
Tonix Pharmaceuticals Holding Corp. 8-K
Exhibit 99.03

Extended Survival of 9 - and 10 - Gene Edited Pig Heart Xenografts with Ischemia Minimization and CD154 Costimulation Blockade – Based Immunosuppression I. Ileka 1 , R. Chaban 1 , K. Kinoshita 1 , G. McGrath 1 , Z. Habibabady 1 , A. Calhoun 1 , A. Maenaka 1 , M. Ma 1 , V. Diaz 1 ,M. Dufault 1 , L. Burdorf 2 , W. Eyestone 2 , K. Whitworth 3 , D.K.C. Cooper 1 R.N. Pierson III 1 1 Center for Transplantation Sciences, Massachusetts General Hospital and Harvard Medical School, Boston, MA 2 Revivicor, Inc., Blacksburg, VA 3 National Swine Resource and Research Center (NSRRC), Columbia, MO

Relevant Disclosures • R. Chaban was supported by the Benjamin Research Fellowship from the German Research Foundation (DFG) • I. Ileka is supported by T32 5T32 AI007529 - 24 • Gene edited pigs were provided by Revivicor and NSRRC (NIH grant U42OD011140) • Tonix Pharmaceuticals provided TNX - 1500*, a humanized, Fc - modified, dimeric anti - CD154 mAb • This work was supported by NIH grants UO1 AI153612 (Pierson), U19 AI090959 (Cooper), and sponsored research agreements with Tonix Pharmaceuticals * TNX - 1500 is an investigational new biologics and is not approved for any indication
Background • Gene - edited (GE) pigs for Xenotransplantation. – Remove CHO antigen targets of preformed Ab • TKO (Gal - 1,3 - a Gal, Neu5Gc, b 4Gal) – Add human regulatory molecules • Complement: CD46, CD55, CD59 • Coagulation: TBM, EPCR, TFPI – Add human anti - inflammatory ‘transgenes’ • CD47, HLA - E/ b 2 m g, HO - 1, A20, CD39
Background • We evaluated hearts from multi - GE pigs in baboon transplants treated with a novel costimulation - based immunosuppressive regimen and cold - perfused ‘ischemia minimization’.

Methods • Current study: – 3 - , 9 - , or 10 - GE pig hearts – novel costimulation - based immunosuppressive regimen – cold - perfused storage technique designed to minimize graft ischemia . • Eight baboons recipients received heterotopic heart transplants using Steen’s cold - perfusion ischemia minimization – 3 Reference pig hearts ( Ntl . Swine Resource & Research Ctr: NSRRC) • 3 - GE pigs (n=3): GTKO. β4 GALNT2KO.hCD55 – 5 Multi - GE pig hearts ( Revivicor ) • 9 - GE pigs (n=3): GalKO . β4 GalNT2KO. GHRKO. hCD46.hCD55 . hTBM.hEPCR . hCD47 .hHO - 1 • 10 - GE pigs (n=2): GalKO . β4 GalNT2KO. GHRKO. CD46.CD55 . hTBM.hEPCR . hCD47.hHO - 1 . CMAHKO
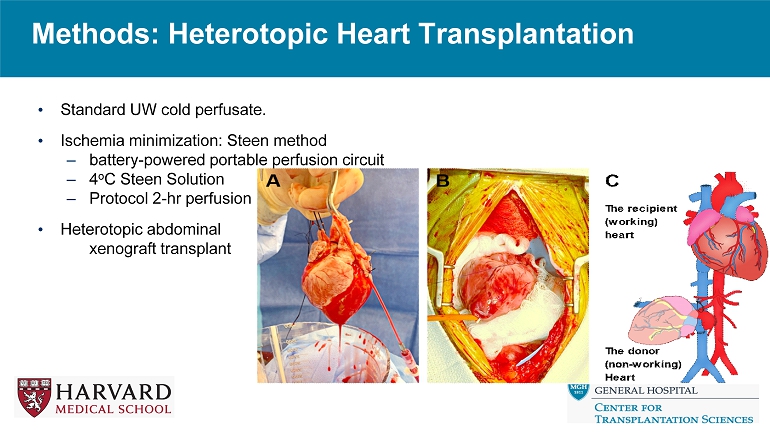
Methods: Heterotopic Heart Transplantation • Standard UW cold perfusate. • Ischemia minimization: Steen method – battery - powered portable perfusion circuit – 4 o C Steen Solution – Protocol 2 - hr perfusion • Heterotopic abdominal xenograft transplant
Methods: Steen Ischemia Minimization 7 • Steen: buffered extracellular solution (laboratory - made) • 240ml of washed human RBCs; 760 ml Steen solution • Aortic perfusion at 4 o C, 40 - 50 mmHg, immersed in reservoir; LV vent
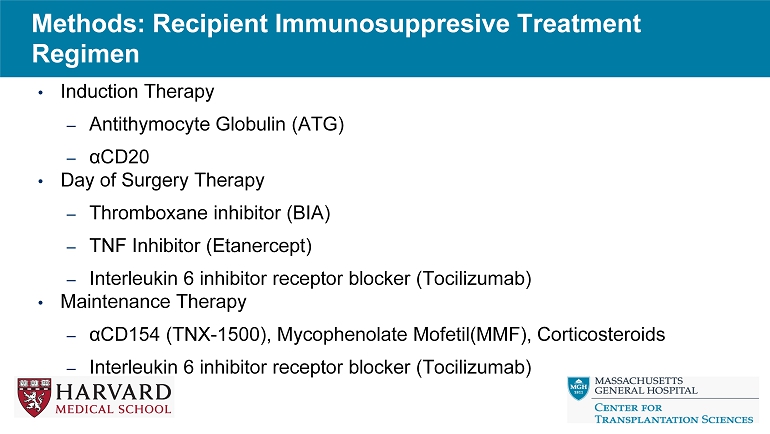
Methods: Recipient Immunosuppresive Treatment Regimen • Induction Therapy – Antithymocyte Globulin (ATG) – α CD20 • Day of Surgery Therapy – Thromboxane inhibitor (BIA) – TNF Inhibitor (Etanercept) – Interleukin 6 inhibitor receptor blocker (Tocilizumab) • Maintenance Therapy – α CD154 (TNX - 1500), Mycophenolate Mofetil(MMF), Corticosteroids – Interleukin 6 inhibitor receptor blocker (Tocilizumab)
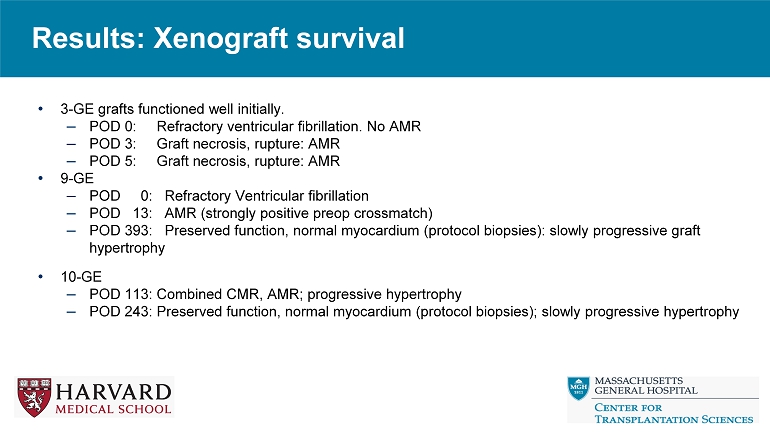
Results: Xenograft survival • 3 - GE grafts functioned well initially. – POD 0: Refractory ventricular fibrillation. No AMR – POD 3: Graft necrosis, rupture: AMR – POD 5: Graft necrosis, rupture: AMR • 9 - GE – POD 0: Refractory Ventricular fibrillation – POD 13: AMR (strongly positive preop crossmatch) – POD 393: Preserved function, normal myocardium (protocol biopsies): slowly progressive graft hypertrophy • 10 - GE – POD 113: Combined CMR, AMR; progressive hypertrophy – POD 243: Preserved function, normal myocardium (protocol biopsies); slowly progressive hypertrophy
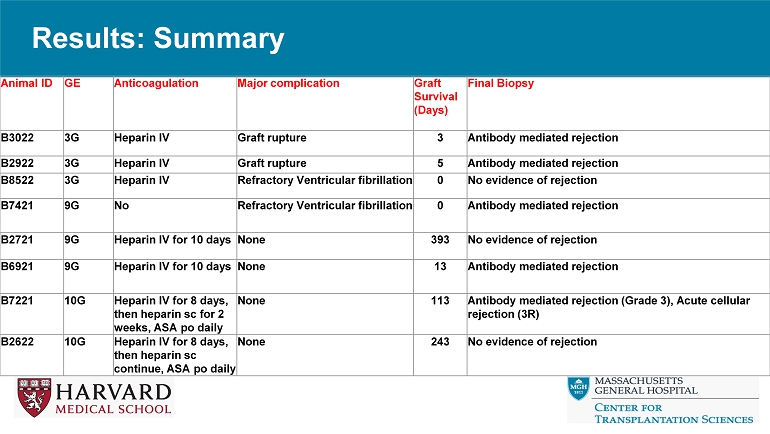
Results: Summary Final Biopsy Graft Survival (Days) Major complication Anticoagulation GE Animal ID Antibody mediated rejection 3 Graft rupture Heparin IV 3G B3022 Antibody mediated rejection 5 Graft rupture Heparin IV 3G B2922 No evidence of rejection 0 Refractory Ventricular fibrillation Heparin IV 3G B8522 Antibody mediated rejection 0 Refractory Ventricular fibrillation No 9G B7421 No evidence of rejection 393 None Heparin IV for 10 days 9G B2721 Antibody mediated rejection 13 None Heparin IV for 10 days 9G B6921 Antibody mediated rejection (Grade 3), Acute cellular rejection (3R) 113 None Heparin IV for 8 days, then heparin sc for 2 weeks, ASA po daily 10G B7221 No evidence of rejection 243 None Heparin IV for 8 days, then heparin sc continue, ASA po daily 10G B2622
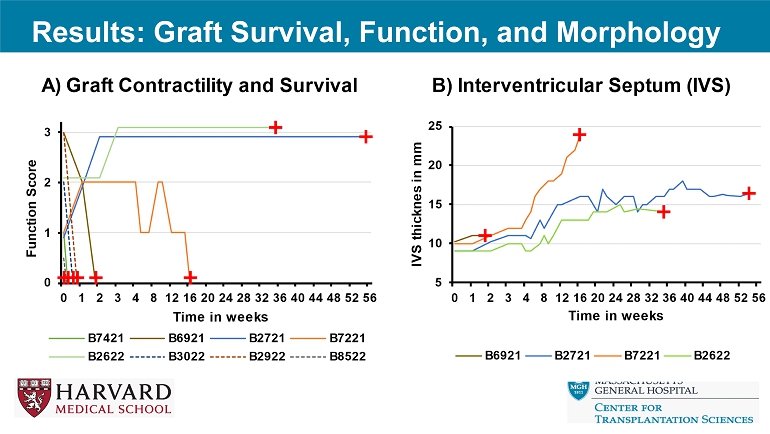
Results: Graft Survival, Function, and Morphology
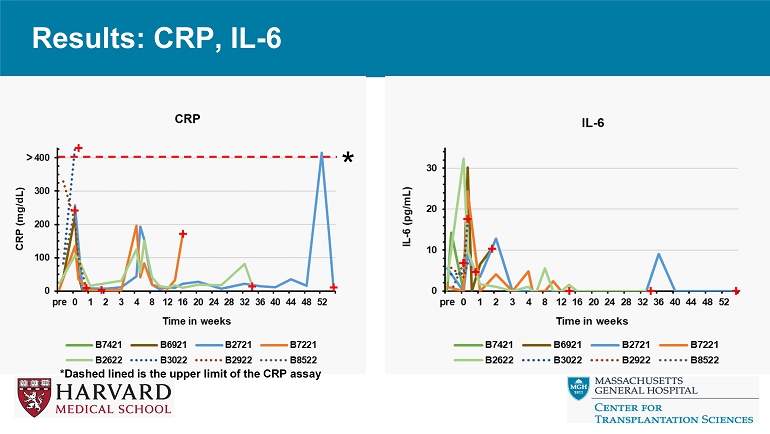
Results: CRP, IL - 6 *Dashed lined is the upper limit of the CRP assay *
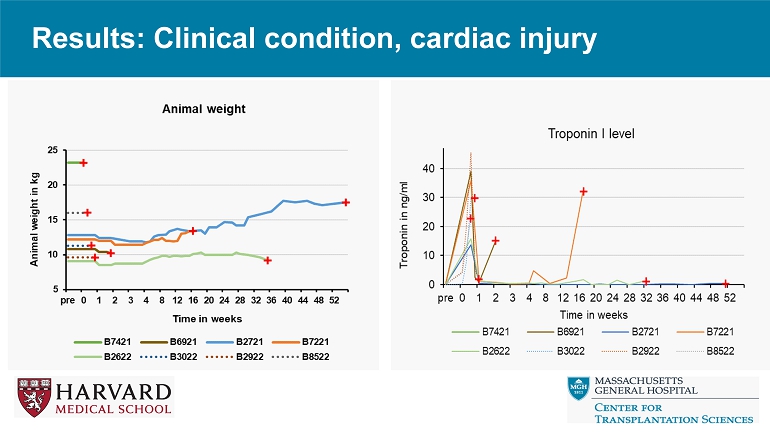
Results: Clinical condition, cardiac injury
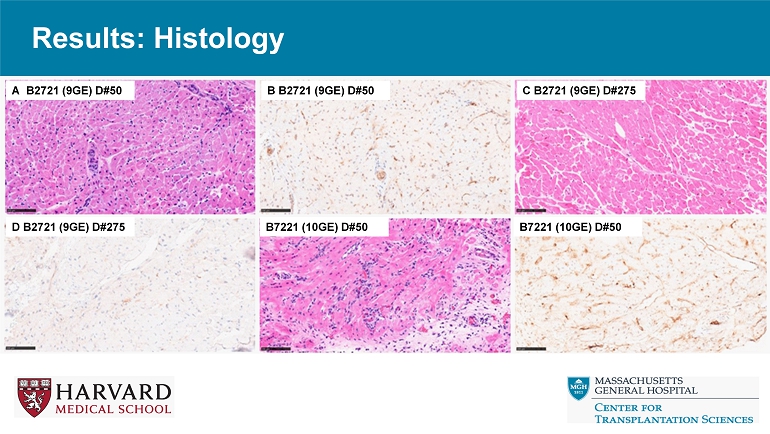
Results: Histology A B2721 (9GE) D#50 B B2721 (9GE) D#50 C B2721 (9GE) D#275 D B2721 (9GE) D#275 B7221 (10GE) D#50 B7221 (10GE) D#50
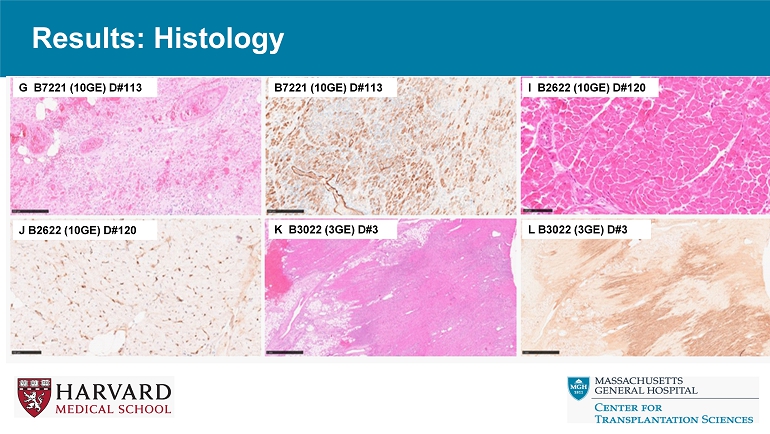
Results: Histology G B7221 (10GE) D#113 B7221 (10GE) D#113 I B2622 (10GE) D#120 J B2622 (10GE) D#120 K B3022 (3GE) D#3 L B3022 (3GE) D#3
Conclusions • Relative to reference genetics without thrombo - regulatory and anti - inflammatory gene expression, 9 - or 10 - GE pig hearts exhibit promising performance in the context of a clinically applicable regimen including ischemia minimization and αCD154 - based immunosuppression. • Despite GE modifications and use of ischemia minimization, peri - transplant myocardial injury (Troponin leak) and systemic inflammation (CRP, IL - 6 elaboration) occur consistently, but do not prevent graft recovery and long - term survival. • These encouraging results justify further evaluation in an orthotopic heart xenotransplant model.

Thank you! Key Contributors: R Chaban A Calhoun V Diaz I Ileka RN Pierson III