
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.02

TNX - 801: A Novel Mpox Vaccine (Horsepox Platform) to Enhance Preparedness and Global Vaccine Equity Zeil Rosenberg MD, MPH Executive Vice President, Medical World Vaccine Congress - Europe October 18, 2023 © 2023 Tonix Pharmaceuticals Holding Corp.

2 © 2023 Tonix Pharmaceuticals Holding Corp. Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995. These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others. These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially. There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements. These factors include, but are not limited to, the risks related to failure to obtain FDA clearances or approvals and noncompliance with FDA regulations; delays and uncertainties caused by the global COVID - 19 pandemic; risks related to the timing and progress of clinical development of our product candidates; our need for additional financing; uncertainties of patent protection and litigation; uncertainties of government or third party payor reimbursement; limited research and development efforts and dependence upon third parties; and substantial competition. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products. The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise. Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law. Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31, 2022, as filed with the Securities and Exchange Commission (the “SEC”) on March 13, 2023, and periodic reports and current reports filed with the SEC on or after the date thereof. All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements.

3 Tonix Pharmaceuticals • NASDAQ traded, commercial stage biopharmaceutical company • Focus: CNS (incl. 2 commercial), Long Covid, and Infectious Disease • Poxvirus – based Vaccine program: • Mpox/Smallpox (TNX - 801) • COVID (TNX - 1800) Infectious Disease R&D Center (RDC) – Frederick, MD Accelerated development of vaccines and antiviral drugs ~48,000 square feet, BSL - 2 and BSL - 3 © 2022 Tonix Pharmaceuticals Holding Corp. Advanced Development Center (ADC) – North Dartmouth, MA Development and clinical scale manufacturing of biologics ~45,000 square feet, BSL - 2 Proprietary, US Government Use Only

4 In 1796 Edward Jenner Successfully Used Vaccination to Protect Against Smallpox • Jenner reasoned infection with illness similar to smallpox, but less deadly, could protect against smallpox ‒ “Jenner “vaccinated” ( vacca , Latin for “cow”) a patient with pustule matter from “cowpox” sores on a milkmaid’s hands; ‒ Patient remained healthy when challenged with smallpox virus • Jenner wrote he suspected that the agent causing cowpox, which he called vaccinia , actually originated in horses and was transferred from horses to cows’ udders by contaminated farm workers’ hands. The College of Physicians of Philadelphia. Accessed July 15, 2021. https:// www.historyofvaccines.org © 2023 Tonix Pharmaceuticals Holding Corp.
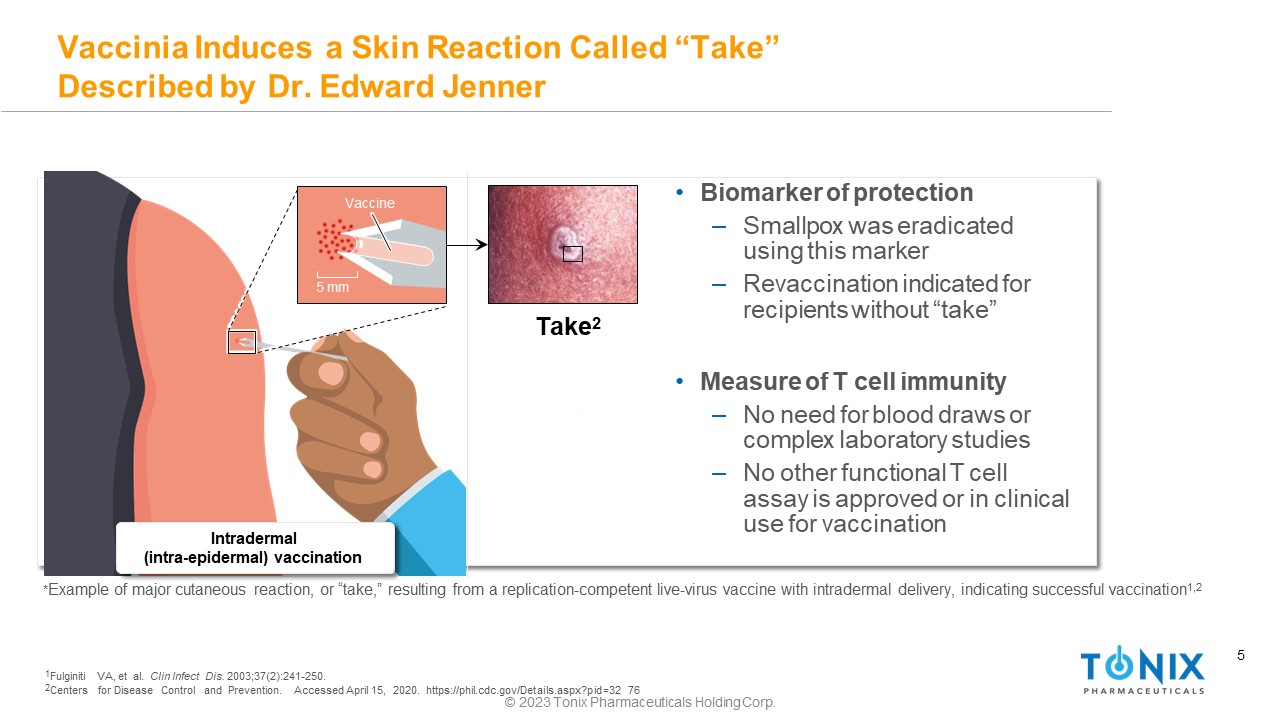
5 Vaccinia Induces a Skin Reaction Called “Take” Described by Dr. Edward Jenner * Example of major cutaneous reaction, or “take,” resulting from a replication - competent live - virus vaccine with intradermal delivery, indicating successful vaccination 1,2 5 mm Vaccine Intradermal (intra - epidermal) vaccination Take 2 © 2023 Tonix Pharmaceuticals Holding Corp. • Biomarker of protection ‒ Smallpox was eradicated using this marker ‒ Revaccination indicated for recipients without “take” • Measure of T cell immunity ‒ No need for blood draws or complex laboratory studies ‒ No other functional T cell assay is approved or in clinical use for vaccination 1 Fulginiti VA, et al. Clin Infect Dis. 2003;37(2):241 - 250. 2 Centers for Disease Control and Prevention. Accessed April 15, 2020. https://phil.cdc.gov/Details.aspx?pid=32 76

6 TNX - 801 Development • U.S. smallpox vaccine manufactured in 1902 (H.K. Mulford) ‒ 99.7% similar to horsepox in core viral sequence 1,2 • Tonix - 801 is based on a sequence of an isolated horsepox (HPXV) clone 3 − Synthesized 4 in 2018 (isolate was unavailable outside of CDC) − No new gene elements introduced • Sequencing showed Tonix - 801 identical to CDC publication of a 1976 horsepox isolate 5 1 Tulman ER, et al. Genome of horsepox virus. J Virol . 2006 80(18):9244 - 58.PMID:16940 536 2 Schrick, L. et al An Early American Smallpox Vaccine Based on Horsepox N Engl J Med 2017; 377:149 3Noyce RS, et al.. Construction of an infectious horsepox virus vaccine from chemically synthesized DNA fragments. PLoS One . 2018 Jan 19;13(1):e0188453 4Trindade GS , et al. Serro 2 Virus Highlights the Fundamental Genomic and Biological Features of a Natural Vaccinia Virus Inf ecting Humans. Viruses 2016 Dec 10;8(12). pii: E328. PMID:27 9733 99 PMCID: PMC5192389 DOI: 10.3390/v8120328 5Noyce, RS, et al. Synthetic Chimeric Horsepox Virus (scHPXV) Vaccination Protects Macaques from Monkeypox* Presented as a poster at the American Society of Microbiology BioThreats Conf erence - January 29, 2020, Arlington, VA. ( https://content.equisolve.net/tonixpharma/media/10929ac27f4fb5f5204f5cf41d59a121.pdf ) © 2023 Tonix Pharmaceuticals Holding Corp.
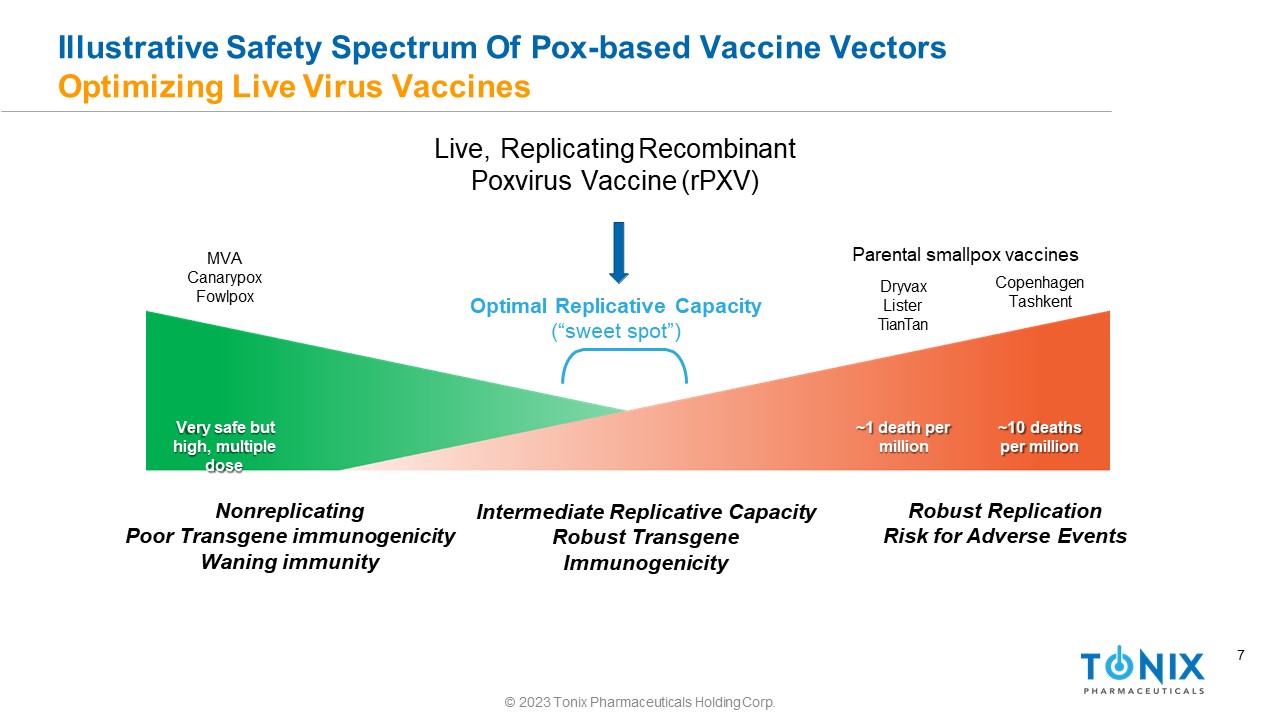
7 Illustrative Safety Spectrum Of Pox - based Vaccine Vectors Optimizing Live Virus Vaccines Very safe but high, multiple dose ~1 death per million ~10 deaths per million Nonreplicating Poor Transgene immunogenicity Waning immunity Robust Replication Risk for Adverse Events Parental smallpox vaccines Dryvax Lister TianTan Copenhagen Tashkent MVA Canarypox Fowlpox Optimal Replicative Capacity (“sweet spot”) Live, Replicating Recombinant Poxvirus Vaccine (rPXV) Intermediate Replicative Capacity Robust Transgene Immunogenicity © 2023 Tonix Pharmaceuticals Holding Corp.
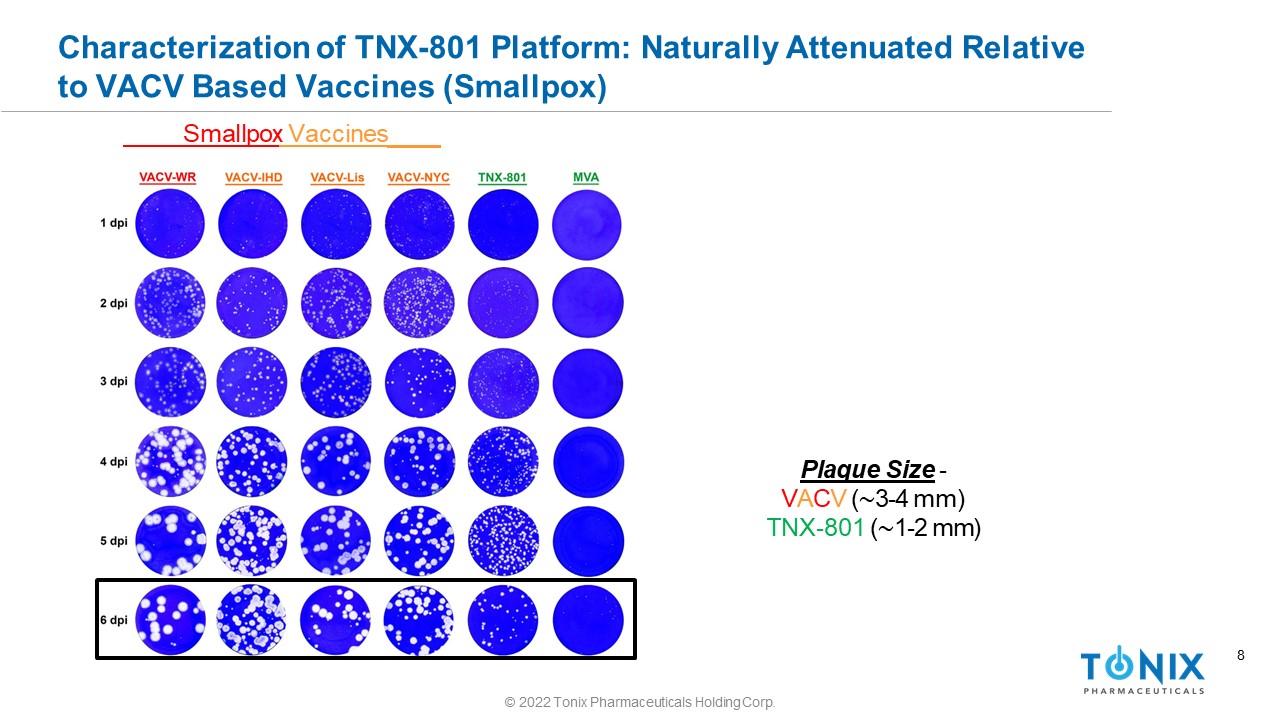
8 Characterization of TNX - 801 Platform: Naturally Attenuated Relative to VACV Based Vaccines (Smallpox) Plaque Size - V A C V ( ∼ 3 - 4 mm) TNX - 801 ( ∼ 1 - 2 mm) Smallpox Vaccines © 2022 Tonix Pharmaceuticals Holding Corp.
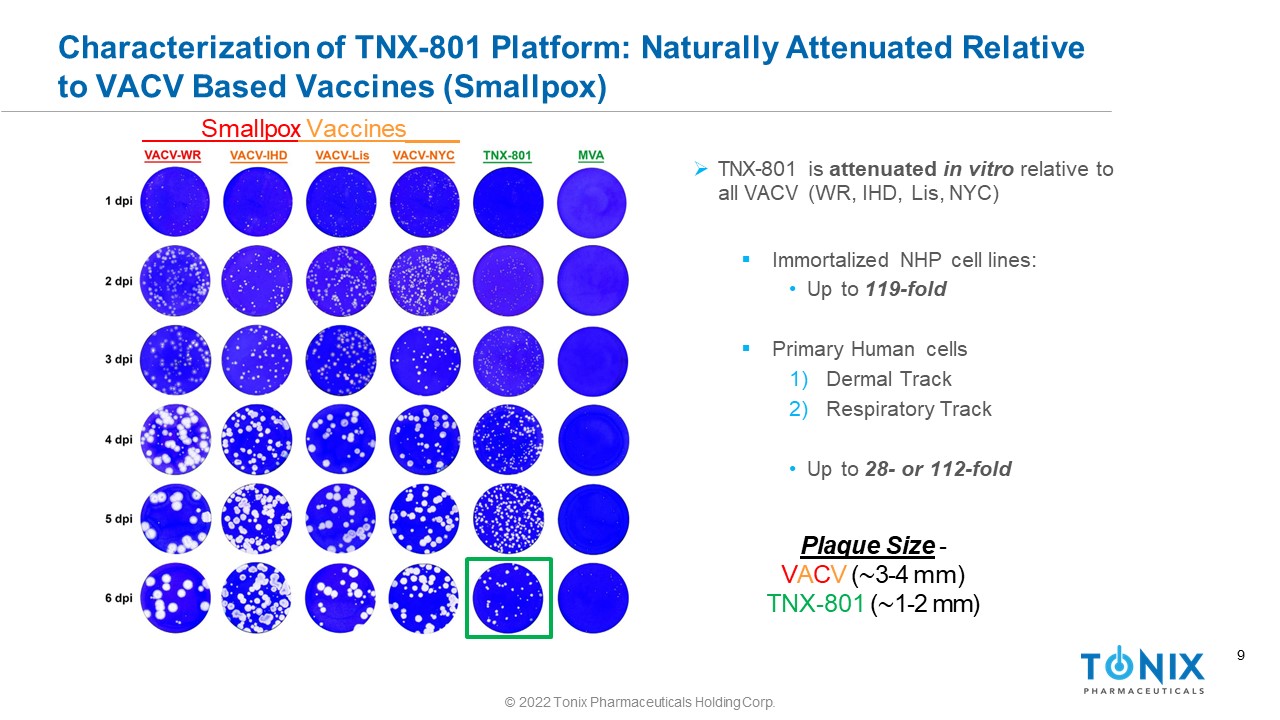
9 Characterization of TNX - 801 Platform: Naturally Attenuated Relative to VACV Based Vaccines (Smallpox) » TNX - 801 is attenuated in vitro relative to all VACV (WR, IHD, Lis, NYC) ▪ Immortalized NHP cell lines: • Up to 119 - fold ▪ Primary Human cells 1) Dermal Track 2) Respiratory Track • Up to 28 - or 112 - fold Plaque Size - V A C V ( ∼ 3 - 4 mm) TNX - 801 ( ∼ 1 - 2 mm) Smallpox Vaccines © 2022 Tonix Pharmaceuticals Holding Corp.
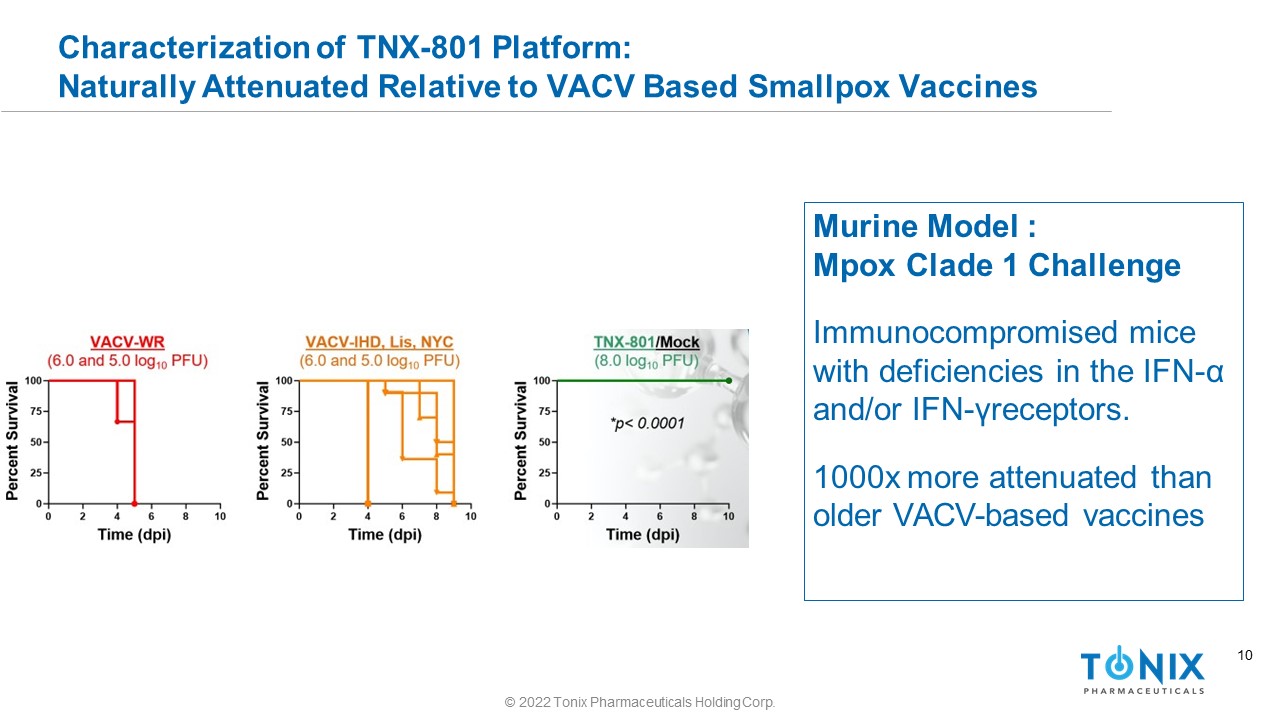
10 Characterization of TNX - 801 Platform: Naturally Attenuated Relative to VACV Based Smallpox Vaccines Murine Model : Mpox Clade 1 Challenge Immunocompromised mice with deficiencies in the IFN - α and/or IFN - γ receptors. 1000x more attenuated than older VACV - based vaccines © 2022 Tonix Pharmaceuticals Holding Corp.

11 © 2023 Tonix Pharmaceuticals Holding Corp.
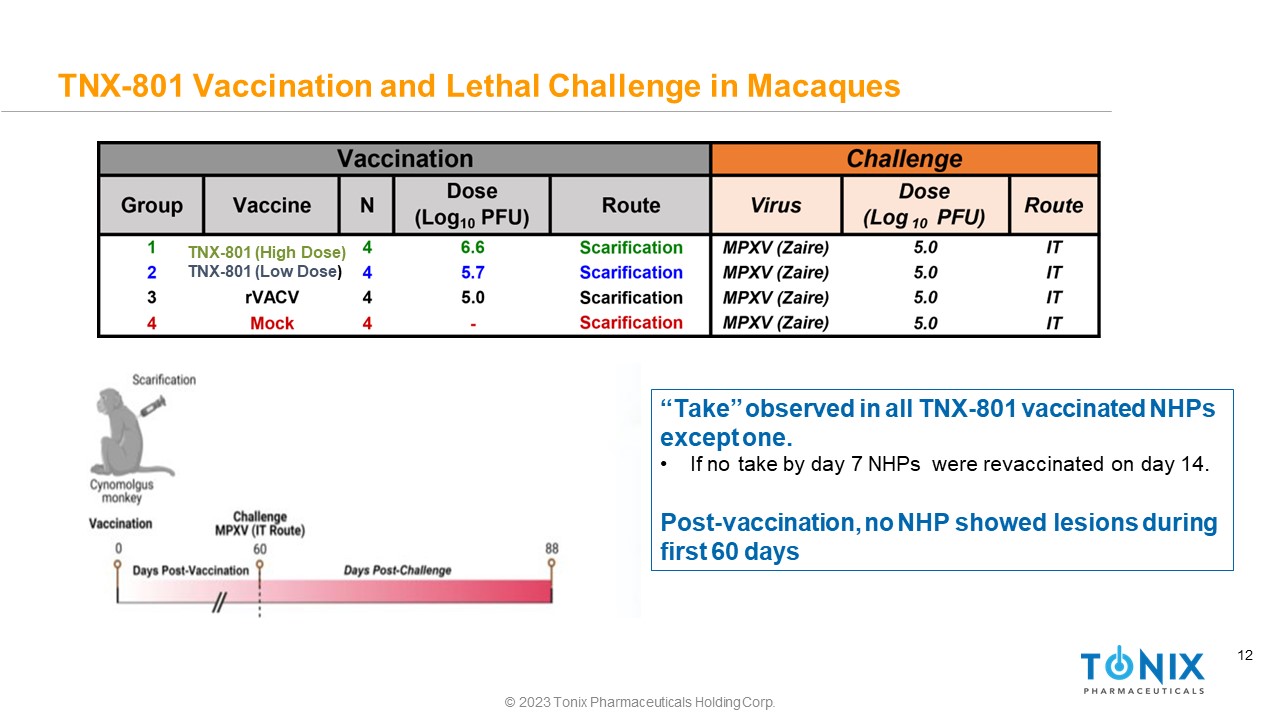
12 TNX - 801 Vaccination and Lethal Challenge in Macaques “Take” observed in all TNX - 801 vaccinated NHPs except one. • If no take by day 7 NHPs were revaccinated on day 14. Post - vaccination, no NHP showed lesions during first 60 days TNX - 801 (High Dose) TNX - 801 (Low Dose ) © 2023 Tonix Pharmaceuticals Holding Corp.
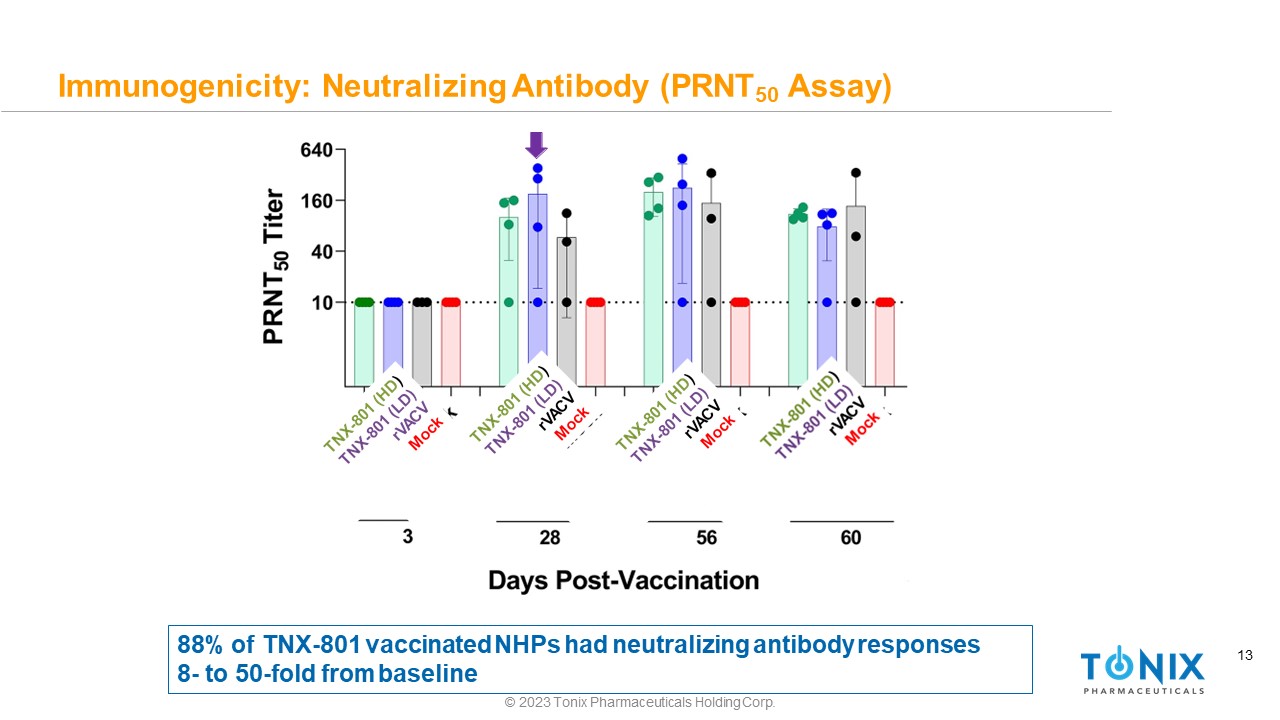
13 Immunogenicity: Neutralizing Antibody (PRNT 50 Assay) 88% of TNX - 801 vaccinated NHPs had neutralizing antibody responses 8 - to 50 - fold from baseline © 2023 Tonix Pharmaceuticals Holding Corp.
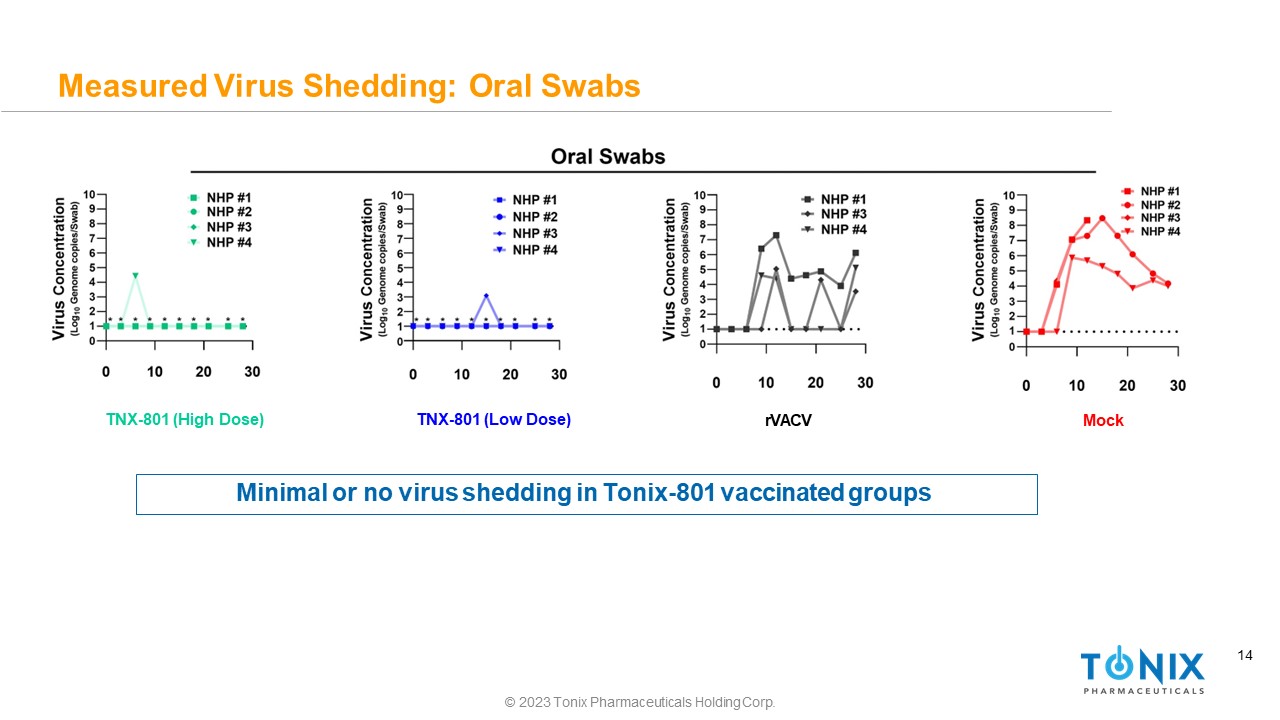
14 Measured Virus Shedding: Oral Swabs TNX - 801 (High Dose) © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 801 (Low Dose) rVACV Mock Minimal or no virus shedding in Tonix - 801 vaccinated groups
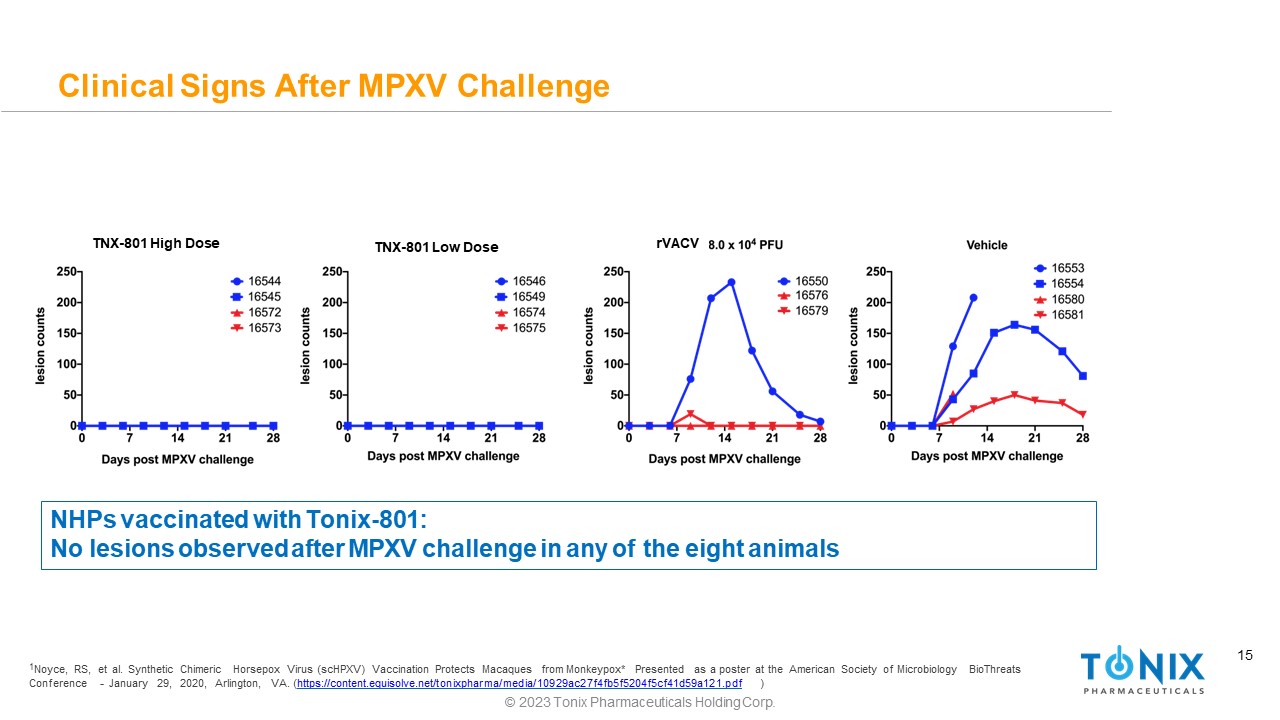
15 Clinical Signs After MPXV Challenge NHPs vaccinated with Tonix - 801: No lesions observed after MPXV challenge in any of the eight animals 1 Noyce, RS, et al. Synthetic Chimeric Horsepox Virus (scHPXV) Vaccination Protects Macaques from Monkeypox* Presented as a poster at the American Society of Microbiology BioThreats Conf erence - January 29, 2020, Arlington, VA. ( https://content.equisolve.net/tonixpharma/media/10929ac27f4fb5f5204f5cf41d59a121.pdf ) © 2023 Tonix Pharmaceuticals Holding Corp. TNX - 801 High Dose TNX - 801 Low Dose rVACV
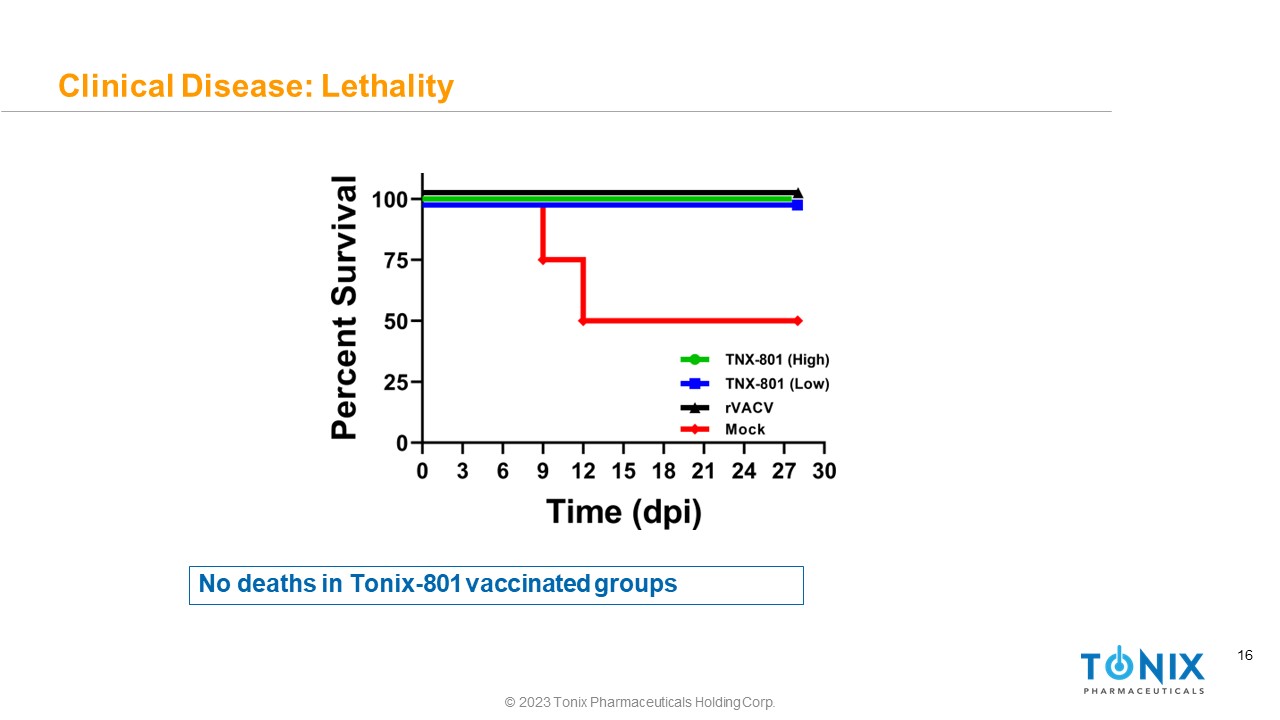
16 Clinical Disease: Lethality No deaths in Tonix - 801 vaccinated groups © 2023 Tonix Pharmaceuticals Holding Corp.
17 © 2023 Tonix Pharmaceuticals Holding Corp. Study Conclusions for TNX - 801 Non - Human Primate Challenge • A single dose vaccination was well tolerated ‒ No severe adverse events • Vaccination was immunogenic • Mpox disease (lesions) was not observed following MPXV (Zaire) challenge • All vaccinated NHPs survived lethal challenge
18 Efficacy of Current Vaccines - RWE Mpox • Jynneos - Most recent data • Earliest study had VE 86% but CI was 59% - 95 %1. • More recent data towards bottom of CI. • CDC US National Study 2 • Epic Database : 173 million persons capturing 2193 mpox cases • 2 dose adjusted VE 66.0% (CI 47.4 - 78.1) • 1 dose adjusted VE 35.8% (CI 22.1 - 47.1) • ACAM2000 (EUA only; not approved by FDA for mpox) • US Military Vaccine Program 3 2.7 million former and current personnel • ACAM2000 Adjusted VE 75% (CI 58 - 85) © 2023 Tonix Pharmaceuticals Holding Corp. 1 Sagy Nature Medicine 2023 2 Deputy et al NEJM 2023 3 Titanji et al NEJM 2023

19 © 2023 Tonix Pharmaceuticals Holding Corp. Do we need an additional approved Mpox vaccine? • Durable Immunity • Attenuated for safety • One dose microneedle • Improve compliance • Reduce administrative burden in epidemic setting • Ring vaccination strategy • Proven effective for disease eradication • Potential to reduce onward transmission • “Take” as biomarker of protection • Global Vaccine Equity ……

20 © 2023 Tonix Pharmaceuticals Holding Corp. Global Vaccine Equity : TNX - 801 • Epidemic continues in Africa 1 • Distribution and Finance are significant barriers 2 • Developed countries ordered nearly all available and future doses of mpox vaccine 3 • Africa access to vaccines is patchy or non - existent 4 TNX - 801 • Potentially lower relative price than incumbent • One dose • Smaller dose & multi - dose packaging stretches supply • High scale manufacturing using existing technologies • Competition in marketplace • Sustainable access • Microneedle one - dose delivery improves accessibility • Free up vaccine supplies to countries now without 1 Koslov Nature Medicine 2023 2 Ogunkola Vaccines 2023 3 LA times May 30, 2023 4 GAVI VaccineWorks June 21, 2023
21 © 2022 Tonix Pharmaceuticals Holding Corp. Investigators and Collaborators Tonix • Seth Lederman • Siobhan Fogarty • Sina Bavari • Scott Goebel • Bruce Daugherty • Farooq Nasar • Helen Stillwell 1 Univ. of Alberta • Ryan Noyce • David Evans Current Addresses 1 University of Pennsylvania 2 IITRI Univ. of Maryland – Institute of Human Virology • José Esparza Southern Research • Fusataka Koide • Landon Westfall 2 • Karen Gilbert 3 LINQ Pharma Consulting • Onesmo Mpanju 3 National Toxicology Program (NTP) at National Institute of Environmental Health Sciences (NIEHS), NIH; Artic Slope Regional Corp.

THANK YOU © 2023 Tonix Pharmaceuticals Holding Corp.