) * *p=0.045, effect size = 0.50
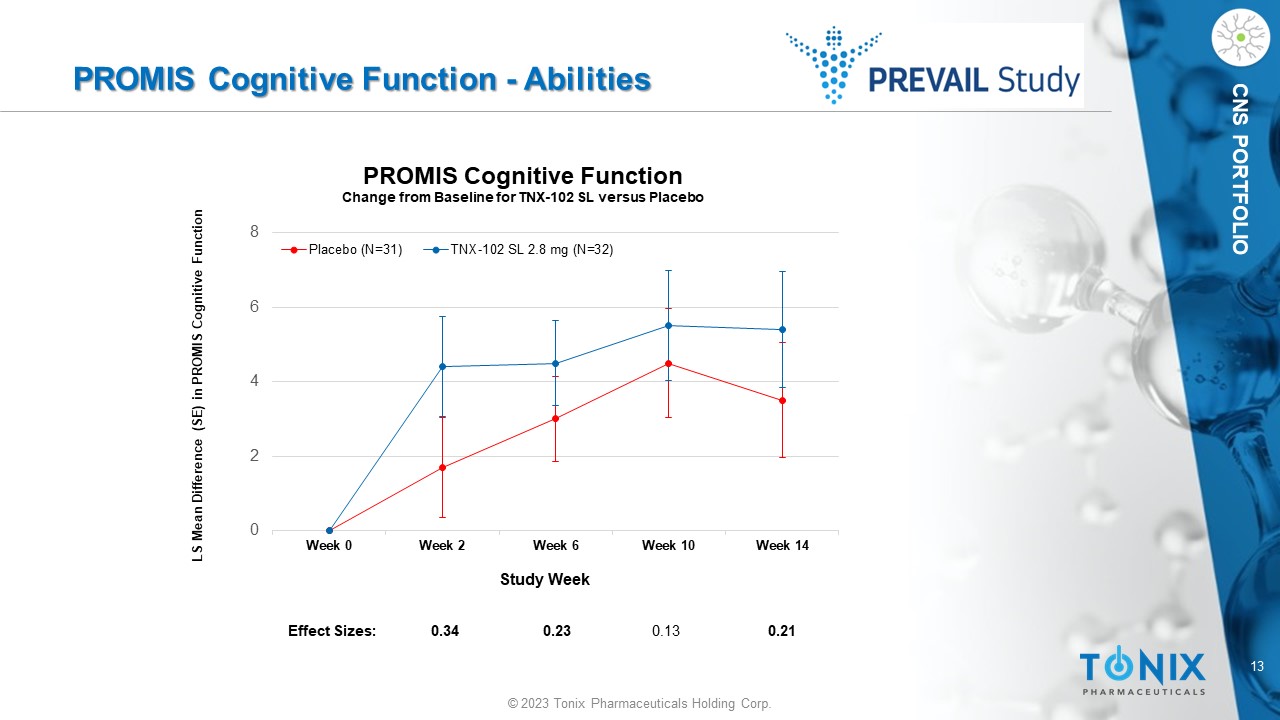
13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROMIS Cognitive Function - Abilities 0 2 4 6 8 Week 0 Week 2 Week 6 Week 10 Week 14 LS Mean Difference (SE) in PROMIS Cognitive Function Study Week PROMIS Cognitive Function Change from Baseline for TNX - 102 SL versus Placebo Placebo (N=31) TNX-102 SL 2.8 mg (N=32) Effect Sizes: 0.34 0.23 0.13 0.21
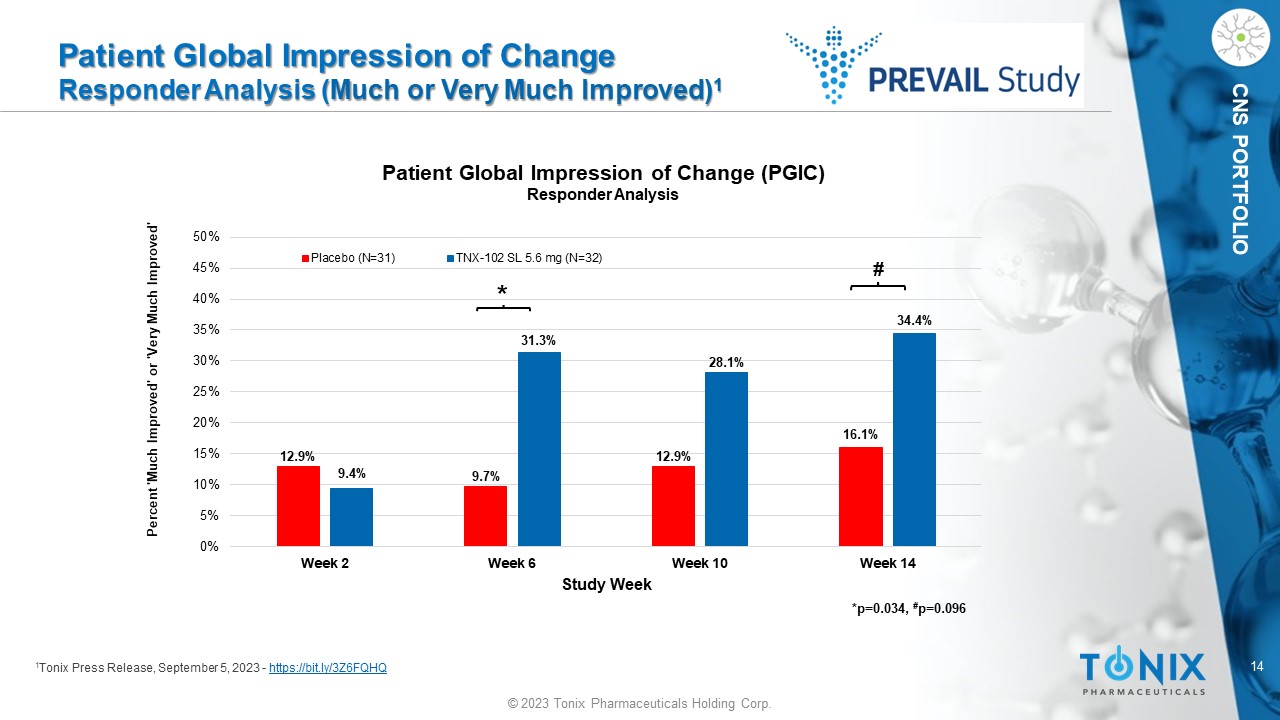
14 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Patient Global Impression of Change Responder Analysis ( Much or Very Much Improved) 1 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ 12.9% 9.7% 12.9% 16.1% 9.4% 31.3% 28.1% 34.4% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% Week 2 Week 6 Week 10 Week 14 Study Week Patient Global Impression of Change (PGIC) Responder Analysis Placebo (N=31) TNX-102 SL 5.6 mg (N=32) Percent 'Much Improved' or 'Very Much Improved' * # *p=0.034, # p= 0.096
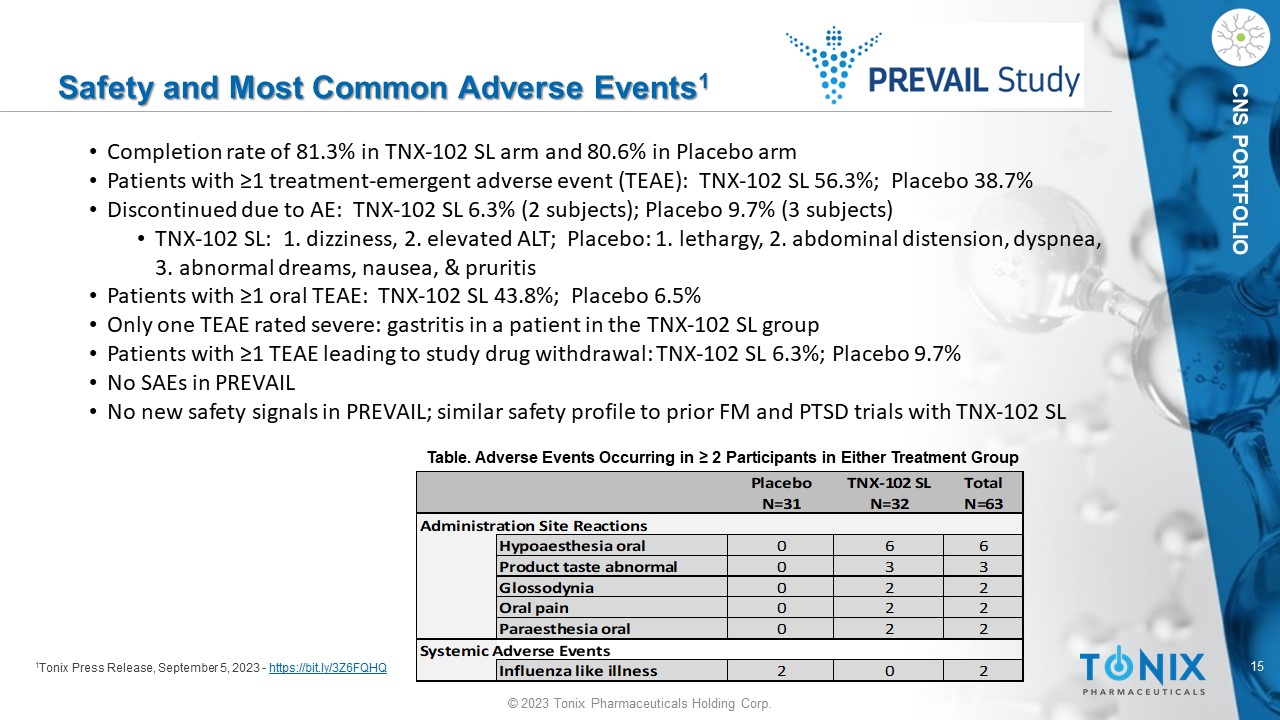
15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Safety and Most Common Adverse Events 1 1 Tonix Press Release, September 5, 2023 - https://bit.ly/3Z6FQHQ Placebo TNX-102 SL Total N=31 N=32 N=63 Administration Site Reactions Hypoaesthesia oral 0 6 6 Product taste abnormal 0 3 3 Glossodynia 0 2 2 Oral pain 0 2 2 Paraesthesia oral 0 2 2 Systemic Adverse Events Influenza like illness 2 0 2 Table. Adverse Events Occurring in ≥ 2 Participants in Either Treatment Group • Completion rate of 81.3% in TNX - 102 SL arm and 80.6% in Placebo arm • Patients with ≥1 treatment - emergent adverse event (TEAE): TNX - 102 SL 56.3%; Placebo 38.7% • Discontinued due to AE: TNX - 102 SL 6.3% (2 subjects); Placebo 9.7% (3 subjects) • TNX - 102 SL: 1. dizziness, 2. elevated ALT; Placebo: 1. lethargy, 2. abdominal distension, dyspnea, 3. abnormal dreams, nausea, & pruritis • Patients with ≥1 oral TEAE: TNX - 102 SL 43.8%; Placebo 6.5% • Only one TEAE rated severe: gastritis in a patient in the TNX - 102 SL group • Patients with ≥1 TEAE leading to study drug withdrawal: TNX - 102 SL 6.3%; Placebo 9.7% • No SAEs in PREVAIL • No new safety signals in PREVAIL; similar safety profile to prior FM and PTSD trials with TNX - 102 SL

16 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Next Steps Tonix plans to meet with FDA to discuss a path to registration ‒ Expected date of End of Phase 2 meeting is 1 st Quarter 2024 F atigue is the principal symptom overlapping with chronic fatigue syndrome/ myalgic encephalomyelitis (CFS/ME) and fibromyalgia syndromes ‒ Expected date of fibromyalgia topline is 4 th Quarter 2023

© 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU

© 2023 Tonix Pharmaceuticals Holding Corp. APPENDIX: ABOUT LONG COVID AND COMMONALITIES WITH FIBROMYALGIA, CFS/ME, AND POST - VIRAL SYNDROMES 18
19 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CHRONIC OVERLAPPING PAIN CONDITIONS (COPC) BELIEVED TO RESULT FROM SHARED BRAIN PROCESSES • COPC is a set of disorders that coaggregate ; these disorders can include but are not limited to 1,2 : • Temporomandibular disorder • Fibromyalgia • Irritable bowel syndrome • Vulvodynia • CFS/ME 3 • Interstitial cystitis/painful bladder syndrome • Endometriosis • Chronic tension - type headache • Migraine headache • Chronic lower back pain 1 Maixner W, et al. J Pain . 2016;17(9 Suppl):T93 - T107. 2 Veasley C, et al. http://www.chronicpainresearch. org/public/CPRA_WhitePaper_2015 - FINAL - Digital.pdf. Published May 2015. Accesse d July 26, 2021. 3 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis • Similar central mechanisms play significant roles in all pain conditions, even those with known peripheral contributions 1,2
20 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Role of Infections in Triggering Fibromyalgia or Chronic fatigue (CFS) - Like Illnesses Infection initiates an autoreactive process, which affects several functions, including brain and energy metabolism 2 - 7 • Infections can trigger any of these conditions in approximately 10% of exposed individuals • The initial location of the infection determines the subsequent pain syndrome • Any type of infectious diarrhea will trigger irritable bowel syndrome (IBS) in 10% to 20% of those exposed 1 Department of Health and Human Services, Office of the Assistant Secretary for Health. 2022. National Research Action Plan on Lo ng COVID, 200 Independence Ave SW, Washington, DC 20201. 2 Blomberg J, et al. Front Immunol. 2018;9:229. Published 2018 Feb 15. 3 Warren JW, et al. Urology. 2008;71(6):1085 - 1090. 4 Buskila D, et al. Autoimmun Rev. 2008;8(1):41 - 43. 5 Hickie I, et al. BMJ. 2006;333(7568):575. 6 Parry SD, et al. Am J Gastroenterol. 2003;98(9):1970 - 1975. 7 Halvorson HA, et al. Am J Gastroenterol. 2006;101(8):1894 - 1942. • Symptoms of Long COVID, like multi - site pain, fatigue and insomnia, are the hallmarks of chronic pain syndromes like fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). • In August 2022, the HHS released the National Research Action Plan on Long COVID 1 which endorses the connection between Long COVID and chronic fatigue syndrome.
21 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO POTENTIAL INCREASE IN MYALGIA FOLLOWING THE COVID - 19 PANDEMIC The specific causes may be due to: Chronic pain increase due to COVID - 19 could be nociplastic , neuropathic, or nociceptive Chronic pain newly triggered in individuals without SARS - CoV - 2 infection by exacerbation of risk factors (poor sleep, inactivity, fear, anxiety, and depression) Chronic pain as part of a post viral syndrome or the result of viral - associated organ damage Worsening of chronic pain due to exacerbation of preexisting pain physical or mental complaints Clauw DJ et al. Pain. 2020;161(8):1694 - 1697.
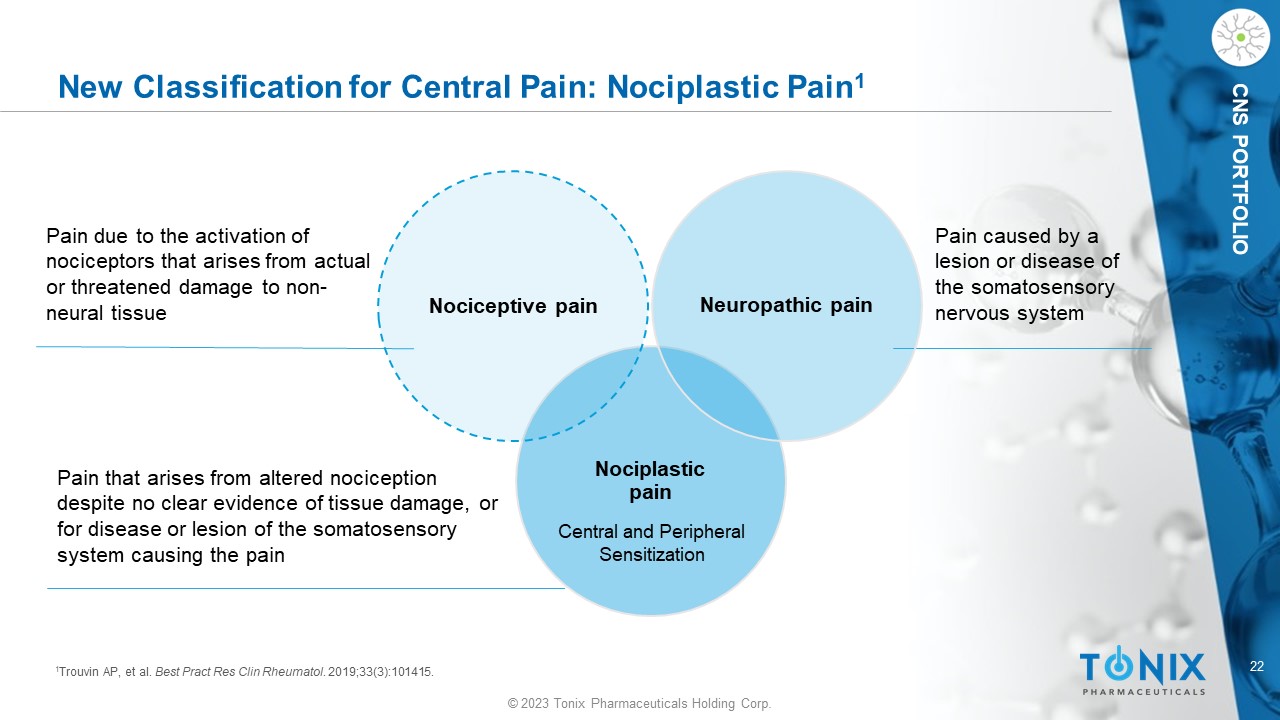
22 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO New Classification for Central Pain: Nociplastic Pain 1 Nociplastic pain Nociceptive pain Neuropathic pain Pain due to the activation of nociceptors that arises from actual or threatened damage to non - neural tissue Pain that arises from altered nociception despite no clear evidence of tissue damage, or for disease or lesion of the somatosensory system causing the pain Pain caused by a lesion or disease of the somatosensory nervous system 1 Trouvin AP, et al. Best Pract Res Clin Rheumatol . 2019;33(3):101415. Central and Peripheral Sensitization
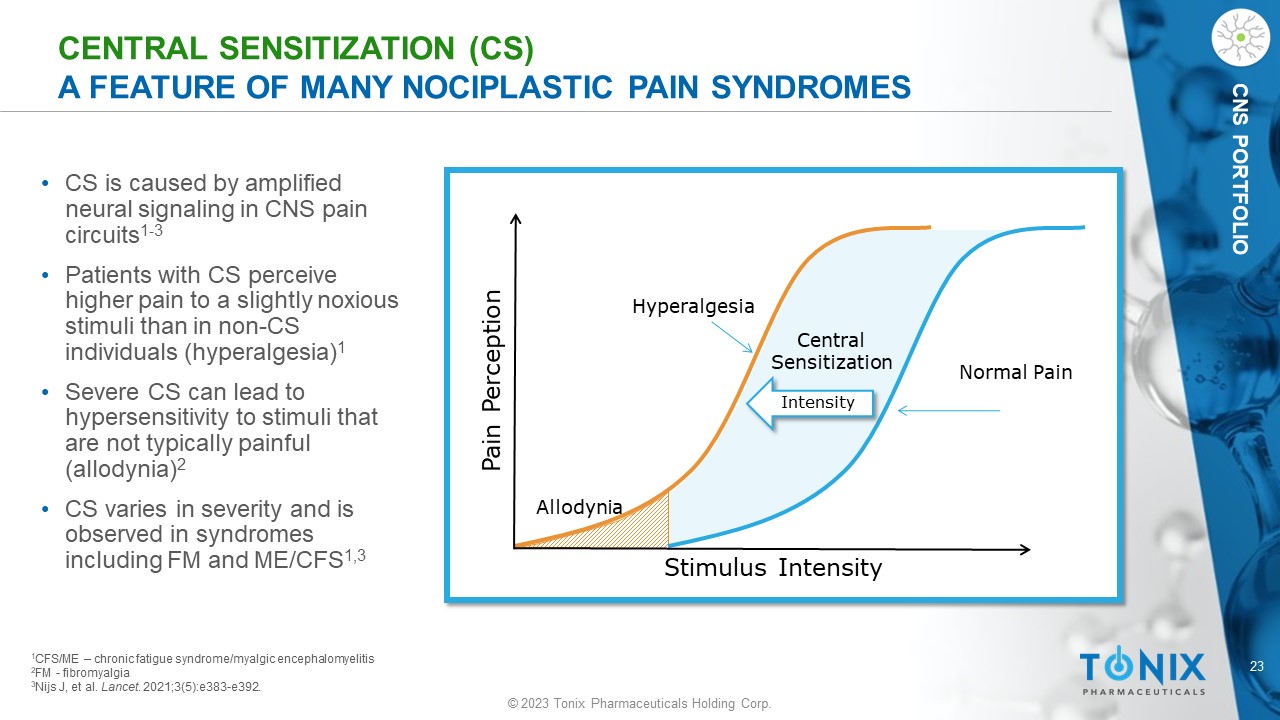
23 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CENTRAL SENSITIZATION (CS) A FEATURE OF MANY NOCIPLASTIC PAIN SYNDROMES • CS is caused by amplified neural signaling in CNS pain circuits 1 - 3 • Patients with CS perceive higher pain to a slightly noxious stimuli than in non - CS individuals (hyperalgesia) 1 • Severe CS can lead to hypersensitivity to stimuli that are not typically painful (allodynia) 2 • CS varies in severity and is observed in syndromes including FM and ME/CFS 1,3 Stimulus Intensity Pain Perception Normal Pain Hyperalgesia Allodynia Central Sensitization Intensity 1 CFS/ME – chronic fatigue syndrome/ myalgic encephalomyelitis 2 FM - fibromyalgia 3 Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
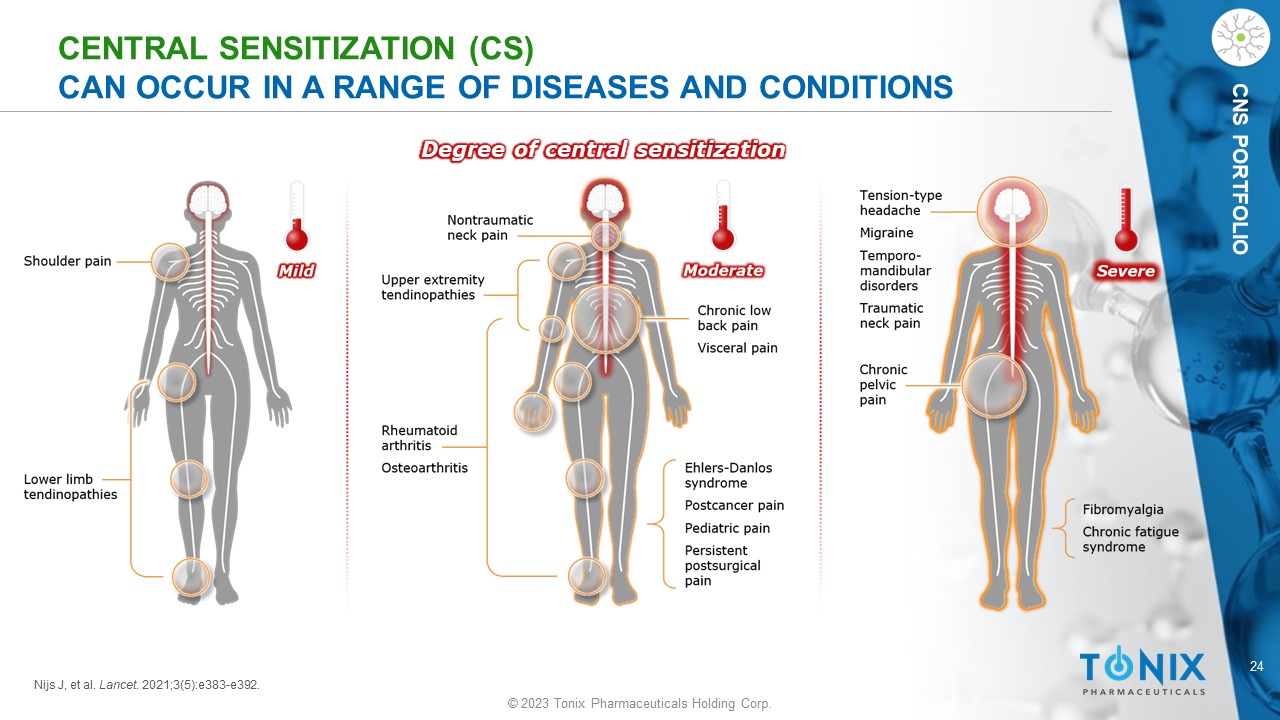
24 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO CENTRAL SENSITIZATION (CS) CAN OCCUR IN A RANGE OF DISEASES AND CONDITIONS Degree of central sensitization Nijs J, et al. Lancet . 2021;3(5):e383 - e392.
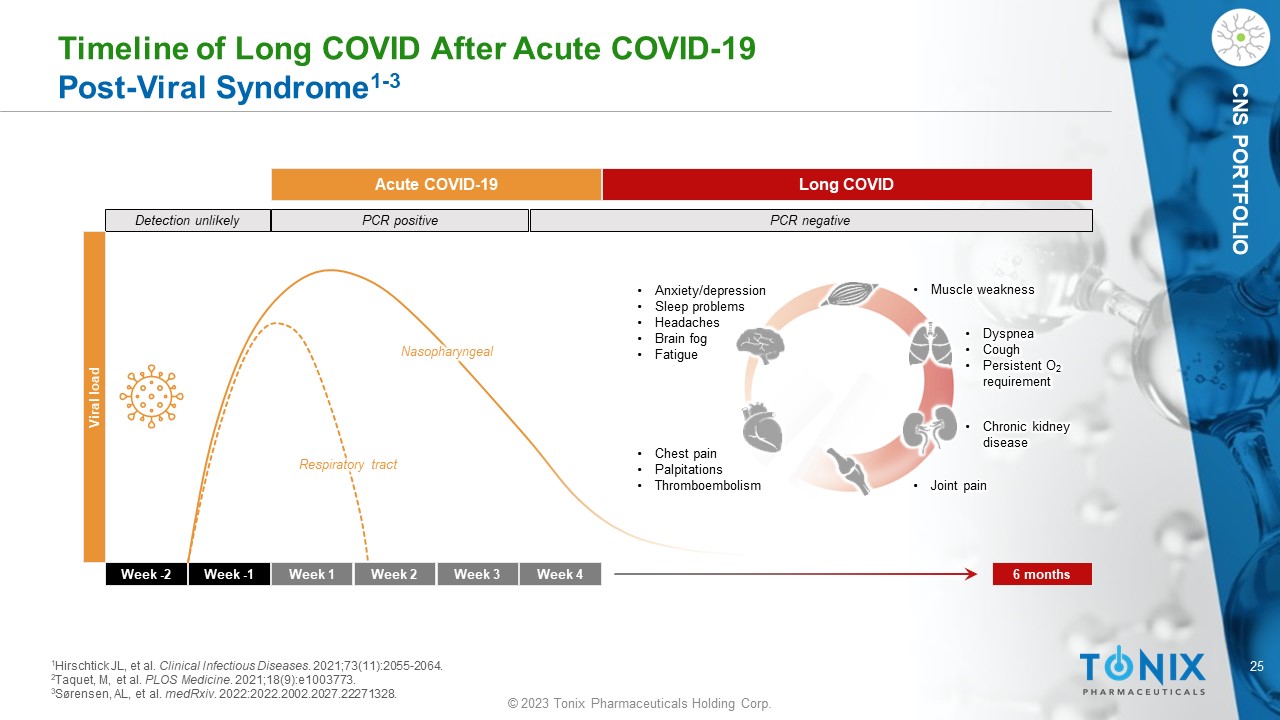
25 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Timeline of Long COVID After Acute COVID - 19 Post - Viral Syndrome 1 - 3 Week - 2 Week - 1 Week 1 Week 2 Week 3 Week 4 6 months Detection unlikely PCR positive PCR negative Acute COVID - 19 Long COVID Viral load Nasopharyngeal Respiratory tract • Chest pain • Palpitations • Thromboembolism • Dyspnea • Cough • Persistent O 2 requirement • Anxiety/depression • Sleep problems • Headaches • Brain fog • Fatigue • Chronic kidney disease • Muscle weakness • Joint pain 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773. 3 Sørensen, AL, et al. medRxiv . 2022:2022.2002.2027.22271328.
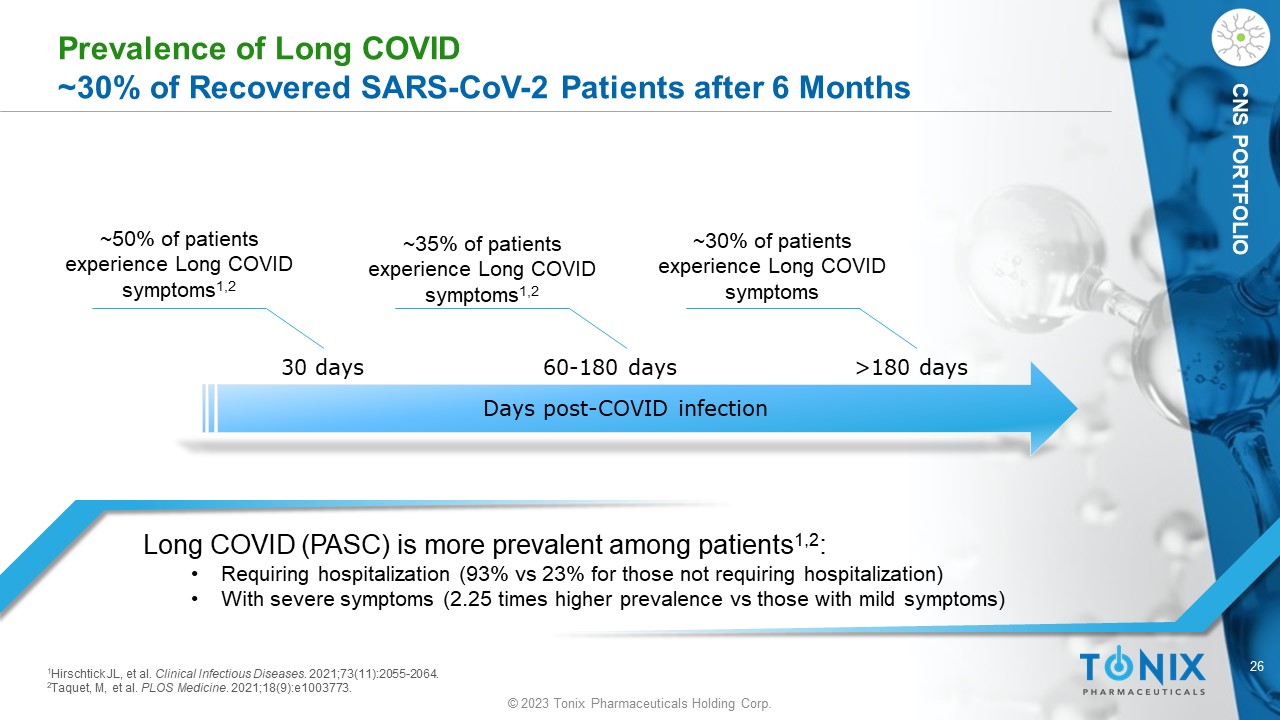
26 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Prevalence of Long COVID ~30% of Recovered SARS - CoV - 2 Patients after 6 Months Long COVID (PASC) is more prevalent among patients 1,2 : • Requiring hospitalization (93% vs 23% for those not requiring hospitalization) • With severe symptoms (2.25 times higher prevalence vs those with mild symptoms) ~50% of patients experience L ong COVID symptoms 1,2 Days post - COVID infection 30 days 60 - 180 days >180 days ~35% of patients experience Long COVID symptoms 1,2 ~30% of patients experience Long COVID symptoms 1 Hirschtick JL, et al. Clinical Infectious Diseases . 2021;73(11):2055 - 2064. 2 Taquet, M, et al. PLOS Medicine . 2021;18(9):e1003773.
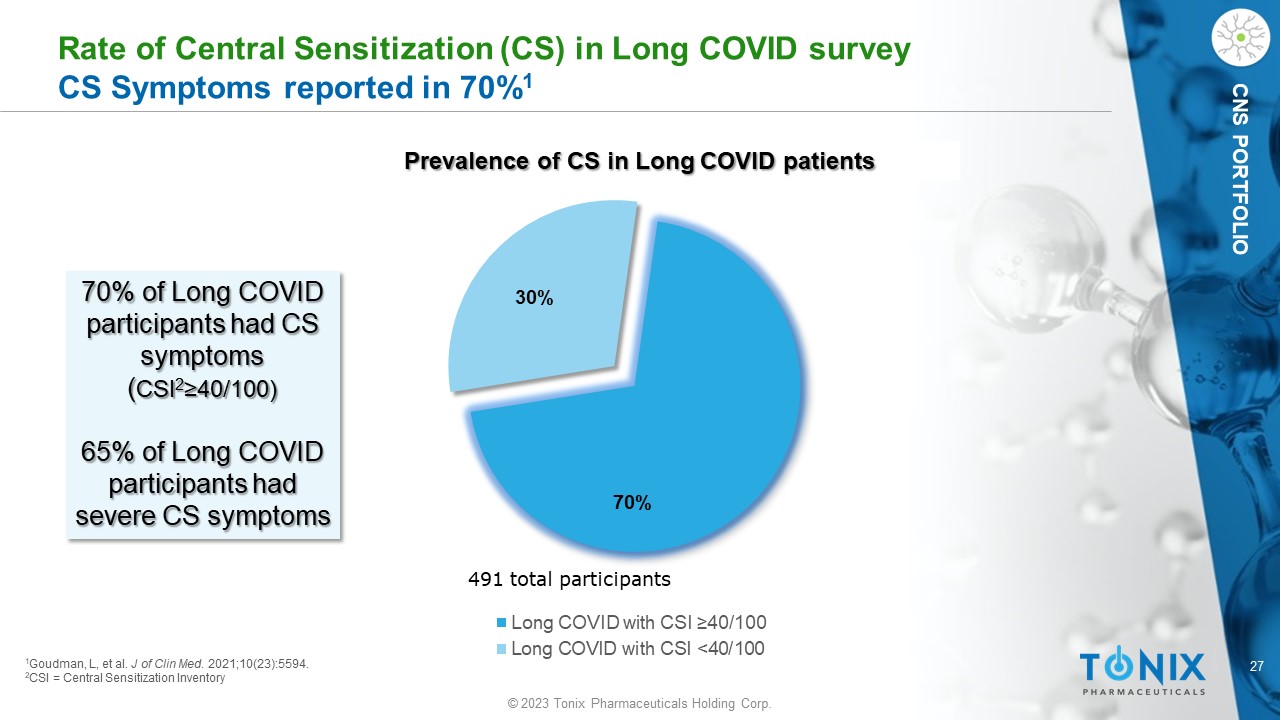
27 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Rate of Central Sensitization (CS) in Long COVID survey CS Symptoms reported in 70% 1 70% 30% Long COVID with CSI ≥40/100 Long COVID with CSI <40/100 491 total participants 1 Goudman , L, et al. J of Clin Med . 2021;10(23):5594. 2 CSI = Central Sensitization Inventory 70% of Long COVID participants had CS symptoms ( CSI 2 ≥40/100) 65% of Long COVID participants had severe CS symptoms Prevalence of CS in Long COVID patients
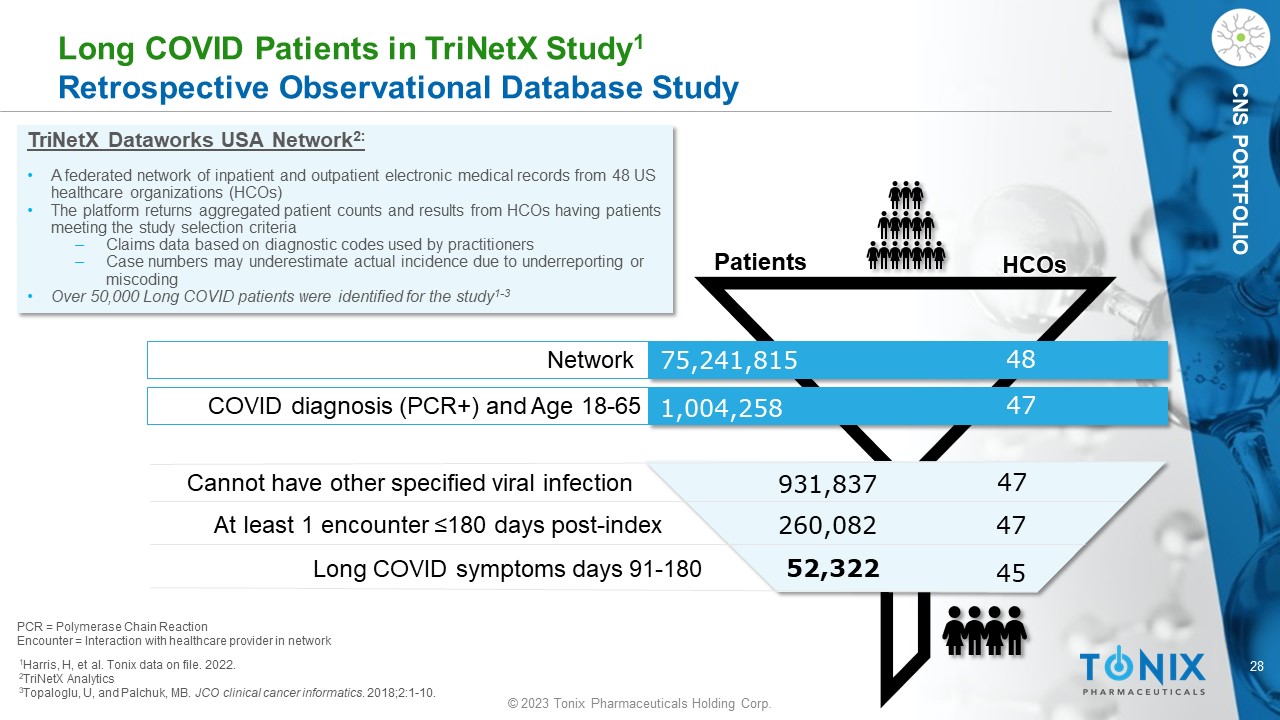
28 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Long COVID Patients in TriNetX Study 1 Retrospective Observational Database Study 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 3 Topaloglu, U, and Palchuk , MB. JCO clinical cancer informatics . 2018;2:1 - 10. TriNetX Dataworks USA Network 2: • A federated network of inpatient and outpatient electronic medical records from 48 US healthcare organizations (HCOs) • The platform returns aggregated patient counts and results from HCOs having patients meeting the study selection criteria ‒ Claims data based on diagnostic codes used by practitioners ‒ Case numbers may underestimate actual incidence due to underreporting or miscoding • Over 50,000 Long COVID patients were identified for the study 1 - 3 52,322 260,082 931,837 47 47 45 48 47 75,241,815 1,004,258 HCOs Patients Network COVID diagnosis (PCR+) and Age 18 - 65 Cannot have other specified viral infection At least 1 encounter ≤180 days post - index Long COVID symptoms days 91 - 180 PCR = Polymerase Chain Reaction Encounter = Interaction with healthcare provider in network
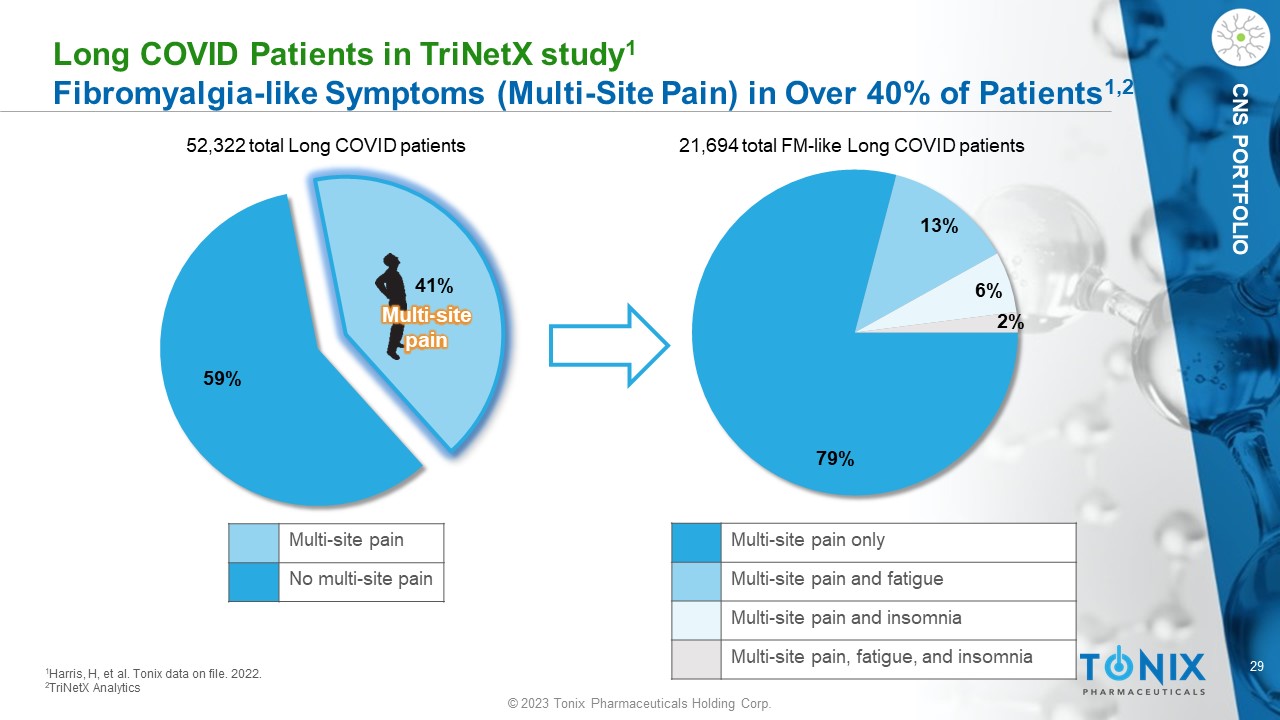
29 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO L ong COVID P atients in T riNetX study 1 F ibromyalgia - like Symptoms (Multi - Site Pain) in Over 40% of Patients 1,2 79% 13% 6% 2% 52,322 total Long COVID patients 21,694 total FM - like Long COVID patients 1 Harris, H, et al. Tonix data on file. 2022. 2 TriNetX Analytics 59% 41% Multi - site pain Multi - site pain No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Multi - site pain, fatigue, and insomnia
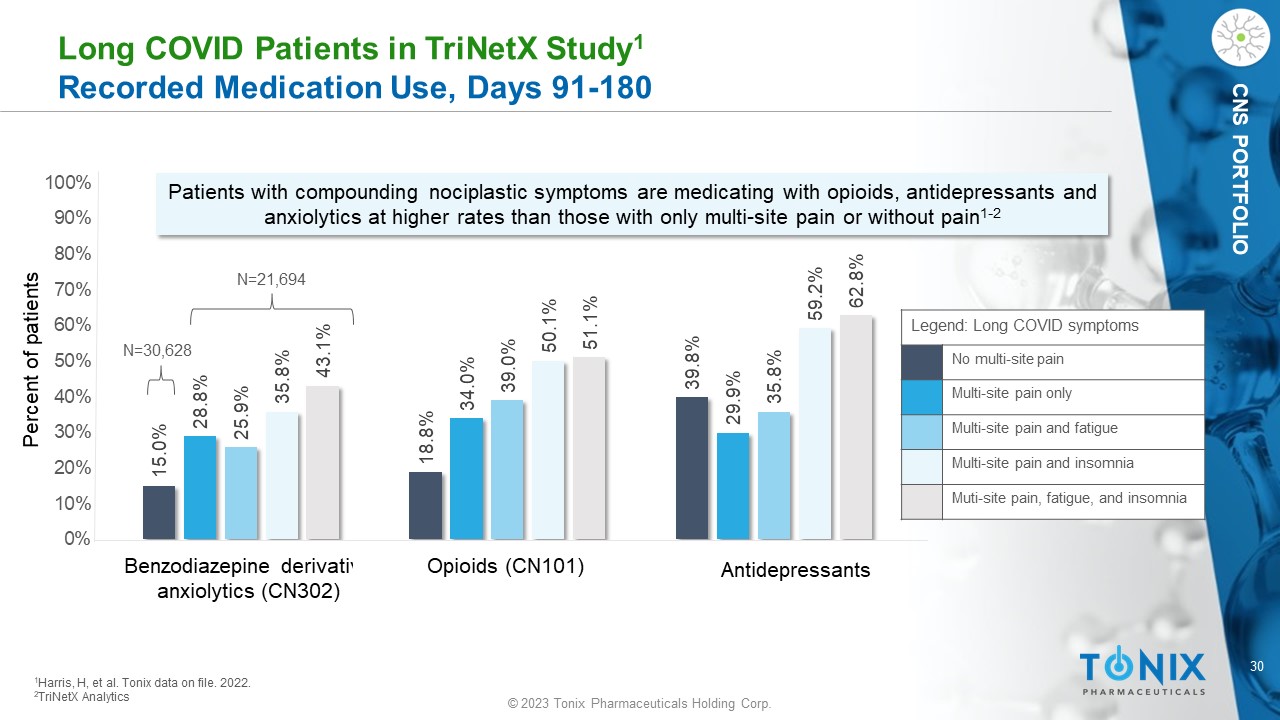
30 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO L ong COVID Patients in TriNetX Study 1 Recorded Medication Use, Days 91 - 180 15.0% 18.8% 39.8% 28.8% 34.0% 29.9% 25.9% 39.0% 35.8% 35.8% 50.1% 59.2% 43.1% 51.1% 62.8% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Patients with compounding nociplastic symptoms are medicating with opioids, antidepressants and anxiolytics at higher rates than those with only multi - site pain or without pain 1 - 2 1 Harris, H, et al. Tonix dat a on file. 2022. 2 TriNetX Analytics Percent of patients Benzodiazepine derivative anxiolytics (CN302) Opioids (CN101) Antidepressants Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia N=21,694 N=30,628
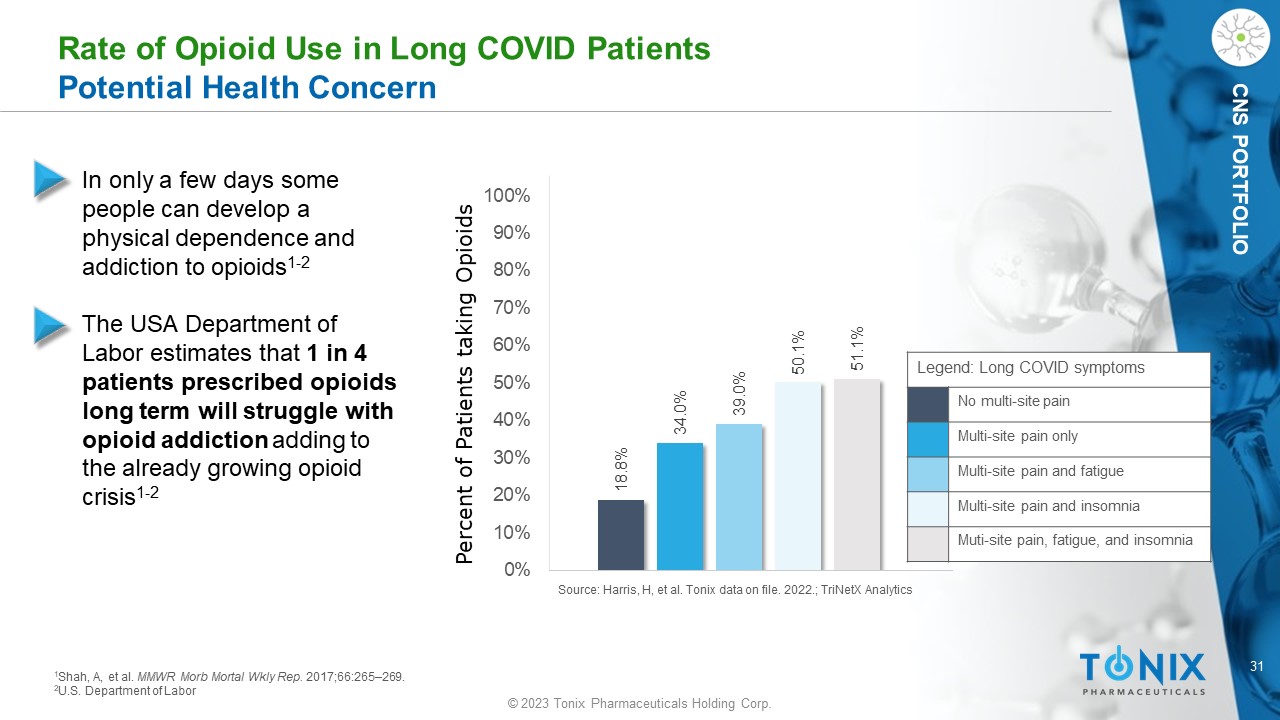
31 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO 18.8% 34.0% 39.0% 50.1% 51.1% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% Percent of Patients taking Opioids Rate of Opioid Use in Long COVID Patients Potential Health Concern • In only a few days some people can develop a physical dependence and addiction to opioids 1 - 2 • The USA Department of Labor estimates that 1 in 4 patients prescribed opioids long term will struggle with opioid addiction adding to the already growing opioid crisis 1 - 2 1 Shah, A, et al. MMWR Morb Mortal Wkly Rep. 2017;66:265 – 269. 2 U.S. Department of Labor Source: Harris, H, et al. Tonix data on file. 2022.; TriNetX Analytics Legend: Long COVID symptoms No multi - site pain Multi - site pain only Multi - site pain and fatigue Multi - site pain and insomnia Muti - site pain, fatigue, and insomnia
32 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Significant Financial Impact of Long COVID for Households and Economies 25% of Long COVID patients are unable to return to work 1 Over 250,000 Quality Adjusted Life - Years (QUALYS) will be lost due to Long COVID in the UK 2 $23.3 billion is estimated to be paid by the UK government to avoid QUALY losses due to Long COVID 2 1 Davis, HE, et al. eClinicalMedicine . 2021;38. 2 Martin, C, et al. PloS one . 2021;16(12):e0260843 - e0260843.
33 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Long COVID Presidential Memorandum President Biden – April 5, 2022 1 Policy • Commits to redoubling efforts to address the long - term effects of COVID - 19 Organizing Government Wide Response • Harnesses the full potential of the Federal Government, in coordination with public - and private - sector partners, to mount a full and effective response National Research Action Plane • Coordinates efforts across the public and private sectors • Orders establishment of the first - ever interagency national research agenda to, among other things, foster development of new treatments based on a better understanding of the pathophysiological mechanisms of the SARS - CoV - 2 virus Previously, Congress awarded NIH $1.15 billion to study Long COVID. 2 • Funded among other things the RECOVER Initiative implemented by the National Institutes of Health. 1 April 5, 2022 President Biden. “Memorandum on Addressing the Long - Term Effects of COVID - 19 - www.whitehouse.gov/briefing - room/pr esidential - actions/2022/04/05/memorandum - on - addressing - the - long - term - effects - of - covid - 19/ 2 The NIH provision of Title III Health and Human Services, Division M -- Coronavirus Response and Relief Supplemental Appropriation s Act, 2021, of H.R. 133, The Consolidated Appropriations Act of 2021. The bill was enacted into law on 27 December 2020, becoming Public Law 116 - 260.