
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

© 2023 Tonix Pharmaceuticals Holding Corp. © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 601 ER Major Depressive Disorder NASDAQ: TNXP Version P04 93 October 16, 2023 (Doc 1327)

© 2023 Tonix Pharmaceuticals Holding Corp. 2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.
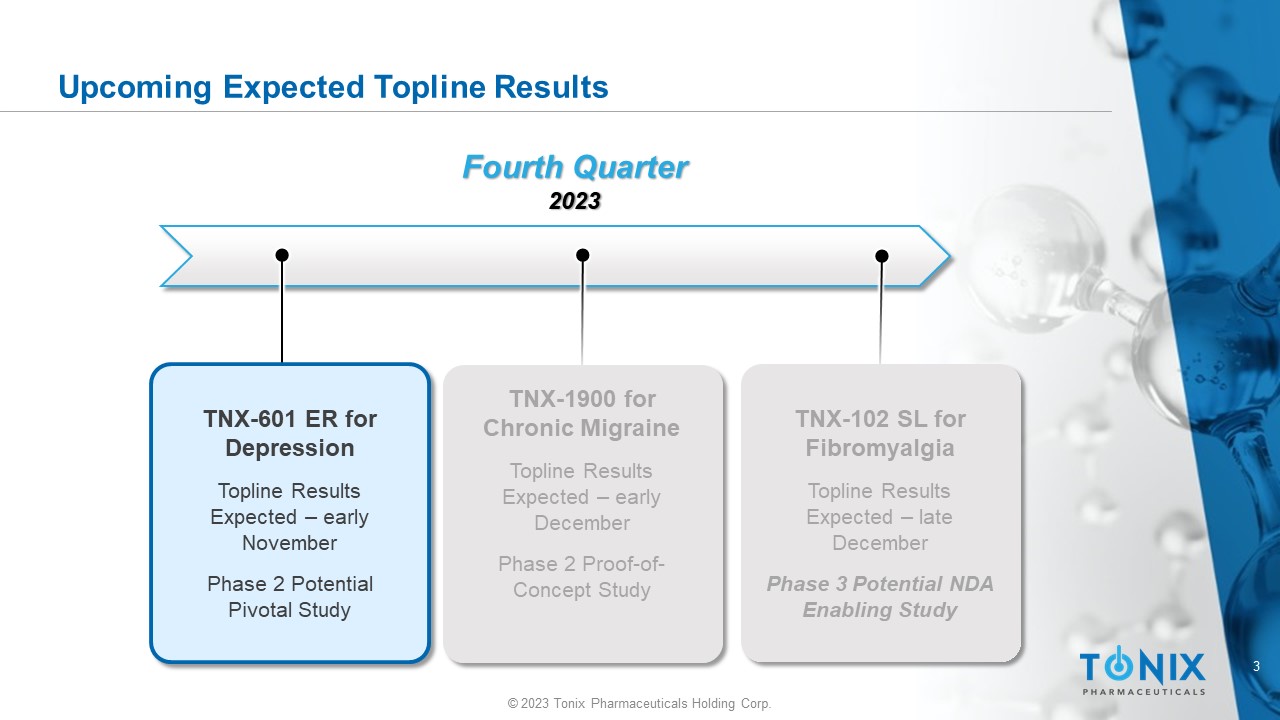
© 2023 Tonix Pharmaceuticals Holding Corp. 3 © 2023 Tonix Pharmaceuticals Holding Corp. Upcoming Expected Topline Results Fourth Quarter 2023 TNX - 1900 for Chronic Migraine Topline Results Expected – early December Phase 2 Proof - of - Concept Study TNX - 601 ER for Depression Topline Results Expected – early November Phase 2 Potential Pivotal Study TNX - 102 SL for Fibromyalgia Topline Results Expected – late December Phase 3 Potential NDA Enabling Study
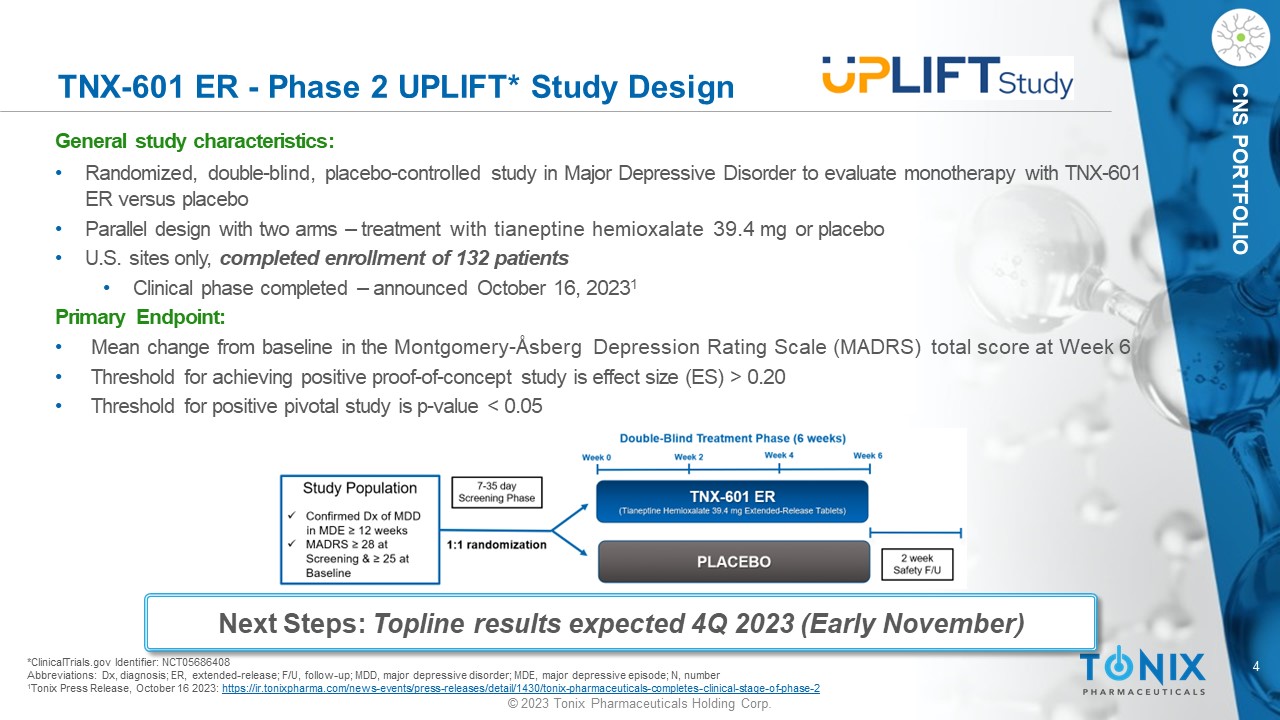
4 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER - Phase 2 UPLIFT* Study Design General s tudy c haracteristics: • Randomized, double - blind, placebo - controlle d study in Major Depressive Disorder to evaluate monotherapy with TNX - 601 ER versus placebo • Parallel design with two arms – treatment with tianeptine hemioxalate 39.4 mg or placebo • U.S. sites only, completed enrollment of 132 patients • Clinical phase completed – announced October 16, 2023 1 Primary Endpoint: • Mean change from baseline in the Montgomery - Åsberg Depression Rating Scale (MADRS) total score at Week 6 • Threshold for achieving positive proof - of - concept study is effect size (ES) > 0.20 • Threshold for positive pivotal study is p - value < 0.05 *ClinicalTrials.gov Identifier: NCT05686408 Abbreviations: Dx, diagnosis; ER, extended - release; F/U, follow - up; MDD, major depressive disorder; MDE, major depressive episo de; N, number 1 Tonix Press Release, October 16 2023: https://ir.tonixpharma.com/news - events/press - releases/detail/1430/tonix - pharmaceuticals - completes - clinical - stage - of - phase - 2 Next Steps: Topline results expected 4Q 2023 (Early November)

© 2023 Tonix Pharmaceuticals Holding Corp. 5 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Major Depressive Disorder (MDD) • Major depressive disorder (MDD) is a leading cause of disability worldwide, with 21 million adults in the US alone experiencing a depressive episode in 2020 1 • L ifetime prevalence of 16%, and associated with important psychological suffering, as well as elevated rates of suicide and worse prognosis of comorbid medical conditions 2,3 • Highly comorbid with other psychiatric disorders (e.g., anxiety disorders, substance use disorders) as well as medical conditions (e.g., cardiovascular disease, metabolic syndromes, respiratory diseases, various deficiencies, infections, collagen disorders, endocrine diseases, etc.) • Hormonal aspects can significantly impact course and treatment (especially evident in post - partum depression) • Most treatment guidelines support use of antidepressants in moderate to severe MDD Epidemiology and Characteristics of Depression 1 Substance Abuse and Mental Health Services Administration (SAMHSA). 2020. Key Substance Use and Mental Health Indicators in t he United States: Results from the 2020 National Survey on Drug Use and Health. 2 Kupfer et al., 2012. The Lancet . 379, 1045 – 1055 3 Otte et al., 2016. Nat. Rev. Dis. Primer . 2:16065
© 2023 Tonix Pharmaceuticals Holding Corp. 6 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO • The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, regarded as the largest antidepressant trial ever conducted, indicated approximately 30% of depressed patients fail to achieve remission , even after multiple treatment attempts 1,2 • SSRIs are currently the most prescribed class of antidepressants, yet only about 50% of patients with MDD respond to initial SSRI treatment , and only 35 - 40% of those patients achieve full remission 1 • Antidepressant treatments often continue for years, and the side effect profiles of the monoaminergic antidepressants are intolerable to many • There is a high unmet need for new classes of antidepressants with different mechanisms of action High Unmet N eed for New C lasses of Antidepressants 1 Rush et al., 2006. Am J Psychiatry. 163:1905 – 1917 2 Rush et al., 2004. Control Clin Trials . 25(1):119 - 42
© 2023 Tonix Pharmaceuticals Holding Corp. 7 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 601 E R*: Depression Tianeptine Hemioxalate Extended - Release Tablets (39.4 mg) CNS PORTFOLIO • A novel, oral, extended - release once - daily tablet • Treatment effect of tianeptine sodium immediate release t.i.d. in depression is well - established • Tianeptine restores neuroplasticity in animal models • PPAR - β / δ and PPAR - γ agonist 1 Differentiators: Relative to tianeptine IR available ex - US: • Once - daily dosing Relative to traditional antidepressants: • Unique mechanism of action – beyond neurotransmitter modulation • Tianeptine sodium IR has similar efficacy but less weight gain or sexual side effects than traditional antidepressants • Tianeptine’s side effects are described in labeling in countries in which it is marketed 2 Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Dysfunction From Corticosteroids, Alzheimer’s Disease 3 Status: Phase 2 MDD study UPLIFT enrollment complete. Clinical phase completed as of October 16, 2023 Next Steps: Topline results expected early November 2023 1 Sullivan G et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 Su mmary of product characteristics ( SmPC), European Medicines Agency, Stablon ®, https://www.servier.com.ve/sites/default/files/spc - pil/spc - stablon.pdf accessed 9 - 25 - 23. 3 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 Abbreviations: IR, immediate release; t .i.d., three times a day *TNX - 601 ER has not been approved for any indication.
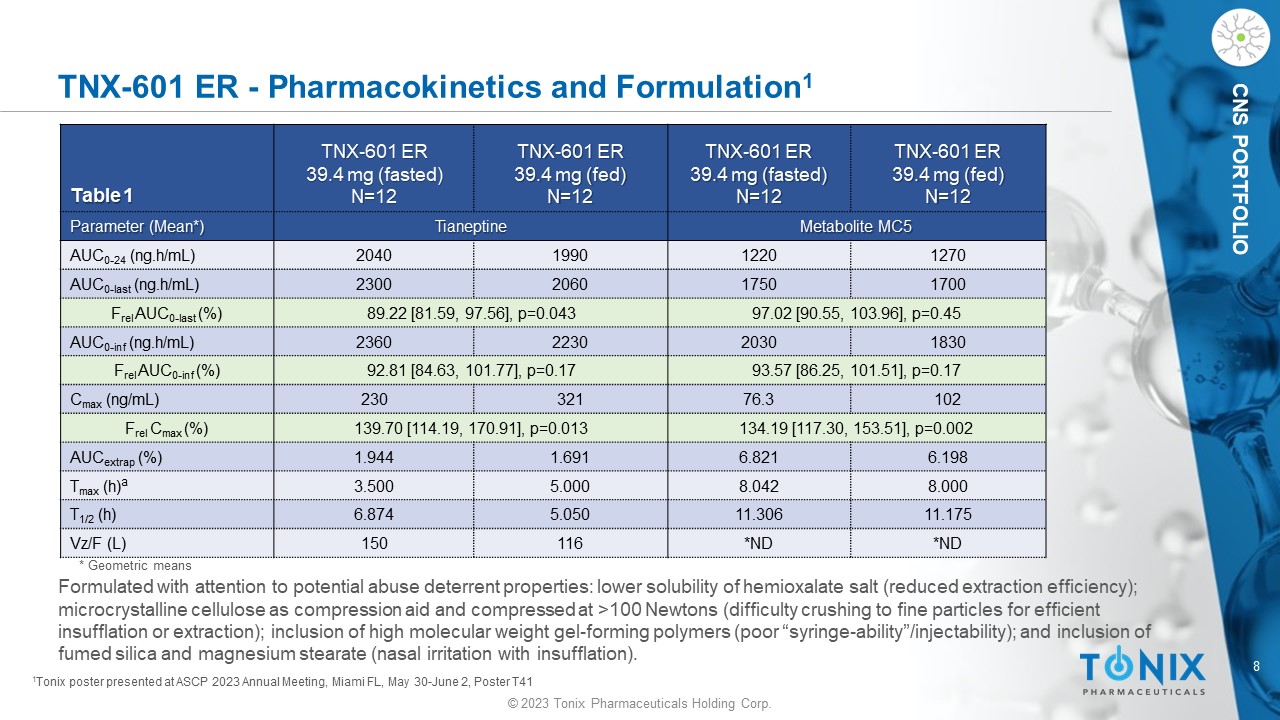
© 2023 Tonix Pharmaceuticals Holding Corp. 8 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER - Pharmacokinetics and Formulation 1 TNX - 601 ER 39.4 mg (fed) N=12 TNX - 601 ER 39.4 mg (fasted) N=12 TNX - 601 ER 39.4 mg (fed) N=12 TNX - 601 ER 39.4 mg (fasted) N=12 Table 1 Metabolite MC5 Tianeptine Parameter (Mean*) 1270 1220 1990 2040 AUC 0 - 24 ( ng.h /mL) 1700 1750 2060 2300 AUC 0 - last ( ng.h /mL) 97.02 [90.55, 103.96], p=0.45 89.22 [81.59, 97.56], p=0.043 F rel AUC 0 - last (%) 1830 2030 2230 2360 AUC 0 - inf ( ng.h /mL) 93.57 [86.25, 101.51], p=0.17 92.81 [84.63, 101.77], p=0.17 F rel AUC 0 - inf (%) 102 76.3 321 230 C max (ng/mL) 134.19 [117.30, 153.51], p=0.002 139.70 [114.19, 170.91], p=0.013 F rel C max (%) 6.198 6.821 1.691 1.944 AUC extrap (%) 8.000 8.042 5.000 3.500 T max (h) a 11.175 11.306 5.050 6.874 T 1/2 (h) *ND *ND 116 150 Vz /F (L) Formulated with attention to potential abuse deterrent properties: lower solubility of hemioxalate salt (reduced extraction efficiency); microcrystalline cellulose as compression aid and compressed at >100 Newtons (difficulty crushing to fine particles for effic ien t insufflation or extraction); inclusion of high molecular weight gel - forming polymers (poor “syringe - ability”/injectability); and inclusion of fumed silica and magnesium stearate (nasal irritation with insufflation). 1 Tonix poster presented at ASCP 2023 Annual Meeting, Miami FL, May 30 - June 2, Poster T41 * Geometric means

© 2023 Tonix Pharmaceuticals Holding Corp. 9 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO History of Tianeptine and TNX - 601 ER CNS PORTFOLIO • Tianeptine sodium (amorphous) immediate release (IR) tablets have been available in Europe and many countries in Asia and Latin America for the treatment of MDD since it was first marketed in France in 1989. Due to its short half - life, tianeptine sodium IR is taken three times daily, which is challenging for patient adherence. • Currently, there is no tianeptine - containing product approved in the U.S. and no extended - release tianeptine product approved anywhere in the world. • Tonix discovered a novel hemioxalate salt of tianeptine that may provide improved stability, consistency, and manufacturability compared to known salt forms of tianeptine. • TNX - 601 ER is taken once daily, increasing patient adherence and is thereby anticipated to improve the overall effectiveness of treatment compared to that of tianeptine sodium IR.
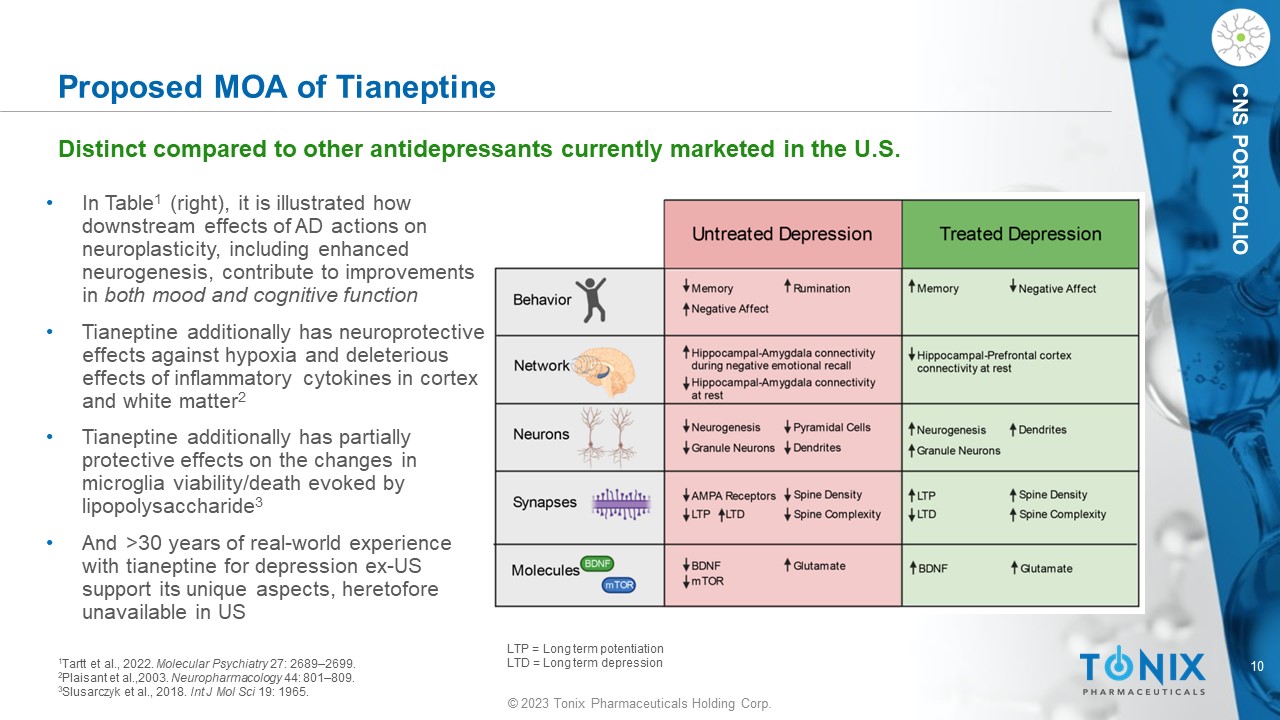
© 2023 Tonix Pharmaceuticals Holding Corp. 10 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO 1 Tartt et al., 2022. Molecular Psychiatry 27: 2689 – 2699. 2 Plaisant et al.,2003. Neuropharmacology 44: 801 – 809. 3 Slusarczyk et al., 2018. Int J Mol Sci 19: 1965. • In Table 1 (right), it is illustrated how downstream effects of AD actions on neuroplasticity, including enhanced neurogenesis, contribute to improvements in both mood and cognitive function • Tianeptine additionally has neuroprotective effects against hypoxia and deleterious effects of inflammatory cytokines in cortex and white matter 2 • Tianeptine additionally has partially protective effects on the changes in microglia viability/death evoked by lipopolysaccharide 3 • And >30 years of real - world experience with tianeptine for depression ex - US support its unique aspects, heretofore unavailable in US Proposed MOA of Tianeptine Distinct compared to other antidepressants currently marketed in the U.S. LTP = Long term potentiation LTD = Long term depression
© 2023 Tonix Pharmaceuticals Holding Corp. 11 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Clinical Trials of Tianeptine Sodium • Antidepressant efficacy confirmed in multicenter double - blind, placebo - controlled, randomized trials 1,2 • Enriched enrollment randomized withdrawal design trial of long - term (16.5 months) treatment demonstrated reduction of MDD relapse and recurrence by 2 - to 3 - fold compared to placebo 3 • Head - to - head comparisons showing equivalent efficacy of tianeptine with: ‒ TCAs ▪ Imipramine 1 ▪ Amitriptyline 4,5,6 ‒ SSRIs ▪ Fluoxetine 4,7 ▪ Sertraline 8 ▪ Paroxetine 9,10,11 ▪ Escitalopram 12 ‒ Mianserin 13 • Rigorous meta - analysis 14,15 of studies comparing tianeptine to SSRIs concluded tianeptine at least as effective as SSRIs, and trend noted for better overall acceptability profile in treatment of depressed patients Placebo - controlled and comparative trials in depression 1 Cassono et al., 1996. Eur Psychiatry. 11(5):254 - 9 2 Costa e Silva et al., 1997. Neuropsychobiology . 35(1):24 - 9 3 Dalery et al., 2001. Hum Psychopharmacol .16(S1):S39 - S47 4 Lôo et al., 1999. Neuropsychobiology .19(2):79 - 85 5 Guelfi et al., 1989. Neuropsychobiology . 22(1):41 - 8 11 Nickel et al., 2003. J Clin Psychopharmacol . 23(2):155 - 68 12 Emsley et al., 2018. J Clin Psychiatry. 79(4):17m11741 13 Brion et al., 1996. Presse Med . 25(9):461 - 8 14 Kasper et al., 2002. Eur Psychiatry . 17 Suppl 3:331 - 40 15 Olié et al., 2003. Encephale . 29(4 Pt 1):322 - 8 6 Invernizzi et al., 1994. Neuropsychobiology . 30(2 - 3):85 - 93 7 Novotny et al., 2002. Hum Psychopharmacol . 17(6):299 - 303 8 Szádóczky et al., 2002. Encephale . 28(4):343 - 9 9 Lepine et al., 2001. Hum Psychopharmacol . 16(3):219 - 227 10 Waintraub et al., 2002. CNS Drugs . 16(1):65 - 75

© 2023 Tonix Pharmaceuticals Holding Corp. 12 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tianeptine’s Off - target Activity • Because of low affinity binding and agonist activity on µ - opioid receptor 1 , there is the potential abuse liability of tianeptine drug substance when available in large quantities by ‒ People seeking a µ - opioid “high” ‒ People self - managing withdrawal effects from opioids • Based on these µ – opioid data and interpretations, 1 unregulated tianeptine entered the US ‒ As a research chemical - not for human use ‒ As an ingredient in food supplements sold over the counter ‒ Without any submitted data or regulatory status, promoted as a “smart drug” (nootropic) sold over the internet Illicit or unregulated introduction of the drug substance to the United States 1 Gassaway et al., 2014. Transl Psychiatry . 4(7):e411
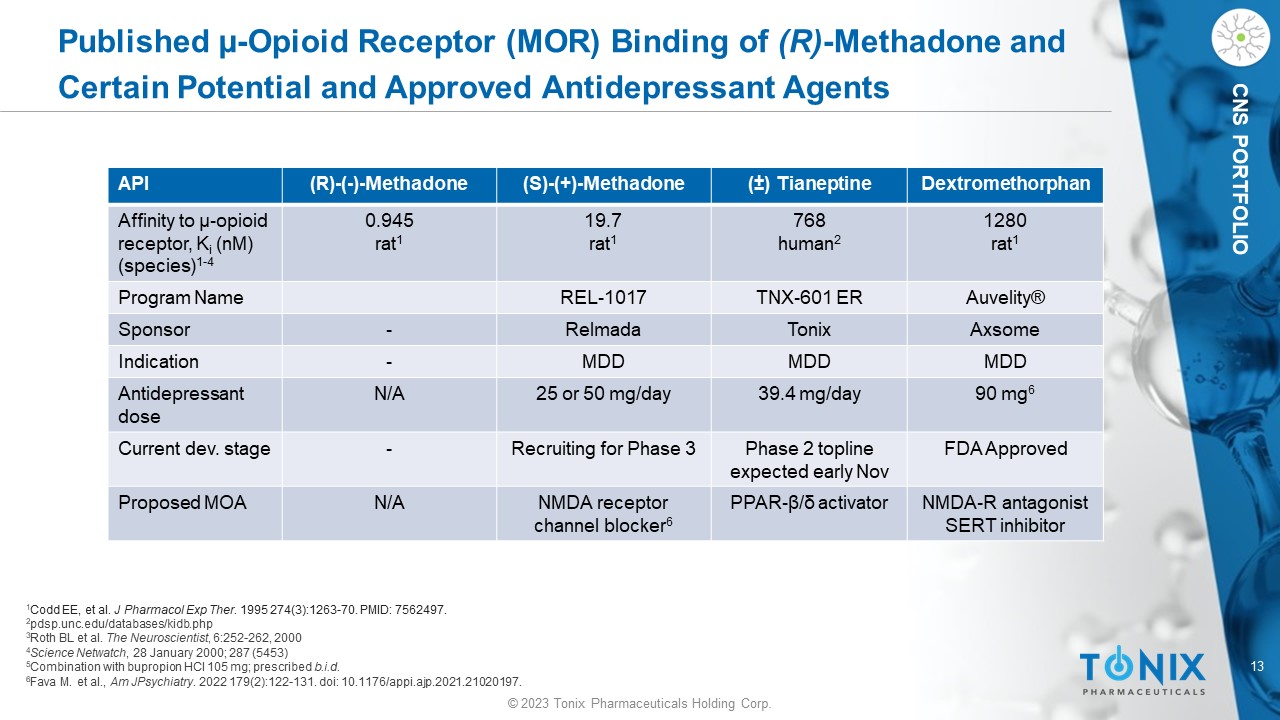
© 2023 Tonix Pharmaceuticals Holding Corp. 13 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Published µ - Opioid Receptor (MOR) Binding of (R) - Methadone and Certain Potential and Approved Antidepressant Agents 1 Codd EE, et al. J Pharmacol Exp Ther . 1995 274(3):1263 - 70. PMID: 7562497. 2 pdsp.unc.edu/databases/ kidb.php 3 Roth BL et al. The Neuroscientist , 6:252 - 262, 2000 4 Science Netwatch , 28 January 2000; 287 (5453) 5 Combination with bupropion HCl 105 mg; prescribed b.i.d. 6 Fava M. et al., Am JPsychiatry . 2022 179(2):122 - 131. doi : 10.1176/appi.ajp.2021.21020197. Dextromethorphan ( ± ) Tianeptine (S) - (+) - Methadone (R) - ( - ) - Methadone API 1280 rat 1 768 human 2 19.7 rat 1 0.945 rat 1 Affinity to µ - opioid receptor, K i ( nM ) (species) 1 - 4 Auvelity ® TNX - 601 ER REL - 1017 Program Name Axsome Tonix Relmada - Sponsor MDD MDD MDD - Indication 90 mg 6 39.4 mg/day 25 or 50 mg/day N/A Antidepressant dose FDA Approved Phase 2 topline expected early Nov Recruiting for Phase 3 - Current dev. stage NMDA - R antagonist SERT inhibitor PPAR - β/δ activator NMDA receptor channel blocker 6 N/A Proposed MOA

© 2023 Tonix Pharmaceuticals Holding Corp. 14 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Prescription Tianeptine has Low Incidence of Abuse • Tianeptine and its MC5 metabolite are weak opioid (µ - opioid) receptor (MOR) agonists 1 that present a potential abuse liability if illicitly misused in large quantities ‒ Typically abused at 8 - 80 times the therapeutic dose on a daily basis 2 . • Post - marketing research in France showed that in patients who were prescribed tianeptine for depression, the incidence of misuse was approximately 1 case per 1,000 patients treated 3 ‒ Suggests low abuse liability when used at the antidepressant dose • Clinical trials have shown that abrupt cessation of a therapeutic course of tianeptine does not result in dependence or withdrawal symptoms following a treat ment duration of : ‒ 6 - weeks 4 - 8 ‒ 3 - months 9 ‒ 12 - months 10 Low activity at µ - opioid receptor is associated with low misuse of prescription oral tianeptine 1 Gassaway et al., 2014. Transl Psychiatry . 4(7):e411 2 Lauhan et al., 2018. Psychosomatics. 59(6), 547 – 53 3 Haute Authorite de Sante. Transparency Committee Opinion. Stablon 12.5 Mg, Coated Tablet, Re - Assessment of Actual Benefit at the Request of the Transparency Committee. December 5, 2012. 4 Emsley et al., 2018. J. Clin. Psychiatry . 79 (4) 5 Bonierbale et al., 2003. Curr Med Res Opin . 19(2):114 - 124 6 Guelfi et al., 1989. Neuropsychobiology . 22 (1), 41 – 48 7 Invernizzi et al., 1994. Neuropsychobiology . 30 (2 – 3), 85 – 93 8 Lepine et al., 2001. Hum. Psychopharmacol . 16 (3), 219 – 227 9 Guelfi et al., 1992. Neuropsychobiology . 25 (3), 140 – 148. 10 Lôo et al., 1992. Br. J. Psychiatry. Suppl. No. 15, 61 – 65.

© 2023 Tonix Pharmaceuticals Holding Corp. 15 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER Drug product • The only abuse - deterrent properties approved for the labels of certain marketed opioids are extended - release formulations with physiochemical barriers +/ - aversive components to abuse • TNX - 601 ER was formulated with attention to these potentially abuse deterrent properties: ‒ Active ingredient, tianeptine oxalate less soluble than sodium salt, reducing extraction efficiency in solvents such as water and alcohol ‒ Microcrystalline cellulose is a compression aid that results in extremely hard tablets, reducing ability to crush to fine particulate matter for insufflation or efficient extraction, pressed at >100 Newtons ‒ Inclusion of high molecular weight gel - forming polymers also adversely affects the “syringe - ability” and injectability of the drug product ‒ Inclusion of hydrophilic fumed silica as well as magnesium stearate may cause nasal irritation if insufflated; in high doses, orally ingested magnesium stearate may cause GI hyperactivity and irritation ‒ All potentially serve to make TNX - 601 ER a non - optimal source of tianeptine for high dose abuse TNX - 601 ER formulated with attention to FDA - guided potential abuse deterrent properties* *https://www.fda.gov/drugs/information - drug - class/final - guidance - evaluation - and - labeling - abuse - deterrent - opioids
© 2023 Tonix Pharmaceuticals Holding Corp. 16 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Summary: TNX - 601 ER vs. Other Antidepressants • Given tianeptine’s unique metabolic pathway, which is independent of the hepatic P450 system, it is anticipated that, like tianeptine sodium, TNX - 601 ER will have a reduced risk of drug - drug interactions compared to most antidepressants • Unique mechanism of action (MOA) compared to available antidepressants in the U.S. • The efficacy of tianeptine sodium IR is comparable to both selective serotonin reuptake inhibitor (SSRI) and tricyclic antidepressants 1,2 while being associated with a low incidence of sexual dysfunction compared with either of those classes 3 , and no associated derangement of sleep architecture, sedative effects, weight gain, or cognitive impairment 1 • Once - daily dosing regimen compared to tianeptine sodium IR at three times a day 1 Wagstaff et al., 2001. CNS Drugs . 15(3), 231 – 259 2 Kasper et al., 2002. Eur Psychiatry. 17 (Suppl 3), 331 - 340 3 Bonierbale et al., 2003. Curr Med Res Opin . 19(2):114 - 124
© 2023 Tonix Pharmaceuticals Holding Corp. 17 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Looking Forward: Additional Potential Indications for TNX - 601 ER • Neurodegenerative disorders ‒ Parkinson’s (and associated conditions, e.g. depression and psychosis) 1 ‒ Alzheimer’s (and associated conditions, e.g. agitation, depression and psychosis) 2 • ADHD 3 • Stress disorders 4 ‒ PTSD, Anxiety • Aging/Neuroprotection 5,6 ‒ Mild Cognitive Impairment • Asthma 7 • Overlapping chronic pain syndromes ‒ Fibromyalgia 8 ‒ Irritable bowel syndrome • Addiction ‒ Opiate use disorder 9 ‒ Alcohol use disorder 1 Levin, 2007. Neurosci Behav Physiol . 37(4):419 - 24 2 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 3 Niederhofer et al., 2004. Neuropsychobiology . 49(3): 130 - 3. 4 Krystal et al., 2009. Drug Discov Today . 14(13 - 14):690 - 697 5 Yoo et al., 2015. J Affect Disord . 185:24 - 30.6 6 Saiz - Ruiz et al., 1998. Prog. Neuro - Psychopharmacol . & Bio. Psychiat . 22(2): 319 - 329 Informed by clinical data and mechanistic insights 7 Lechin et al., 2004. Methods Find Exp Clin Pharmacol . 26(9): 697 – 701 8 “ISRCTN16400909 – Tianeptine for the treatment of fibromyalgia: a prospective double - blind, randomised , single - centre , placebo - controlled, parallel group study" . Controlled - trials.com. Archived from the original on 21 July 2010. Retrieved 13 August 2010 9 Chu et al., 2010. Behav Pharmacol . 21(5 - 6):523 - 9
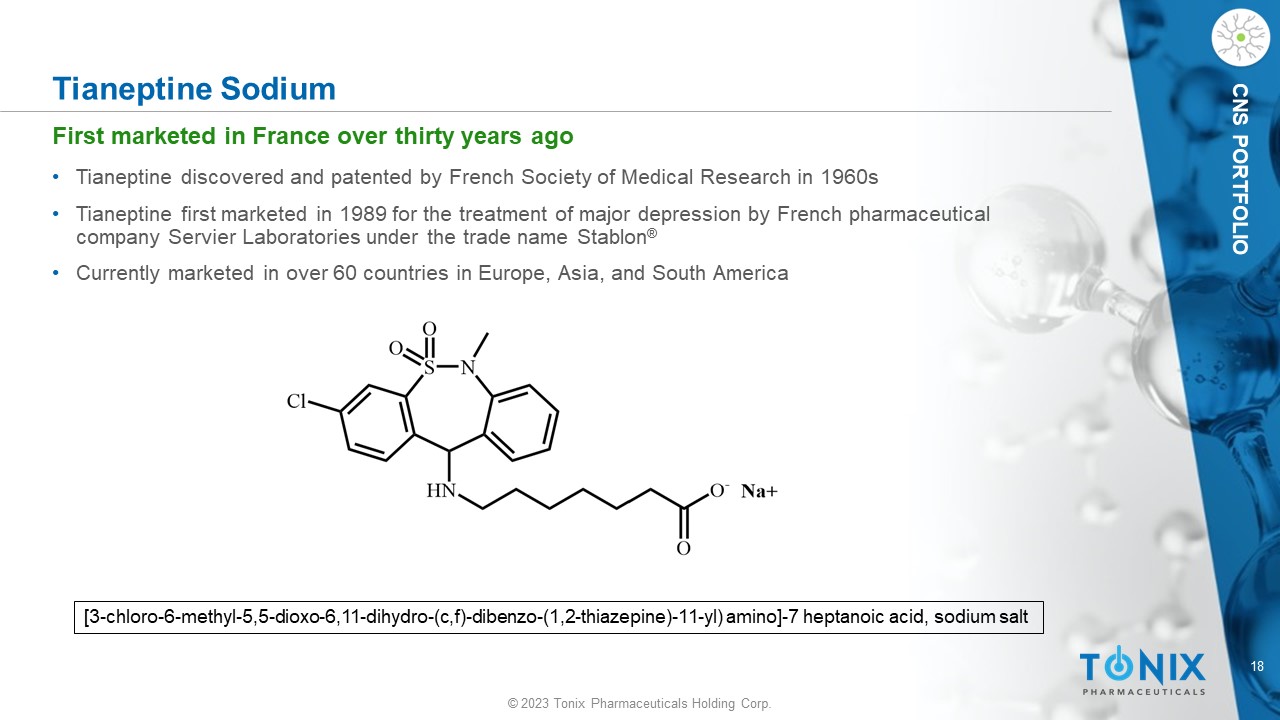
© 2023 Tonix Pharmaceuticals Holding Corp. 18 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tianeptine Sodium • Tianeptine discovered and patented by French Society of Medical Research in 1960s • Tianeptine first marketed in 1989 for the treatment of major depression by French pharmaceutical company Servier Laboratories under the trade name Stablon ® • Currently marketed in over 60 countries in Europe, Asia, and South America [3 - chloro - 6 - methyl - 5,5 - dioxo - 6,11 - dihydro - ( c,f ) - dibenzo - (1,2 - thiazepine) - 11 - yl) amino] - 7 heptanoic acid, sodium salt First marketed in France over thirty years ago
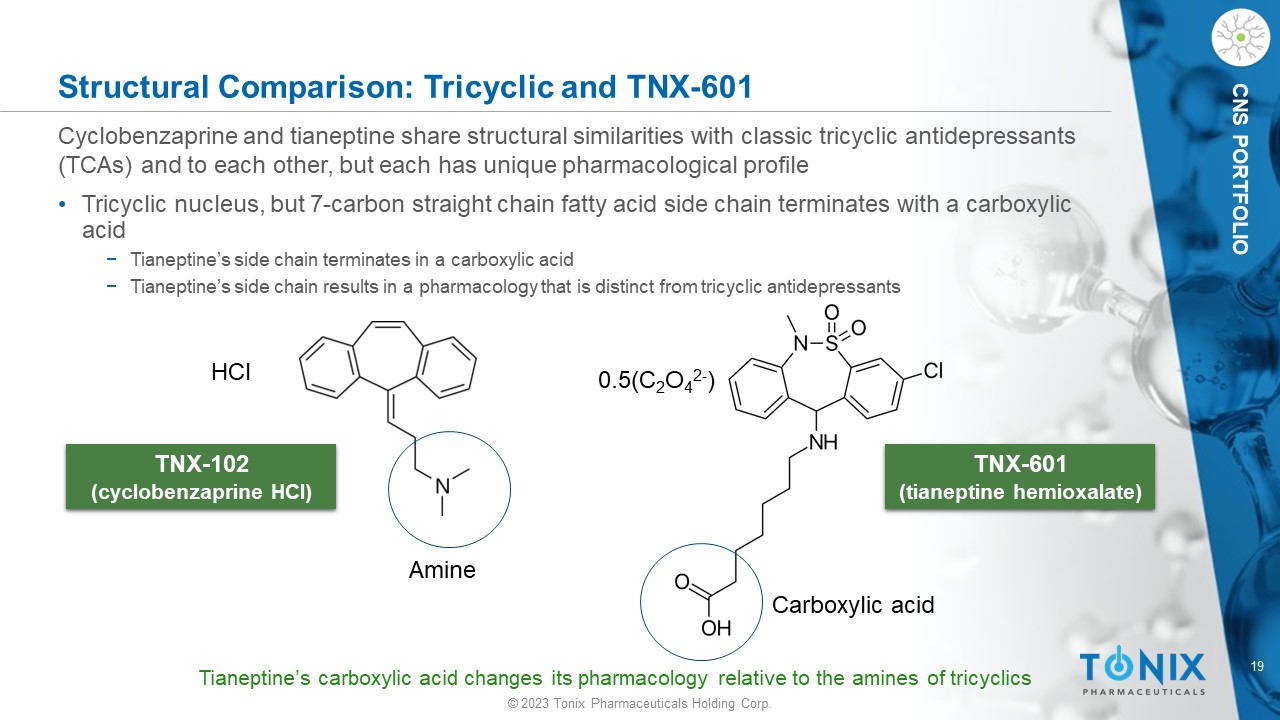
© 2023 Tonix Pharmaceuticals Holding Corp. 19 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Structural Comparison: Tricyclic and TNX - 601 Cyclobenzaprine and tianeptine share structural similarities with classic tricyclic antidepressants (TCAs) and to each other, but each has unique pharmacological profile • Tricyclic nucleus, but 7 - carbon straight chain fatty acid side chain terminates with a carboxylic acid − Tianeptine’s side chain terminates in a carboxylic acid − Tianeptine’s side chain results in a pharmacology that is distinct from tricyclic antidepressants TNX - 102 (cyclobenzaprine HCl) TNX - 601 (tianeptine hemioxalate ) HCl 0.5(C 2 O 4 2 - ) Tianeptine’s carboxylic acid changes its pharmacology relative to the amines of tricyclics Carboxylic acid Amine
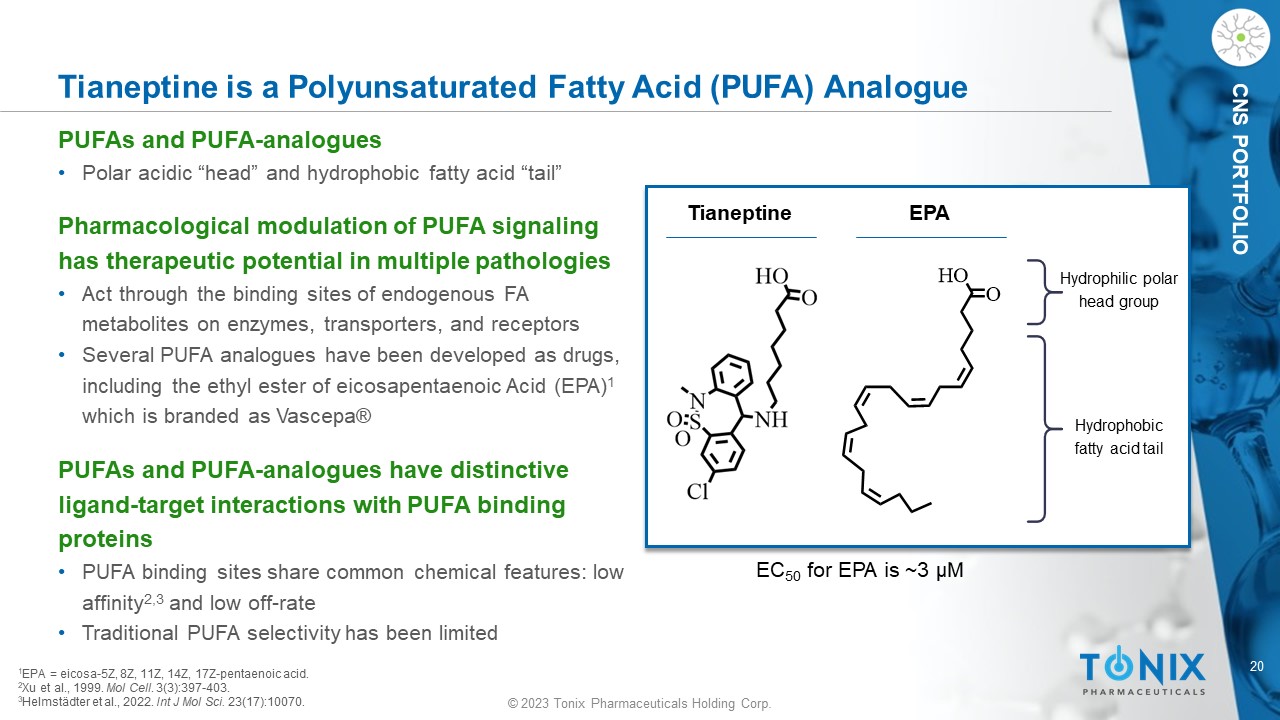
© 2023 Tonix Pharmaceuticals Holding Corp. 20 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tianeptine is a Polyunsaturated Fatty Acid (PUFA) Analogue PUFAs and PUFA - analogues • Polar acidic “head” and hydrophobic fatty acid “tail” Pharmacological modulation of PUFA signaling has therapeutic potential in multiple pathologies • Act through the binding sites of endogenous FA metabolites on enzymes, transporters, and receptors • Several PUFA analogues have been developed as drugs, including the ethyl ester of eicosapentaenoic Acid (EPA) 1 which is branded as Vascepa ® PUFAs and PUFA - analogues have distinctive ligand - target interactions with PUFA binding proteins • PUFA binding sites share common chemical features: low affinity 2,3 and low off - rate • Traditional PUFA selectivity has been limited EPA Hydrophilic polar head group Hydrophobic fatty acid tail 1 EPA = e icosa - 5Z, 8Z, 11Z, 14Z, 17Z - pentaenoic acid. 2 Xu et al., 1999. Mol Cell . 3(3):397 - 403. 3 Helmstädter et al., 2022. Int J Mol Sci. 23(17):10070. Tianeptine EC 50 for EPA is ~3 µM
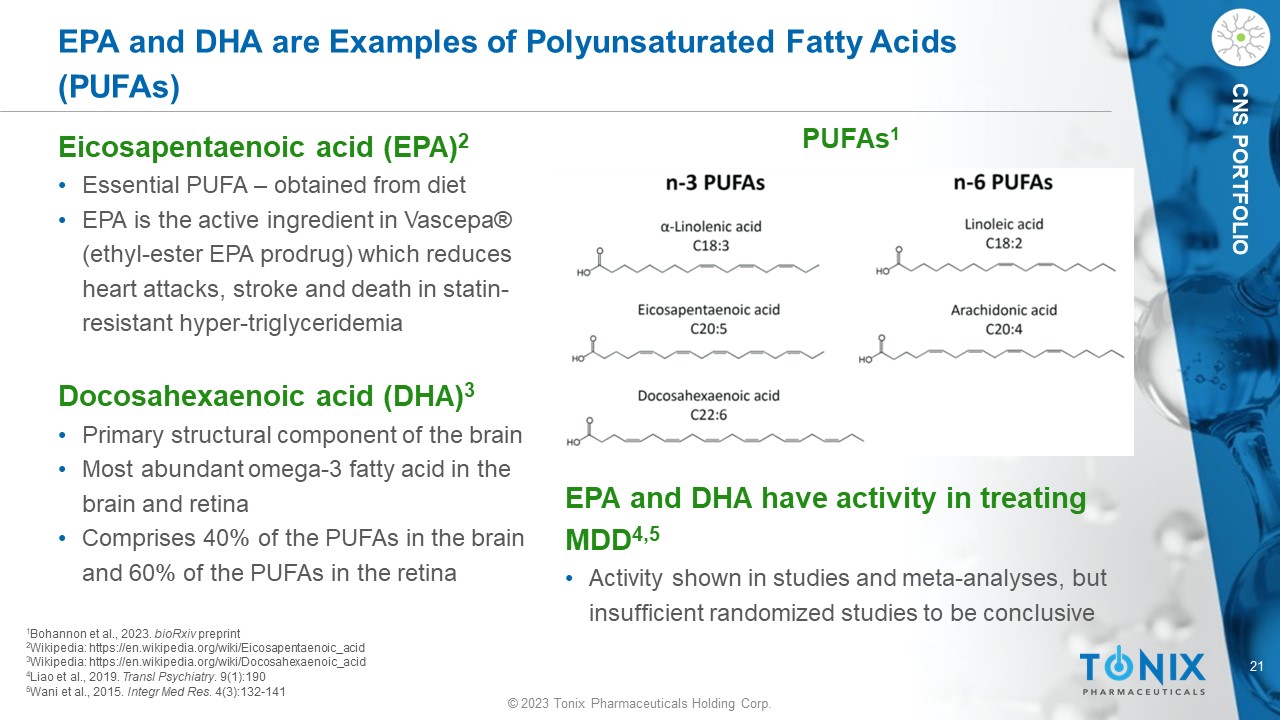
© 2023 Tonix Pharmaceuticals Holding Corp. 21 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO EPA and DHA are Examples of Polyunsaturated Fatty Acids (PUFAs) PUFAs 1 1 Bohannon et al., 2023. bioRxiv preprint 2 Wikipedia: https://en.wikipedia.org/wiki/Eicosapentaenoic_acid 3 Wikipedia: https://en.wikipedia.org/wiki/Docosahexaenoic_acid 4 Liao et al., 2019. Transl Psychiatry . 9(1):190 5 Wani et al., 2015. Integr Med Res . 4(3):132 - 141 E icosapentaenoic acid (EPA ) 2 • Essential PUFA – obtained from diet • EPA is the active ingredient in Vascepa ® (ethyl - ester EPA prodrug) which reduces heart attacks, stroke and death in statin - resistant hyper - triglyceridemia Docosahexaenoic acid (DHA) 3 • Primary structural component of the brain • Most abundant omega - 3 fatty acid in the brain and retina • Comprises 40% of the PUFAs in the brain and 60% of the PUFAs in the retina EPA and DHA have activity in treating MDD 4,5 • Activity shown in studies and meta - analyses, but insufficient randomized studies to be conclusive
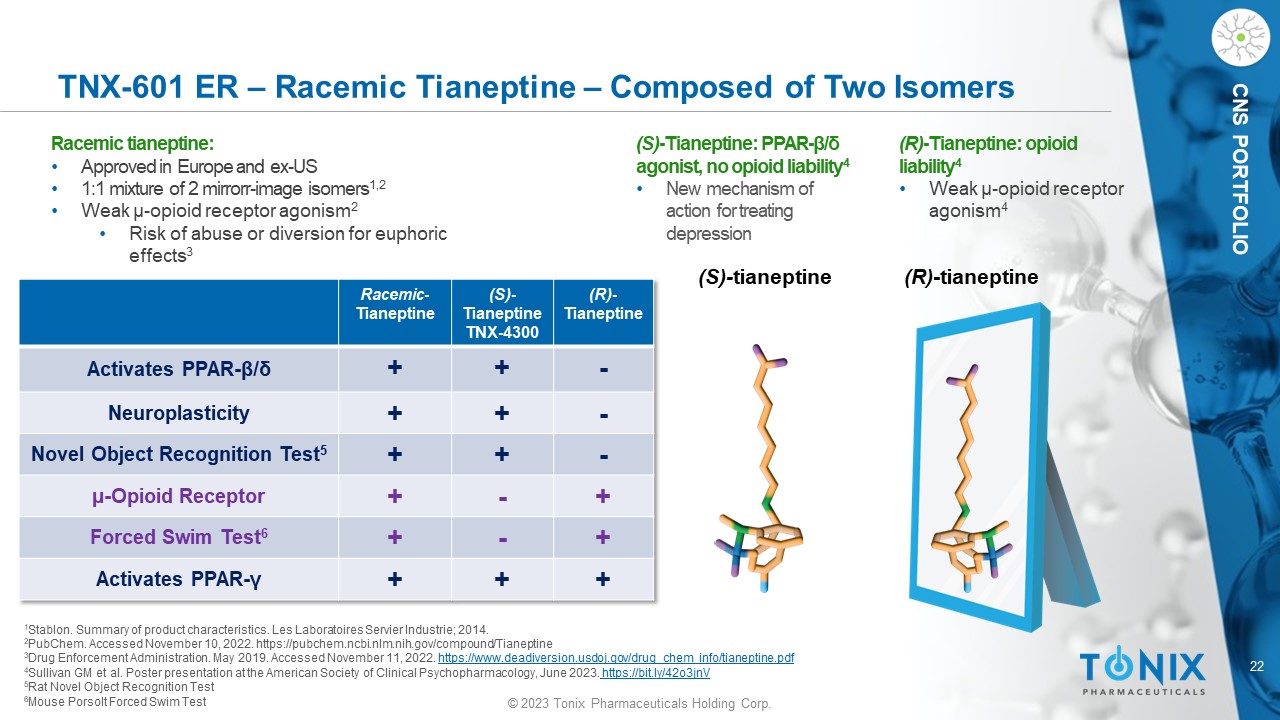
© 2023 Tonix Pharmaceuticals Holding Corp. 22 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO TNX - 601 ER – Racemic Tianeptine – Composed of Two Isomers Racemic tianeptine : • Approved in Europe and ex - US • 1:1 mixture of 2 mirrorr - image isomers 1,2 • Weak µ - o pioid receptor agonism 2 • Risk of abuse or diversion for euphoric effects 3 (S) - t ianeptine (R) - tianeptine 1 Stablon. Summary of product characteristics. Les Laboratoires Servier Industrie ; 2014. 2 PubChem. Accessed November 10, 2022. https://pubchem.ncbi.nlm.nih.gov/compound/Tianeptine 3 Drug Enforcement Administration. May 2019. Accessed November 11, 2022 . https://www.deadiversion.usdoj.gov/drug_chem_info/tianeptine.pdf 4 Sullivan GM et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023 . https://bit.ly/42o3jnV 5 Rat Novel Object Recognition Test 6 Mouse Porsolt Forced Swim Test (R) - Tianeptine (S) - Tianeptine TNX - 4300 Racemic - Tianeptine - + + Activates PPAR - β / δ - + + Neuroplasticity - + + Novel Object Recognition Test 5 + - + µ - Opioid Receptor + - + Forced Swim Test 6 + + + Activates PPAR - γ (S) - Tianeptine: PPAR - β / δ agonist, no opioid liability 4 • New mechanism of action for treating depression (R) - Tianeptine: opioid liability 4 • Weak µ - o pioid receptor agonism 4
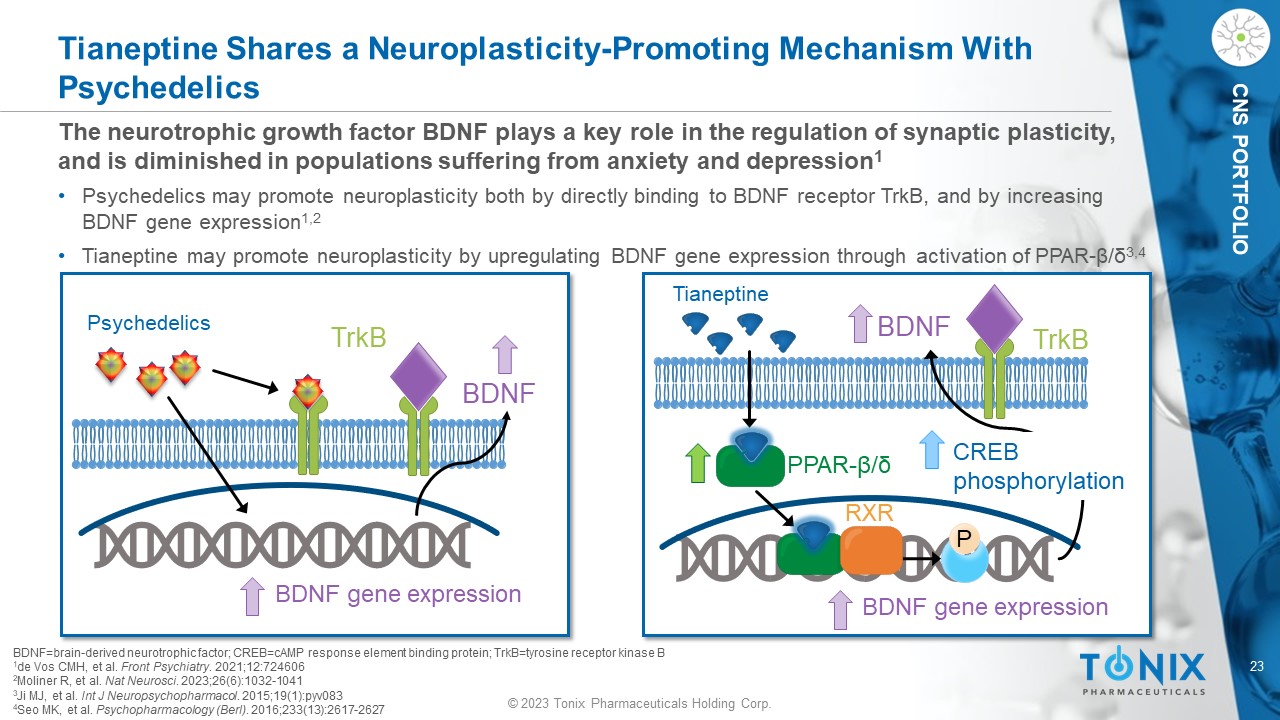
© 2023 Tonix Pharmaceuticals Holding Corp. 23 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO The n eurotrophic growth factor BDNF plays a key role in the regulation of synaptic plasticity, and is diminished in populations suffering from anxiety and depression 1 • Psychedelics may promote neuroplasticity both by directly binding to BDNF receptor TrkB , and by increasing BDNF gene expression 1,2 • Tianeptine may promote neuroplasticity by upregulating BDNF gene expression through activation of PPAR - β/δ 3,4 Tianeptine Shares a Neuroplasticity - Promoting Mechanism With Psychedelics BDNF=brain - derived neurotrophic factor; CREB=cAMP response element binding protein; TrkB =tyrosine receptor kinase B 1 de Vos CMH, et al. Front Psychiatry . 2021;12:724606 2 Moliner R, et al. Nat Neurosci . 2023;26(6):1032 - 1041 3 Ji MJ, et al. Int J Neuropsychopharmacol . 2015;19(1):pyv083 4 Seo MK, et al. Psychopharmacology ( Berl ) . 2016;233(13):2617 - 2627 Tianeptine PPAR - β/δ RXR P BDNF BDNF gene expression CREB phosphorylation TrkB Psychedelics BDNF BDNF gene expression TrkB
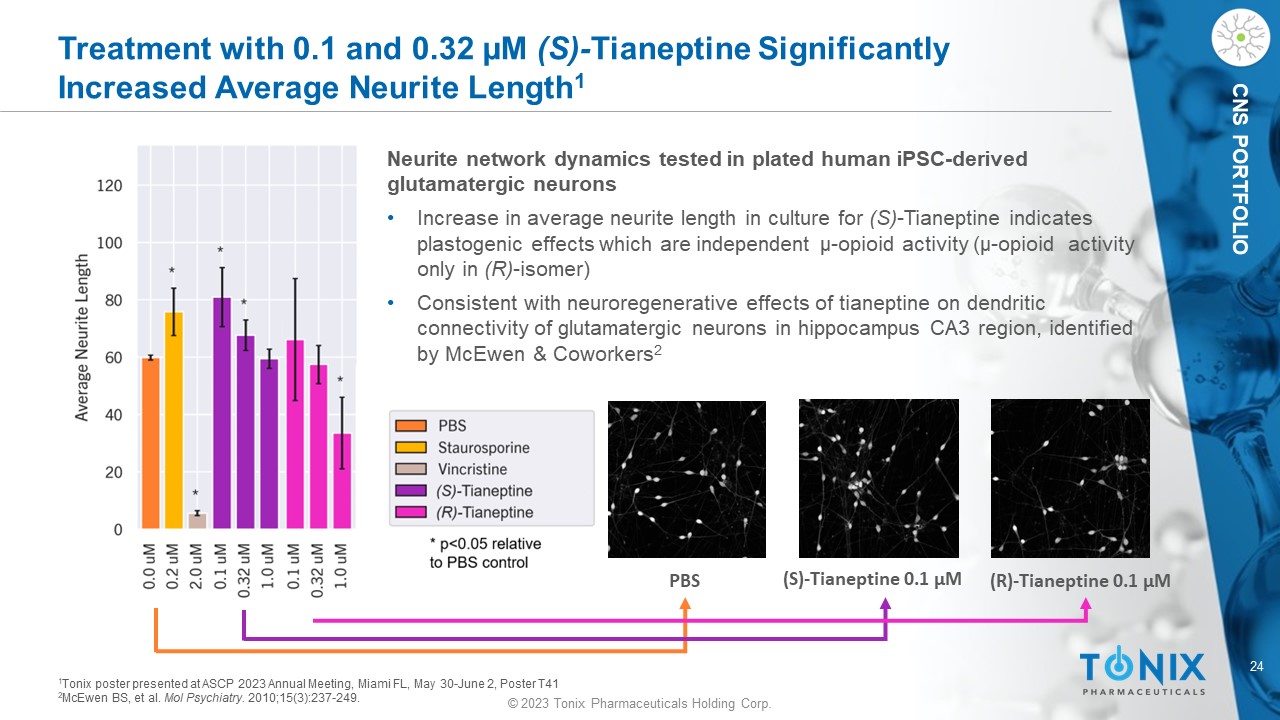
© 2023 Tonix Pharmaceuticals Holding Corp. 24 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Treatment with 0.1 and 0.32 µM (S) - Tianeptine Significantly I ncreased A verage N eurite L ength 1 PBS (S) - Tianeptine 0.1 µM (R) - Tianeptine 0.1 µM Neurite network dynamics tested in plated human iPSC - derived glutamatergic neurons • Increase in average neurite length in culture for (S) - Tianeptine indicates plastogenic effects which are independent µ - opioid activity (µ - opioid activity only in (R) - isomer) • Consistent with neuroregenerative effects of tianeptine on dendritic connectivity of glutamatergic neurons in hippocampus CA3 region, identified by McEwen & Coworkers 2 1 Tonix poster presented at ASCP 2023 Annual Meeting, Miami FL, May 30 - June 2, Poster T41 2 McEwen BS, et al. Mol P sychiatry. 2010;15(3):237 - 249.
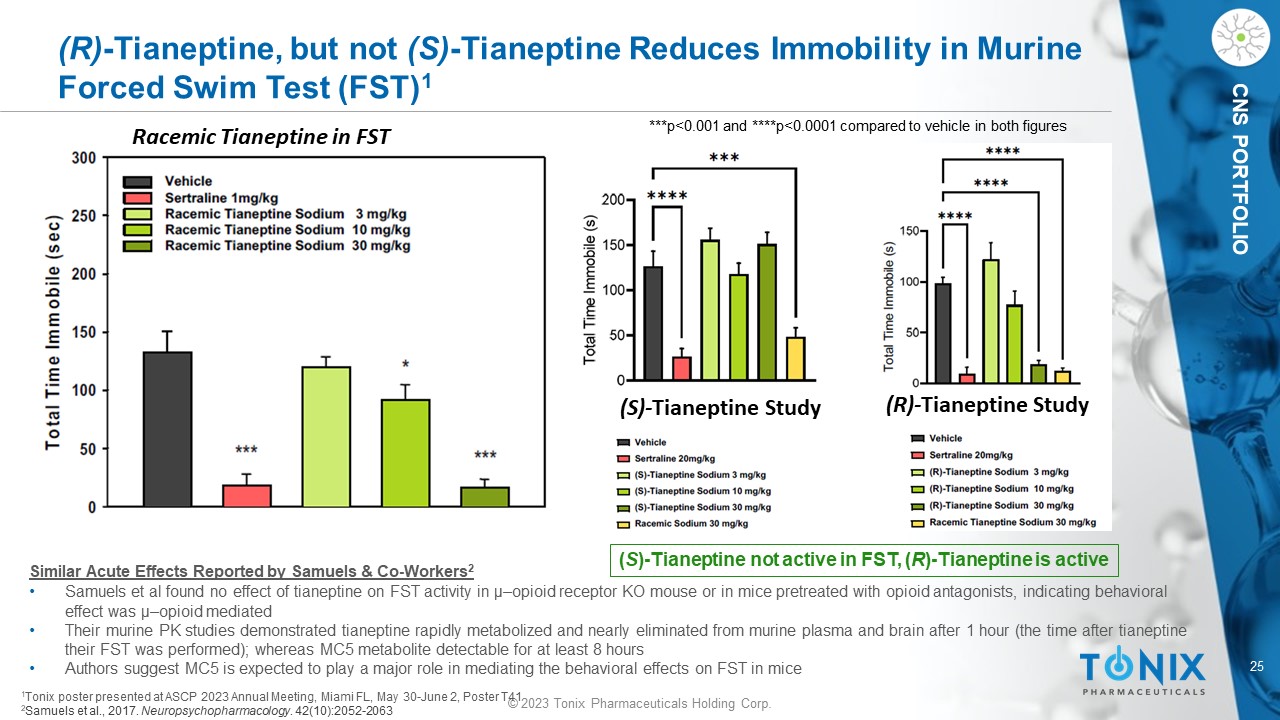
© 2023 Tonix Pharmaceuticals Holding Corp. 25 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO (R) - Tianeptine, but not (S) - Tianeptine Reduces Immobility in Murine Forced Swim Test (FST) 1 (S) - Tianeptine Study (R) - Tianeptine Study Racemic Tianeptine in FST Similar Acute Effects Reported by Samuels & Co - Workers 2 • Samuels et al found no effect of tianeptine on FST activity in µ – opioid receptor KO mouse or in mice pretreated with opioid anta gonists, indicating behavioral effect was µ – opioid mediated • Their murine PK studies demonstrated tianeptine rapidly metabolized and nearly eliminated from murine plasma and brain after 1 h our (the time after tianeptine their FST was performed); whereas MC5 metabolite detectable for at least 8 hours • Authors suggest MC5 is expected to play a major role in mediating the behavioral effects on FST in mice ( S ) - Tianeptine not active in FST, ( R ) - Tianeptine is active 1 Tonix poster presented at ASCP 2023 Annual Meeting, Miami FL, May 30 - June 2, Poster T41 2 Samuels et al., 2017. Neuropsychopharmacology . 42(10):2052 - 2063 ***p<0.001 and ****p<0.0001 compared to vehicle in both figures
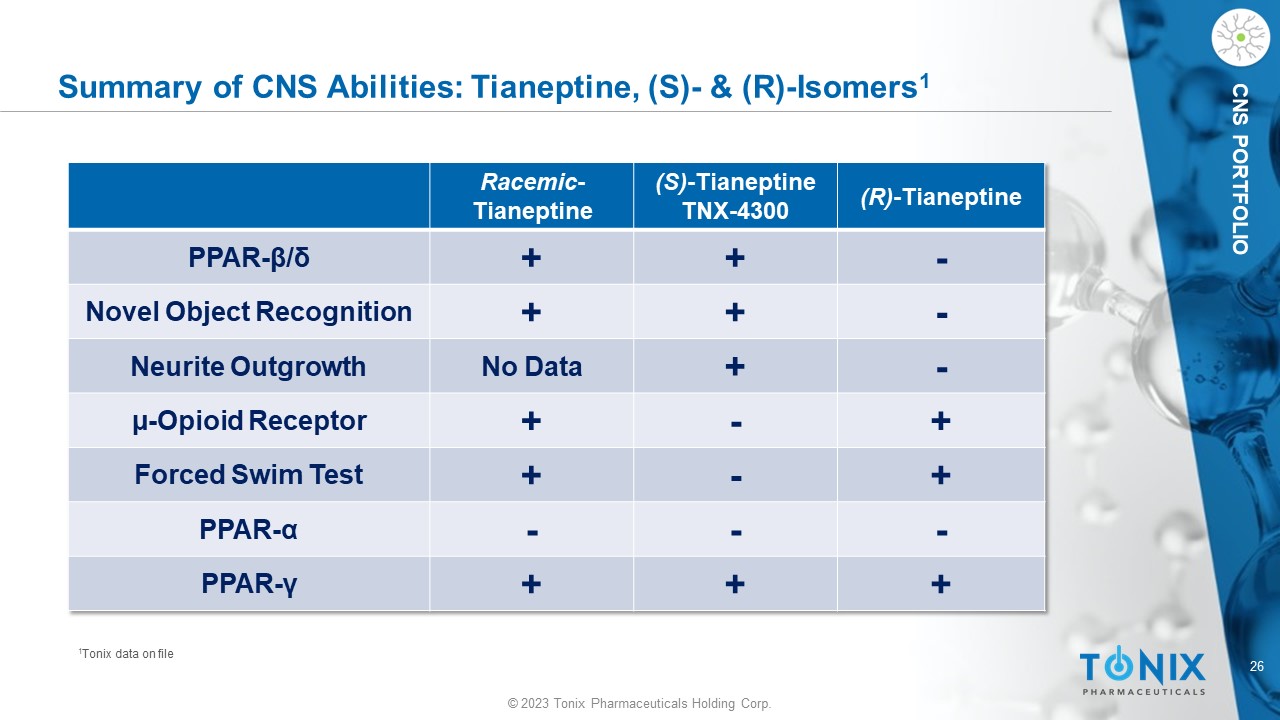
© 2023 Tonix Pharmaceuticals Holding Corp. 26 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Summary of CNS Abilities: Tianeptine, (S) - & (R) - Isomers 1 (R) - Tianeptine (S) - Tianeptine TNX - 4300 Racemic - Tianeptine - + + PPAR - β / δ - + + Novel Object Recognition - + No Data Neurite Outgrowth + - + µ - Opioid Receptor + - + Forced Swim Test - - - PPAR - α + + + PPAR - γ 1 Tonix data on file

© 2023 Tonix Pharmaceuticals Holding Corp. 27 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PROFILE DEVELOPMENT PROGRAM Patents Issued TNX - 4300*: Depression, Alzheimer’s & Parkinson’s diseases Estianeptine (Single (S) - isomer of Tianeptine) CNS PORTFOLIO • Single isomer, oral treatment • Proposed mechanism of action from lab studies indicates estianeptine is the active ingredient of TNX - 601 ER 1 • PPAR - β / δ and PPAR - γ agonist • Free of µ - opioid receptor activity • Estianeptine restores neuroplasticity in tissue culture Differentiators: Relative to racemic tianeptine IR or TNX - 601 ER: • Lack of opioid liability Relative to traditional antidepressants: • Unique mechanism of action – beyond neurotransmitter modulation • Racemic tianeptine sodium IR has similar efficacy but fewer side effects than traditional antidepressants Market Entry: Major Depressive Disorder (MDD) Additional Indications: PTSD, Neurocognitive Disorder From Corticosteroids, Alzheimer’s Disease 2 Status: Pre - clinical Next Steps: Potential fo r IND to be supported by pre - clinical and clinical data from TNX - 601 (racemic tianeptine) development 1 Sullivan GM et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023 . https://bit.ly/42o3jnV 2 García - Alberca et al., 2022. J Alzheimers Dis . 88(2):707 - 720 *TNX - 4300 is in the pre - IND stage of development and has not been approved for any indication
© 2023 Tonix Pharmaceuticals Holding Corp. 28 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PPAR - β / δ Targeted by Two Other Programs • Bezafibrate is a dual PPAR - β /δ / PPAR - γ agonist in a P - o - C trial for bipolar depression 1,2 ‒ Study underway at Mass General ‒ PPAR δ/ δ and PPAR - γ co - activator 1 α are targets validated by pre - clinical and clinical studies 3 • T3D - 959 is a dual is a dual PPAR - β /δ / PPAR - γ agonist in development for Alzheimer’s 4 - 8 ‒ T3D Therapeutics is developing it for Alzheimers after previous work (by DARA BioSciences as DB959 in Phase 1 trials for dyslipidemia and Type 2 diabetes) ‒ Phase 1 and 2 studies reportedly encouraging 5 - 8 1 Nierenberg, AA, Principal Investigator, Massachusetts General Hospital. “ BezafibrateTreatment for Bipolar Depression: A Proof of Concept Study.” h ttps://classic.clinicaltrials.gov/ct2/show/NCT02481245 2 Tenenbaum A, et al. Cardiovasc Diabetol . 2005 4:14. doi : 10.1186/1475 - 2840 - 4 - 14. PMID: 16168052; PMCID: PMC1236941. 3 Nierenberg AA, et al. Biol Psychiatry. 2018 83(9):761 - 769. doi : 10.1016/j.biopsych.2017.12.014. Epub 2018 Jan 10. PMID: 29502862. 4 Chamberlain S, et al. J Alzheimers Dis. 2020;73(3):1085 - 1103. doi : 10.3233/JAD - 190864. PMID: 31884472; PMCID: PMC7081093. 5 Tong M, et al. J Alzheimers Dis Parkinsonism . 2016 6(3):238. doi : 10.4172/2161 - 0460.1000238. Epub 2016 Jun 3. PMID: 27525190; PMCID: PMC4979550. 6 www.t3dtherapeutics.com/wp - content/uploads/2017/01/CTAD - Presentation - 09 - Dec - 2016_website - posting.pdf 7 www.t3dtherapeutics.com/2023/10/04/t3d - therapeutics - selected - to - present - topline - results - from - the - phase - 2 - pioneer - study - of - t3d - 95 9 - as - late - breaking - news - at - the - 16th - clinical - trials - on - alzheimers - disease - conference - ctad/ 8 www.t3dtherapeutics.com/lead - product - candidate - t3d - 959/
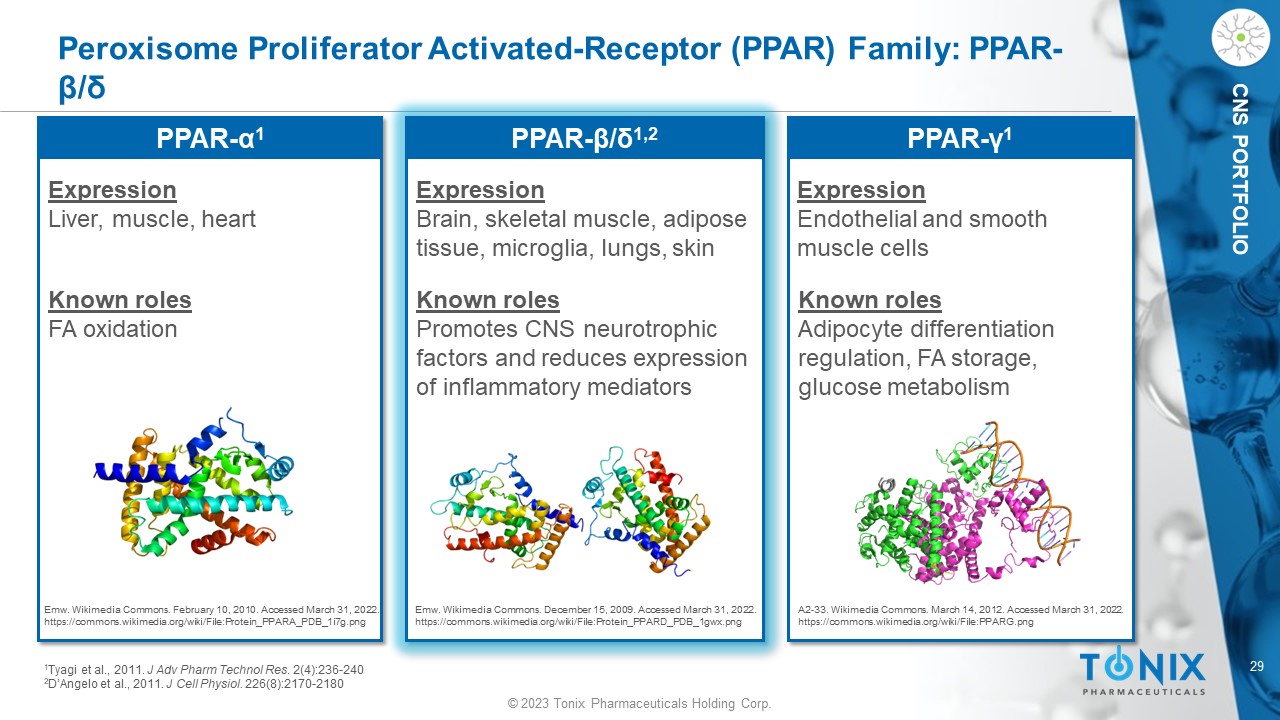
© 2023 Tonix Pharmaceuticals Holding Corp. 29 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Peroxisome Proliferator Activated - Receptor (PPAR) Family: PPAR - β/δ Emw . Wikimedia Commons . February 10, 2010. Accessed March 31, 2022. https://commons.wikimedia.org/wiki/File:Protein_PPARA_PDB_1i7g.png Emw . Wikimedia Commons . December 15, 2009. Accessed March 31, 2022. https://commons.wikimedia.org/wiki/File:Protein_PPARD_PDB_1gwx.png A2 - 33. Wikimedia Commons . March 14, 2012. Accessed March 31, 2022. https://commons.wikimedia.org/wiki/File:PPARG.png Expression Liver, muscle, heart Known roles FA oxidation Expression Brain, skeletal muscle, adipose tissue, microglia, lungs, skin Known roles Promotes CNS neurotrophic factors and reduces expression of inflammatory mediators Expression Endothelial and smooth muscle cells Known roles Adipocyte differentiation regulation, FA storage, glucose metabolism PPAR - α 1 PPAR - β/δ 1,2 PPAR - γ 1 1 Tyagi et al., 2011. J Adv Pharm Technol Res . 2(4):236 - 240 2 D’Angelo et al., 2011. J Cell Physiol. 226(8):2170 - 2180

© 2023 Tonix Pharmaceuticals Holding Corp. 30 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PPAR - β / δ Protects Against Pathology in CNS Animal Models • PPAR - β /δ normally protects against pathophysiological processes in the nervous system 1 ‒ PPAR - β /δ also plays roles in neuronal development and function • PPAR - β /δ - deficient mice exhibited abnormal neurophysiological processes ‒ Decreased myelination, augmented inflammatory reactions and low score in memory tests 2 ‒ Tau (τ) hyperphosphorylation, astrogliosis and CNS inflammation 3 ‒ Worse outcome after cerebral ischemia with defective antioxidant responses 4,5 • Selective PPAR - β /δ agonists improve outcome after: ‒ Experimental Autoimmune Encephalomyelitis 6 ‒ Experimental cerebral ischemia 7 ‒ Transgenic model of Alzheimer’s 8 ‒ Spinal cord trauma 9 ‒ Ischemic stroke related vascular dysfunction 10 ‒ Chemically induced Parkinson’s 7,11 1 Kahremany et al., 2015. Br J Pharmacol . 172(3):754 - 70 2 Peters et al., 2000. Mol Cell Biol. 20:5119 – 5128 3 Barroso et al., 2013. Biochim Biophys Acta. 1832:1241 – 1248 4 Arsenijevic D, et al. J Cereb Blood Flow Metab . 2006;26:433 – 445 5 Pialat et al., 2007. NMR Biomed. 20:335 – 342 6 Polak et al., 2005. J Neuroimmunol . 168:65 – 75 7 Iwashita et al., 2007. J Pharmacol Exp Ther . 320:1087 – 1096 8 Kalinin et al., 2009. Curr Alzheimer Res. 6:431 – 437 9 Paterniti et al., 2010. J Pharmacol Exp Ther . 333:465 – 477 10 Yin et al., 2010. J Neurosci . 30:6398 – 6408 11 Martin et al., 2013. Neuroscience. 240:191 – 203
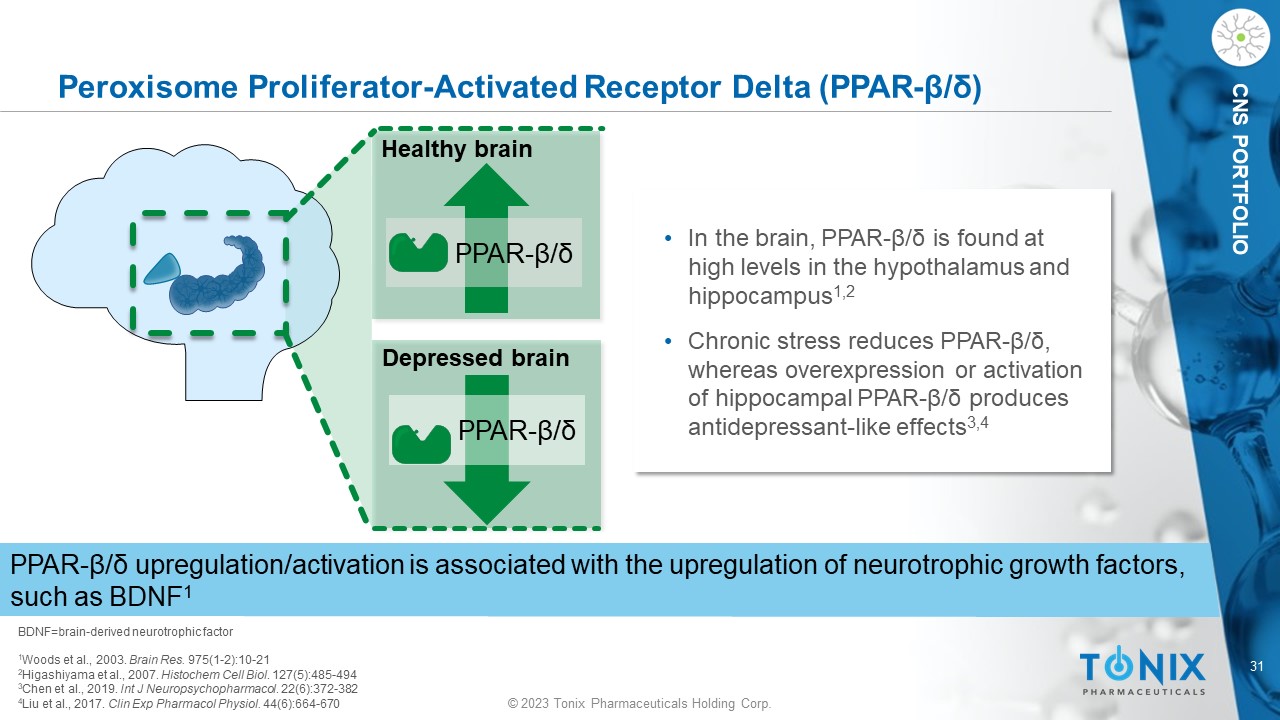
© 2023 Tonix Pharmaceuticals Holding Corp. 31 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Peroxisome Proliferator - Activated Receptor Delta (PPAR - β/δ) BDNF=brain - derived neurotrophic factor 1 Woods et al., 2003. Brain Res. 975(1 - 2):10 - 21 2 Higashiyama et al., 2007. Histochem Cell Biol . 127(5):485 - 494 3 Chen et al., 2019. Int J Neuropsychopharmacol . 22(6):372 - 382 4 Liu et al., 2017. Clin Exp Pharmacol Physiol. 44(6):664 - 670 • In the brain, PPAR - β/δ is found at high levels in the hypothalamus and hippocampus 1,2 • Chronic stress reduces PPAR - β/δ, whereas overexpression or activation of hippocampal PPAR - β/δ produces antidepressant - like effects 3,4 PPAR - β/δ upregulation/activation is associated with the upregulation of neurotrophic growth factors, such as BDNF 1 PPAR - β/δ Healthy brain PPAR - β/δ Depressed brain
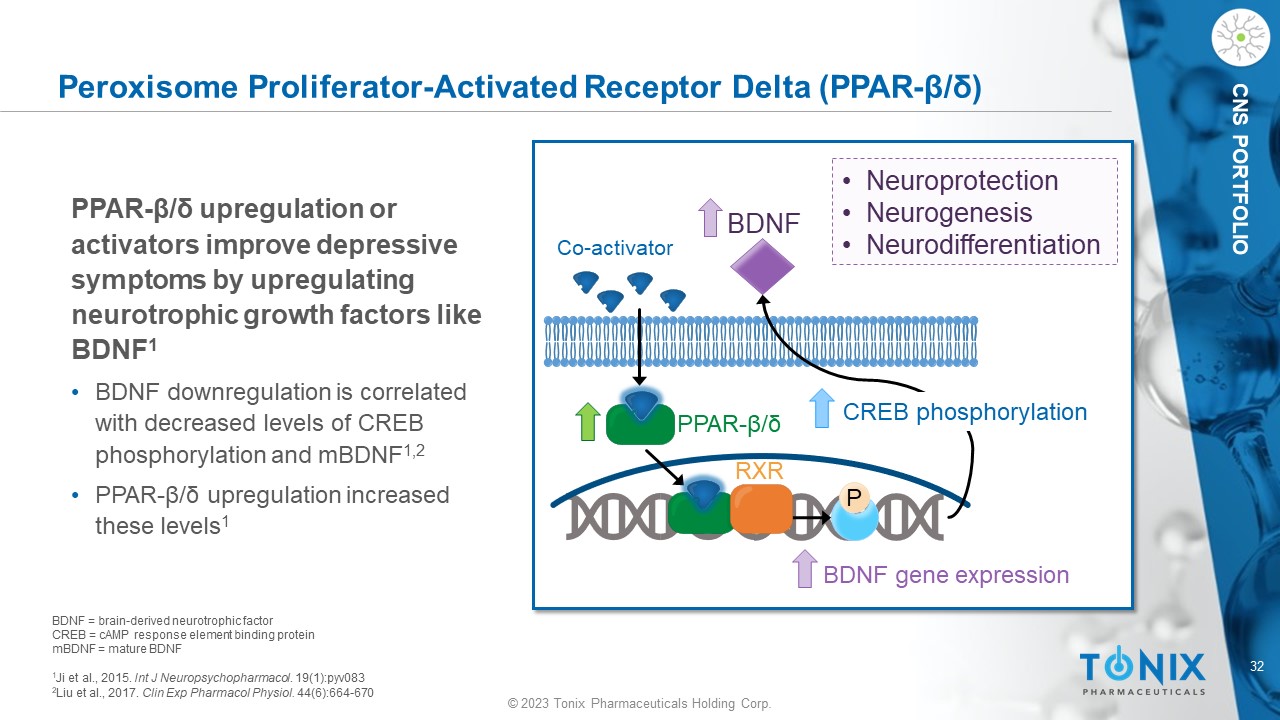
© 2023 Tonix Pharmaceuticals Holding Corp. 32 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Peroxisome Proliferator - Activated Receptor Delta (PPAR - β/δ) BDNF = brain - derived neurotrophic factor CREB = cAMP response element binding protein mBDNF = mature BDNF 1 Ji et al., 2015. Int J Neuropsychopharmacol . 19(1):pyv083 2 Liu et al., 2017. Clin Exp Pharmacol Physiol. 44(6):664 - 670 Co - activator PPAR - β/δ RXR P BDNF BDNF gene expression • Neuroprotection • Neurogenesis • Neurodifferentiation CREB phosphorylation PPAR - β/δ upregulation or activators improve depressive symptoms by upregulating neurotrophic growth factors like BDNF 1 • BDNF downregulation is correlated with decreased levels of CREB phosphorylation and mBDNF 1,2 • PPAR - β/δ upregulation increased these levels 1
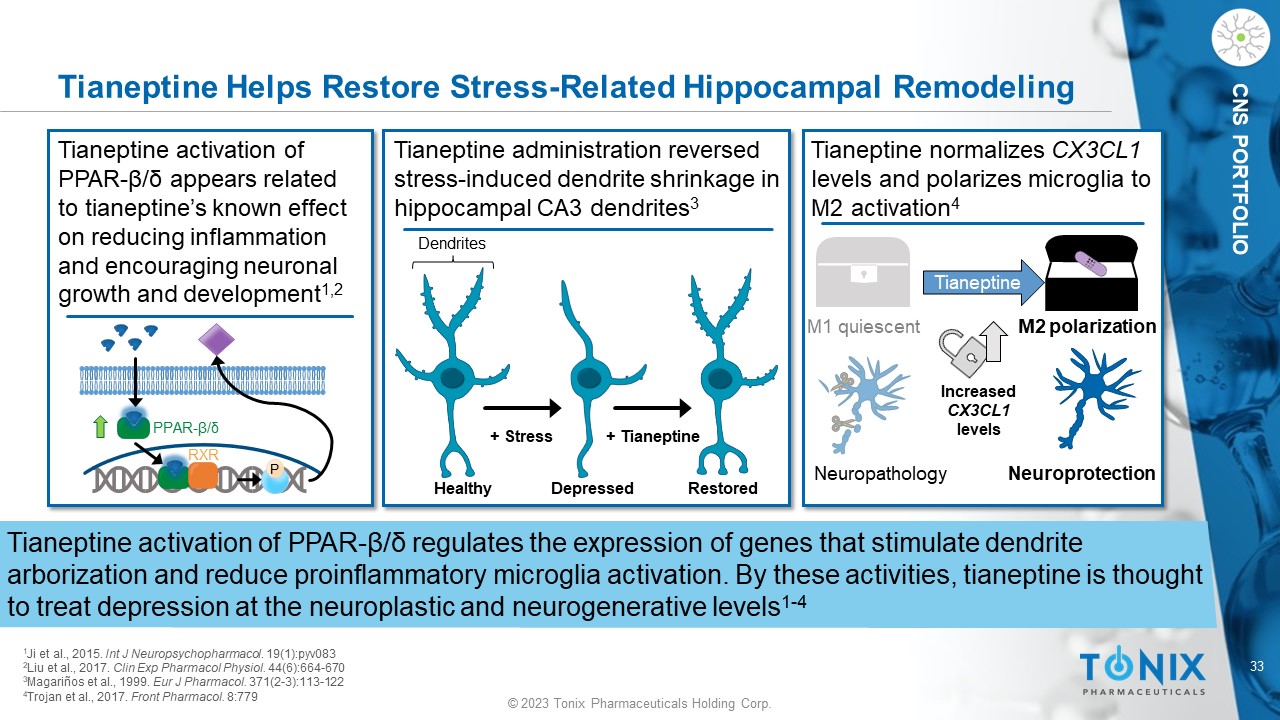
© 2023 Tonix Pharmaceuticals Holding Corp. 33 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tianeptine Helps Restore Stress - Related Hippocampal Remodeling Tianeptine activation of PPAR - β / δ regulates the expression of genes that stimulate dendrite arborization and reduce proinflammatory microglia activation. By these activities, tianeptine is thought to treat depression at the neuroplastic and neurogenerative levels 1 - 4 Tianeptine administration reversed stress - induced dendrite shrinkage in hippocampal CA3 dendrites 3 Restored Healthy Tianeptine activation of PPAR - β/δ appears related to tianeptine’s known effect on reducing inflammation and encouraging neuronal growth and development 1,2 P Depressed Dendrites PPAR - β/δ RXR + Stress + Tianeptine Tianeptine normalizes CX3CL1 levels and polarizes microglia to M2 activation 4 Increased CX3CL1 levels Tianeptine M1 quiescent Neuroprotection Neuropathology M2 polarization 1 Ji et al., 2015. Int J Neuropsychopharmacol . 19(1):pyv083 2 Liu et al., 2017. Clin Exp Pharmacol Physiol. 44(6):664 - 670 3 Magariños et al., 1999. Eur J Pharmacol . 371(2 - 3):113 - 122 4 Trojan et al., 2017. Front Pharmacol . 8:779
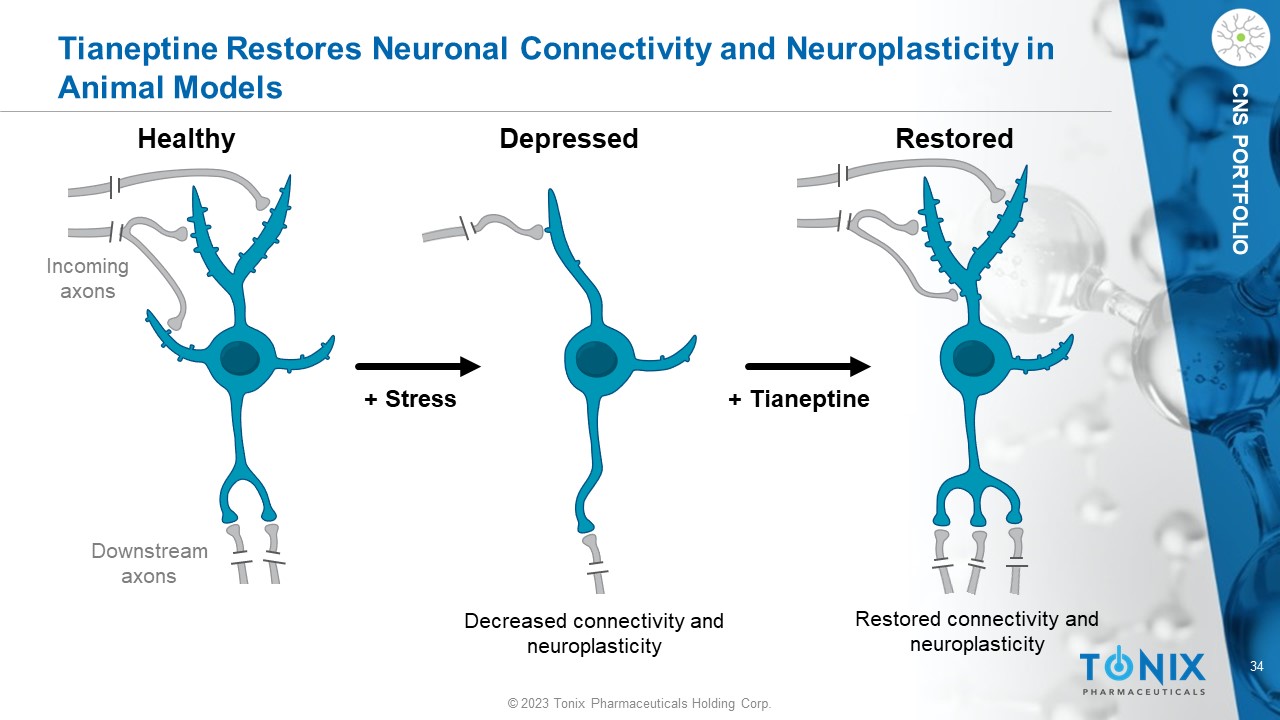
© 2023 Tonix Pharmaceuticals Holding Corp. 34 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tianeptine Restores Neuronal Connectivity and Neuroplasticity in Animal Models Healthy Depressed Restored Decreased connectivity and neuroplasticity Restored connectivity and neuroplasticity + Stress + Tianeptine Incoming axons Downstream axons
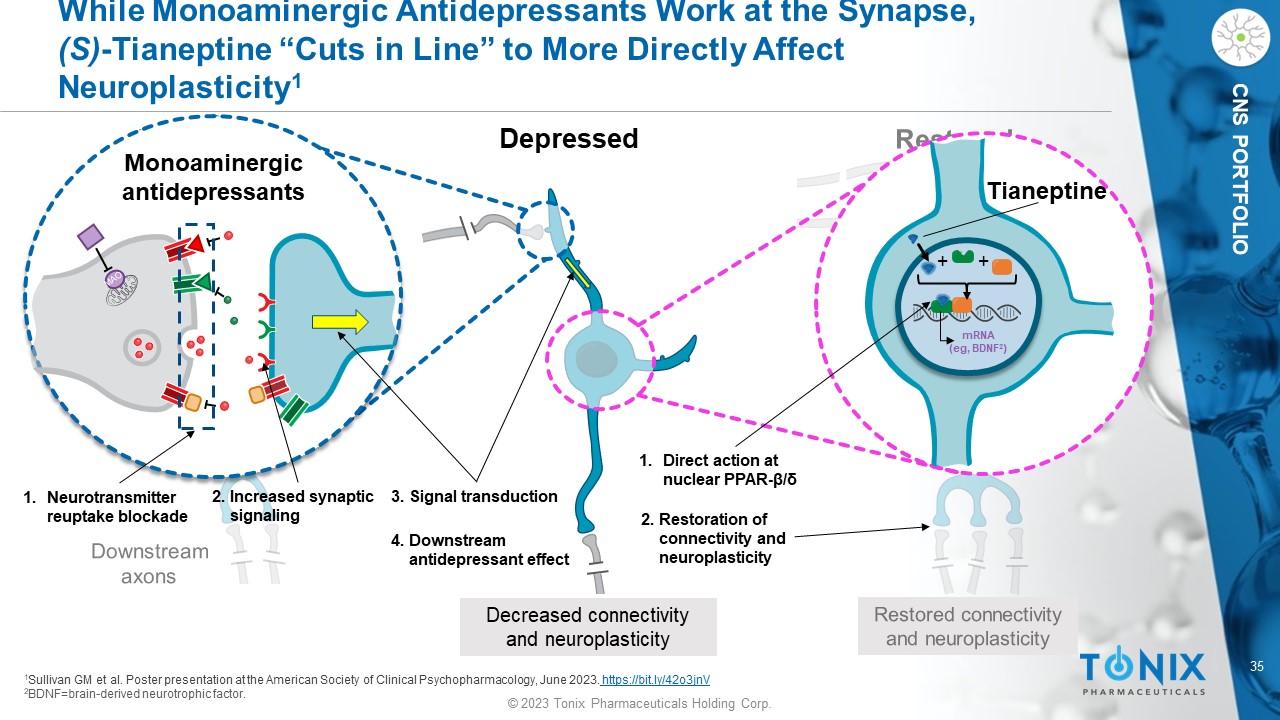
© 2023 Tonix Pharmaceuticals Holding Corp. 35 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO While Monoaminergic Antidepressants Work at the Synapse, (S) - Tianeptine “Cuts in Line” to More Directly Affect Neuroplasticity 1 Depressed Decreased connectivity and neuroplasticity Tianeptine + + mRNA ( eg , BDNF 2 ) 1 Sullivan GM et al. Poster presentation at the American Society of Clinical Psychopharmacology, June 2023. https://bit.ly/42o3jnV 2 BDNF=brain - derived neurotrophic factor. Monoaminergic antidepressants 1. Neurotransmitter reuptake blockade 1. Direct action at nuclear PPAR - β/δ 2. Increased synaptic signaling 3. Signal transduction 4. Downstream antidepressant effect 2. Restoration of connectivity and neuroplasticity
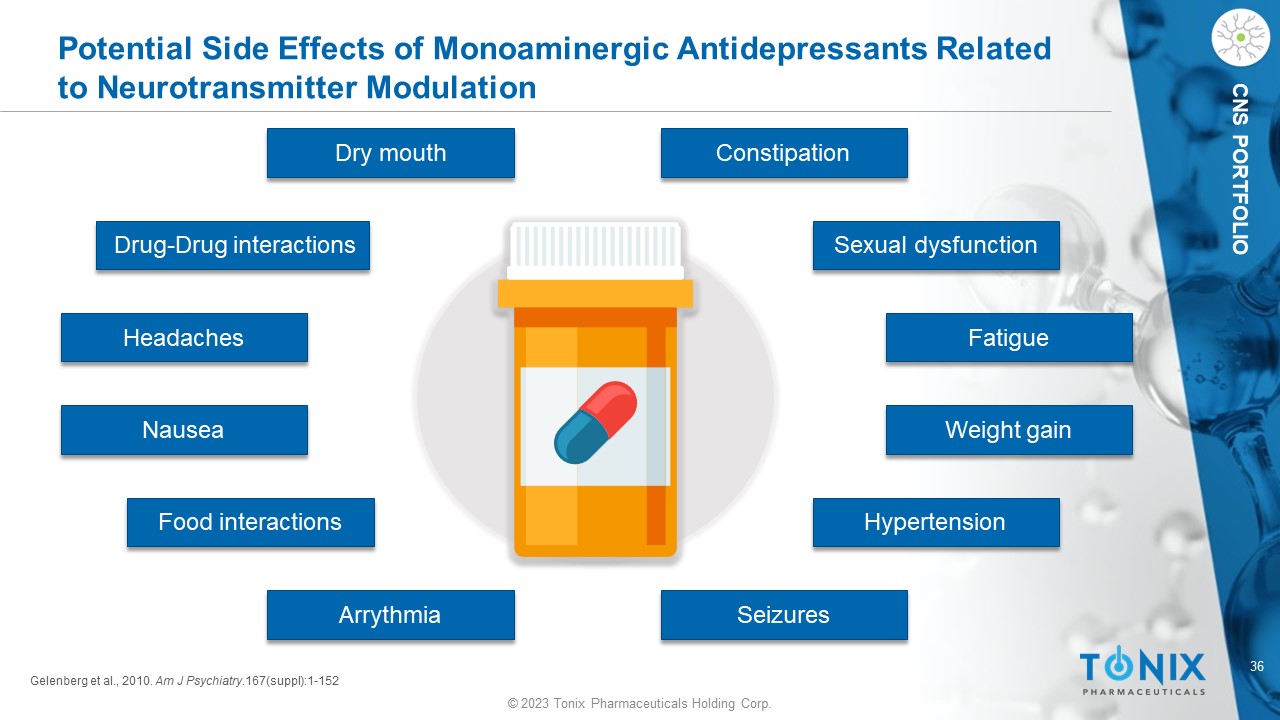
© 2023 Tonix Pharmaceuticals Holding Corp. 36 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Potential Side Effects of Monoaminergic Antidepressants Related to Neurotransmitter Modulation Gelenberg et al., 2010. Am J Psychiatry .167(suppl):1 - 152 Dry mouth Headaches Nausea Hypertension Weight gain Constipation Fatigue Arrythmia Seizures Sexual dysfunction Drug - Drug interactions Food interactions
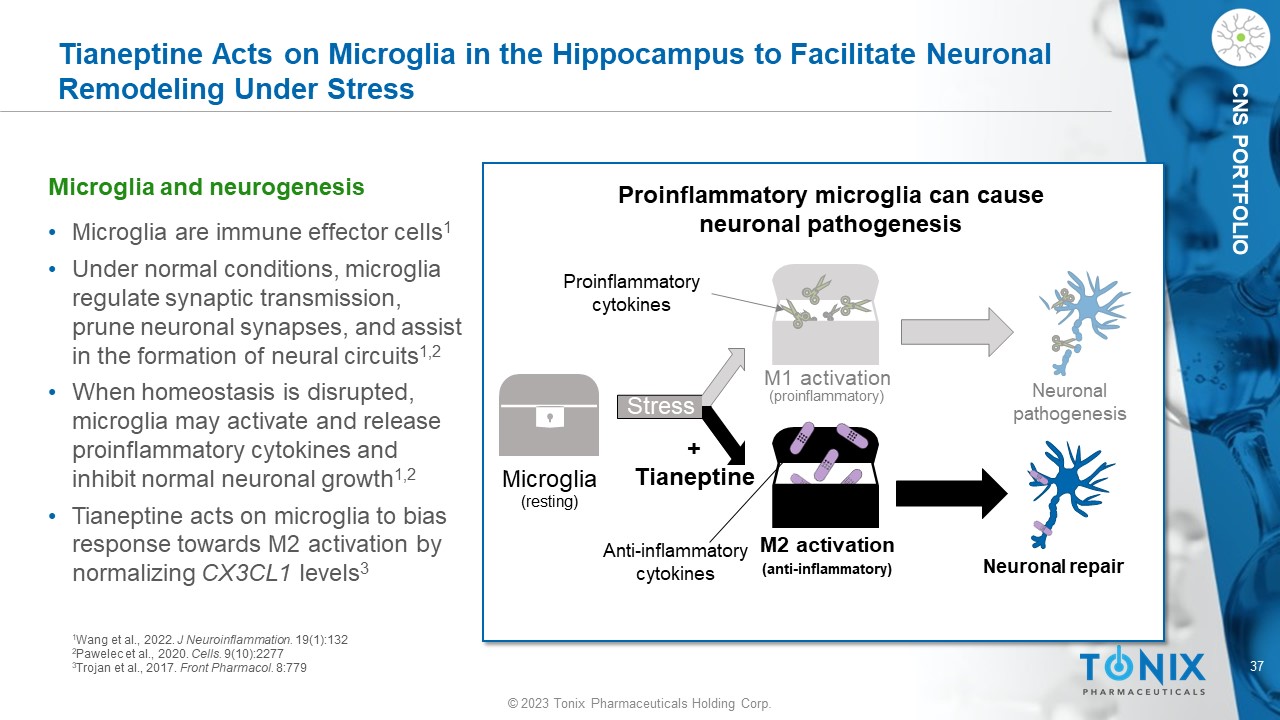
© 2023 Tonix Pharmaceuticals Holding Corp. 37 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO • Microglia are immune effector cells 1 • Under normal conditions, microglia regulate synaptic transmission, prune neuronal synapses, and assist in the formation of neural circuits 1,2 • When homeostasis is disrupted, microglia may activate and release proinflammatory cytokines and inhibit normal neuronal growth 1,2 • Tianeptine acts on microglia to bias response towards M2 activation by normalizing CX3CL1 levels 3 Tianeptine Acts on Microglia in the Hippocampus to Facilitate Neuronal Remodeling Under Stress Microglia and neurogenesis Microglia (resting) Stress M1 activation (proinflammatory) M2 activation (anti - inflammatory) Neuronal pathogenesis Neuronal repair Proinflammatory cytokines Anti - inflammatory cytokines Proinflammatory microglia can cause neuronal pathogenesis + Tianeptine 1 Wang et al., 2022. J Neuroinflammation. 19(1):132 2 Pawelec et al., 2020. Cells. 9(10):2277 3 Trojan et al., 2017. Front Pharmacol . 8:779
© 2023 Tonix Pharmaceuticals Holding Corp. 38 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO PPAR Family Members: PPAR - γ Emw . Wikimedia Commons . February 10, 2010. Accessed March 31, 2022. https://commons.wikimedia.org/wiki/File:Protein_PPARA_PDB_1i7g.png Emw . Wikimedia Commons . December 15, 2009. Accessed March 31, 2022. https://commons.wikimedia.org/wiki/File:Protein_PPARD_PDB_1gwx.png A2 - 33. Wikimedia Commons . March 14, 2012. Accessed March 31, 2022. https://commons.wikimedia.org/wiki/File:PPARG.png Expression Liver, muscle, heart Known roles FA oxidation Expression Brain, skeletal muscle, adipose tissue, microglia, lungs, skin Known roles Promotes CNS neurotrophic factors and reduces expression of inflammatory mediators Expression Endothelial and smooth muscle cells Known roles Adipocyte differentiation regulation, FA storage, glucose metabolism PPAR - α 1 PPAR - β/δ 1,2 PPAR - γ 1 1 Tyagi et al., 2011. J Adv Pharm Technol Res . 2(4):236 - 240 2 D’Angelo et al., 2011. J Cell Physiol. 226(8):2170 - 2180
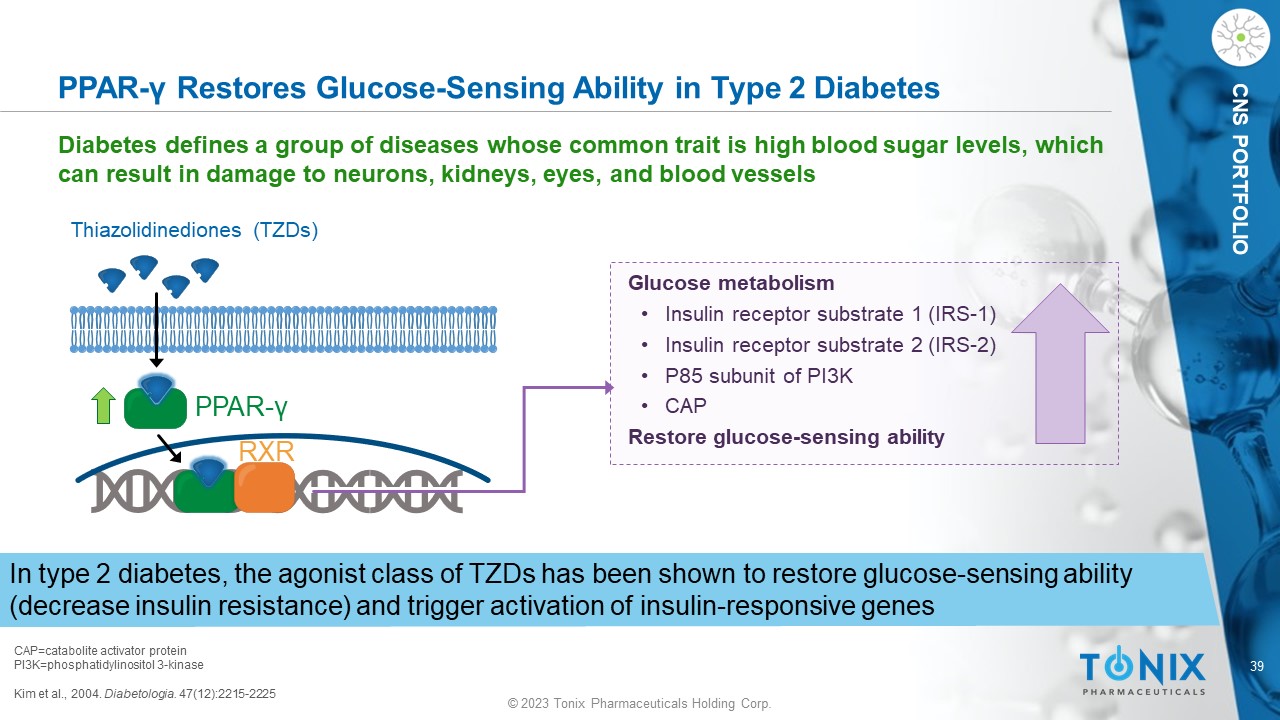
© 2023 Tonix Pharmaceuticals Holding Corp. 39 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Glucose metabolism • Insulin receptor substrate 1 (IRS - 1) • Insulin receptor substrate 2 (IRS - 2) • P85 subunit of PI3K • CAP Restore glucose - sensing ability PPAR - γ Restores Glucose - Sensing Ability in Type 2 Diabetes CAP=catabolite activator protein PI3K=phosphatidylinositol 3 - kinase Kim et al., 2004. Diabetologia . 47(12):2215 - 2225 Diabetes defines a group of diseases whose common trait is high blood sugar levels, which can result in damage to neurons, kidneys, eyes, and blood vessels In type 2 diabetes, the agonist class of TZDs has been shown to restore glucose - sensing ability (decrease insulin resistance) and trigger activation of insulin - responsive genes PPAR - γ RXR Thiazolidinediones ( TZDs )
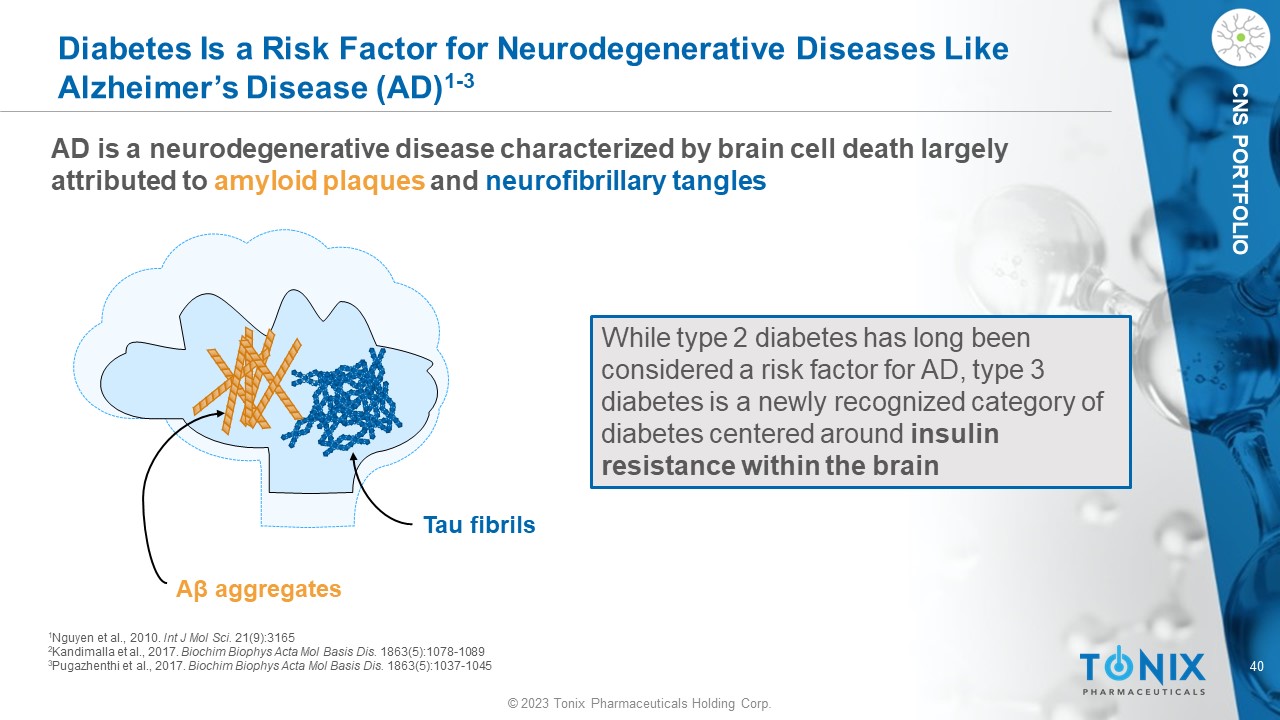
© 2023 Tonix Pharmaceuticals Holding Corp. 40 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Diabetes Is a Risk Factor for Neurodegenerative Diseases Like Alzheimer’s Disease (AD) 1 - 3 AD is a neurodegenerative disease characterized by brain cell death largely attributed to amyloid plaques and neurofibrillary tangles While type 2 diabetes has long been considered a risk factor for AD, type 3 diabetes is a newly recognized category of diabetes centered around insulin resistance within the brain Tau fibrils A β aggregates 1 Nguyen et al., 2010. Int J Mol Sci. 21(9):3165 2 Kandimalla et al., 2017. Biochim Biophys Acta Mol Basis Dis. 1863(5):1078 - 1089 3 Pugazhenthi et al., 2017. Biochim Biophys Acta Mol Basis Dis. 1863(5):1037 - 1045
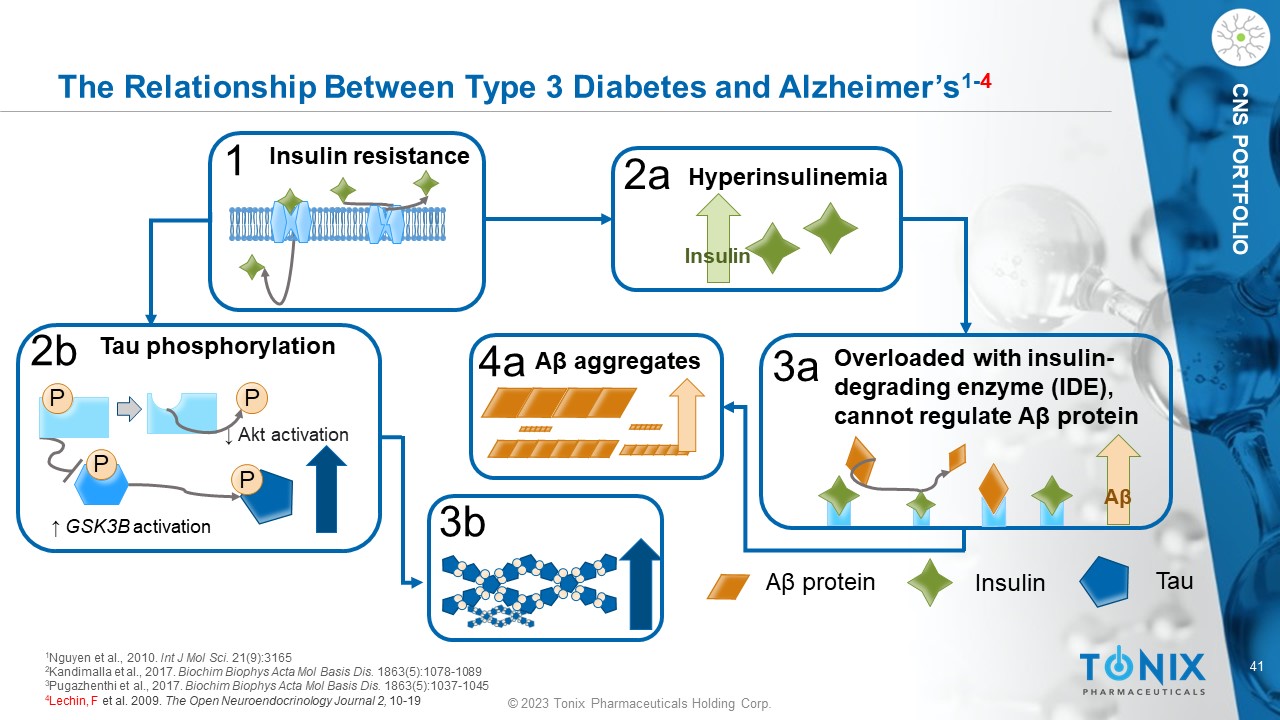
© 2023 Tonix Pharmaceuticals Holding Corp. 41 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO The Relationship Between Type 3 Diabetes and Alzheimer’s 1 - 4 3b ↑ GSK3B activation P P P P Tau phosphorylation ↓ Akt activation 2b 4a Insulin A β protein Tau Overloaded with insulin - degrading enzyme (IDE), cannot regulate A β protein A β 3a Hyperinsulinemia Insulin 2a Insulin resistance 1 A β aggregates 1 Nguyen et al., 2010. Int J Mol Sci. 21(9):3165 2 Kandimalla et al., 2017. Biochim Biophys Acta Mol Basis Dis. 1863(5):1078 - 1089 3 Pugazhenthi et al., 2017. Biochim Biophys Acta Mol Basis Dis. 1863(5):1037 - 1045 4 Lechin , F et al. 2009. The Open Neuroendocrinology Journal 2, 10 - 19
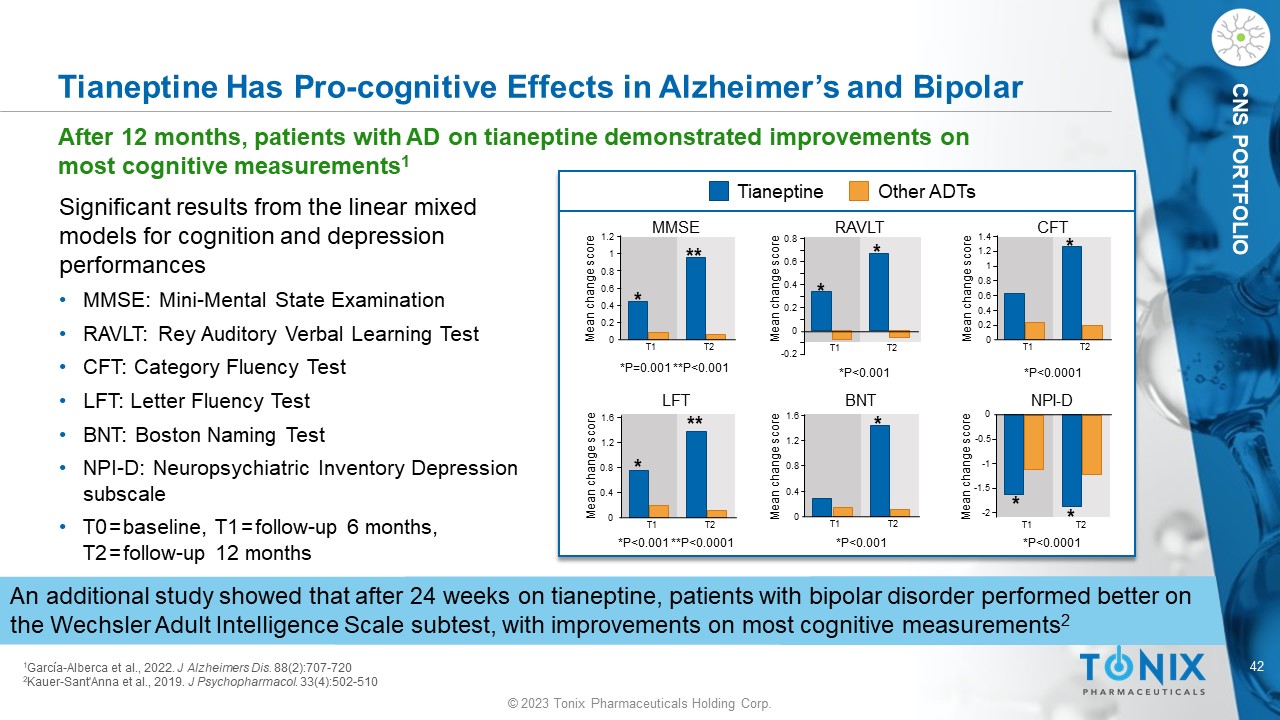
© 2023 Tonix Pharmaceuticals Holding Corp. 42 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Tianeptine Has Pro - cognitive Effects in Alzheimer’s and Bipolar After 12 months, patients with AD on tianeptine demonstrated improvements on most cognitive measurements 1 1 García - Alberca et al., 2022. J Alzheimers Dis. 88(2):707 - 720 2 Kauer - Sant'Anna et al., 2019. J Psychopharmacol . 33(4):502 - 510 Significant results from the linear mixed models for cognition and depression performances • MMSE: Mini - Mental State Examination • RAVLT: Rey Auditory Verbal Learning Test • CFT: Category Fluency Test • LFT: Letter Fluency Test • BNT: Boston Naming Test • NPI - D: Neuropsychiatric Inventory Depression subscale • T0 = baseline, T1 = follow - up 6 months, T2 = follow - up 12 months An additional study showed that after 24 weeks on tianeptine, patients with bipolar disorder performed better on the Wechsler Adult Intelligence Scale subtest, with improvements on most cognitive measurements 2 Tianeptine Other ADTs 1.2 0.8 1 0.6 0.4 0.2 0 MMSE T1 T2 Mean change score * ** *P=0.001 **P<0.001 0.8 0.4 0.6 0.2 0 - 0.2 RAVLT T1 T2 Mean change score * * 1.2 0.8 1 0.6 0.4 0.2 0 CFT T1 T2 Mean change score * 1.4 Mean change score 1.2 0.8 0.4 0 LFT T1 T2 * ** 1.6 1.6 0.8 1.2 0 0.4 Mean change score BNT T1 T2 * Mean change score 0 - 0.5 - 1 - 1.5 - 2 NPI - D T1 T2 * * *P<0.001 *P<0.0001 *P<0.001 **P<0.0001 *P<0.001 *P<0.0001

© 2023 Tonix Pharmaceuticals Holding Corp. 43 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Observations that Relate Tianeptine’s Action to PPAR Activation • Tianeptine selectively activates PPAR - β / δ and PPAR - γ, but not PPAR - α 1 − Regulates PPAR - β / δ and PPAR - γ driven transcription − Tianeptine metabolite MC5 does not activate PPAR - β / δ or PPAR - γ • Tianeptine’s neuroplastic effects on cultured neurons correlate with PPAR - β / δ and PPAR - γ agonism 1 − TNX - 4300 ( estianeptine ) is a new chemical entity, that activates PPAR - β / δ and PPAR - γ and restores neuroplasticity in cultured neurons • Company plans to submit data supporting tianeptine’s mechanism of action to upcoming scientific conferences and for publication Tianeptine is an agonist at PPAR - β / δ and PPAR - γ 1 Tonix poster presented at ASCP 2023 Annual Meeting, Miami FL, May 30 - June 2, Poster T41

© 2023 Tonix Pharmaceuticals Holding Corp. 44 © 2023 Tonix Pharmaceuticals Holding Corp. CNS PORTFOLIO Observations that Relate Tianeptine’s Action to µ - Opioid Receptors • In 2014, tianeptine was reported to be a weak µ – opioid agonist 1 − K i = 383 nM and EC 50 = 194 nM 1 − Others have found even lower binding and activity, e.g., K i = 768 nM 2 or EC 50 >3 uM 3 • In 2017, tianeptine’s µ – opioid activity was implicated as central to its mechanism of treating depression 4,5 − The effect of tianeptine at 30 mg/kg on the Porsolt Forced Swim Test (FST) was decreased by naloxone treatment or in knock - out mice lacking the µ – opioid receptor − Some µ – opioid receptor agonists have a signal in the FST 6 - 8 , which complicates the interpretation of tianeptine effects • In 2023, using medicinal chemistry and pharmacology, scientists at Tonix found no connection between tianeptine’s neuroplastic effects on cultured neurons and its weak µ – opioid receptor agonism 9 − TNX - 4300 ( estianeptine ) restores neuroplasticity in cultured neurons and is free from µ – opioid receptor activity 9 1 Gassaway et al., 2014. Transl Psychiatry . 4(7):e411 2 BL Roth PDSP K i database; https://pdsp.unc.edu/databases 3 Vandeputte et al., 2020. Arch Toxicol . 94(11):3819 - 3830 4 Samuels et al., 2017. Neuropsychopharmacology . 42(10):2052 - 2063 5 Han et al., 2022. Neuropsychopharmacology . 47(7):1387 - 1397 Tianeptine is a weak µ - opiate receptor agonist 6 Szumiec L, et al. Behav Brain Res. 2023. 3:114466. 7 Zomkowski AD, et al., Neurosci Lett. 2005. 381(3):279 - 83. 8 Falcon E, et al. Psychopharmacology ( Berl ). 2015 232(5):907 - 15. 9 Tonix poster presented at ASCP 2023 Annual Meeting, Miami FL, May 30 - June 2, Poster T41

© 2023 Tonix Pharmaceuticals Holding Corp. © 2023 Tonix Pharmaceuticals Holding Corp. THANK YOU