
Exhibit 99.01

NASDAQ: TNXP Investor Presentation September 2016 © 2016 Tonix Pharmaceuticals Holding Corp. Version: P0028 09-06-2016

Cautionary note on forward-looking statements Certainstatementsinthispresentationregardingstrategicplans,expectationsandobjectivesforfutureoperationsorresultsare“forward-lookingstatements”asdefinedbythePrivateSecuritiesLitigationReformActof1995.Thesestatementsmaybeidentifiedbytheuseofforward-lookingwordssuchas“anticipate,”“believe,”“forecast,”“estimate,”and“intend,”amongothers.Theseforward-lookingstatementsarebasedonTonix’scurrentexpectationsandactualresultscoulddiffermaterially.Thereareanumberoffactorsthatcouldcauseactualeventstodiffermateriallyfromthoseindicatedbysuchforward-lookingstatements.Thesefactorsinclude,butarenotlimitedto,substantialcompetition;ourneedforadditionalfinancing;uncertaintiesofpatentprotectionandlitigation;uncertaintiesofgovernmentorthirdpartypayorreimbursement;limitedresearchanddevelopmenteffortsanddependenceuponthirdparties;andrisksrelatedtofailuretoobtainU.SFoodandDrugAdministrationclearancesorapprovalsandnoncompliancewithitsregulations.Aswithanypharmaceuticalunderdevelopment,therearesignificantrisksinthedevelopment,regulatoryapprovalandcommercializationofnewproducts.Theforward-lookingstatementsinthispresentationaremadeasofthedateofthispresentation,evenifsubsequentlymadeavailablebytheCompanyonitswebsiteorotherwise.Tonixdoesnotundertakeanobligationtoupdateorreviseanyforward-lookingstatement,exceptasrequiredbylaw.InvestorsshouldreadtheriskfactorssetforthintheAnnualReportonForm10-KfortheyearendedDecember31,2015,asfiledwiththeSecuritiesandExchangeCommission(the“SEC”)onMarch3,2016,andfutureperiodicreportsfiledwiththeSEConorafterthedatehereof.AlloftheCompany'sforward-lookingstatementsareexpresslyqualifiedbyallsuchriskfactorsandothercautionarystatements. 2 © Copyright 2016 Tonix Pharmaceuticals

Developinginnovative medicines for large and growing markets Targeting common central nervous system disordersOne clinical-stage proprietary candidate targeting posttraumatic stress disorder (PTSD) Differentiated product with potential for sustainable competitive advantages PTSD –Phase 2 trial reported May 2016TNX-102 SL15.6 mg was active in treating military-related PTSD Serious mental health problem2 Planning Phase 3 program in military-related PTSD Planning Phase 3 program in civilian PTSD All intellectual property owned by Tonix 1TNX-102 SL (cyclobenzaprine HClsublingual tablets) is an Investigational New Drug and is not approved for any indication. 2Schnurr, PP et al., Contemporary Clinical Trials 2015;41:75. 3 © Copyright 2016 Tonix Pharmaceuticals

Overview: PTSD program Results from multi-center, double-blind, randomized, placebo-controlled Phase 2 trial in military-related PTSD reported May 2016TNX-102 SL 5.6 mg was active in treating military-related PTSDReduction in symptoms and disease severity [(Clinician-Administered PTSD Scale) CAPS-5] Improvement in clinical global impression (CGI-I) Improvement in function (Sheehan Disability Scale domains for work/school and social/leisure) Systemic side effects include somnolence, dry mouth, headache and sedation Administrative site reactions were common (transient tongue numbness) Completion rate for TNX-102 SL 5.6 mg was 84% and for placebo was 73% Phase 3 program plannedU.S. Food and Drug Administration (FDA) acceptance of proposed Phase 3 program at August 2016 EOP2/Pre-Phase 3 meeting TNX-102 SL 5.6 mg is the appropriate dose for confirmatory study Confirmatory study design similar to Phase 2 AtEasestudy, except CAPS-5 enrollment threshold will be changed from ≥ 29 to ≥ 33 Potential for Breakthrough Designation Two confirmatory trials being planned Q1 2017: military-related PTSD Q2 2017: predominantly civilian PTSD 4 © Copyright 2016 Tonix Pharmaceuticals

Tonix is developing TNX-102 SL for PTSD Advanced sublingual tablet containing low-dose cyclobenzaprine (CBP) Designed for daily bedtime administration with no titration Efficient transmucosalabsorption Avoidance of first-pass metabolism reduces formation of long-lived metabolite TNX-102 SL’s pharmacologic action is believed to improve sleep qualityDisturbed sleep is a common clinical feature of PTSD TNX-102 SL targets receptors believed to play key roles in sleep physiology Phase 2 “AtEase” study was successfully completed in May 2016 5 © Copyright 2016 Tonix Pharmaceuticals
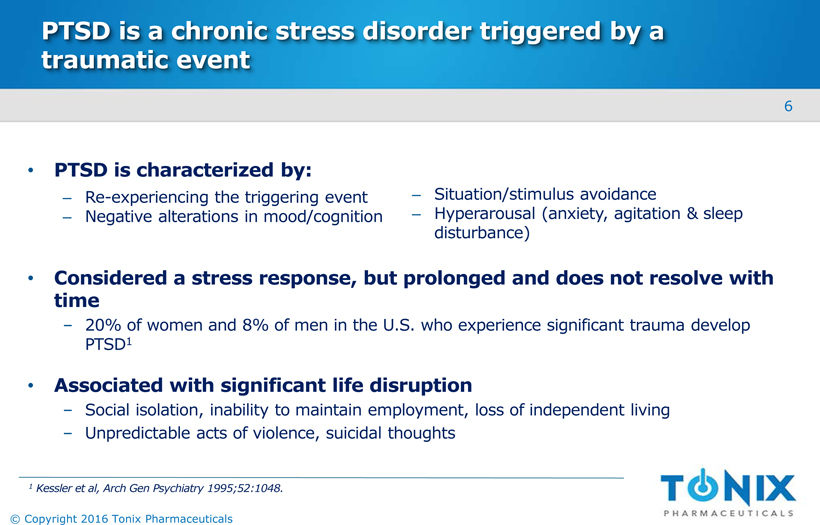
PTSD is a chronic stress disorder triggered by a traumatic event PTSD is characterized by: Considered a stress response, but prolonged and does not resolve with time20% of women and 8% of men in the U.S. who experience significant trauma develop PTSD1 Associated with significant life disruptionSocial isolation, inability to maintain employment, loss of independent living Unpredictable acts of violence, suicidal thoughts Re-experiencing the triggering event Negative alterations in mood/cognition Situation/stimulus avoidance Hyperarousal (anxiety, agitation & sleep disturbance) 1Kessler et al, Arch Gen Psychiatry 1995;52:1048. 6 © Copyright 2016 Tonix Pharmaceuticals
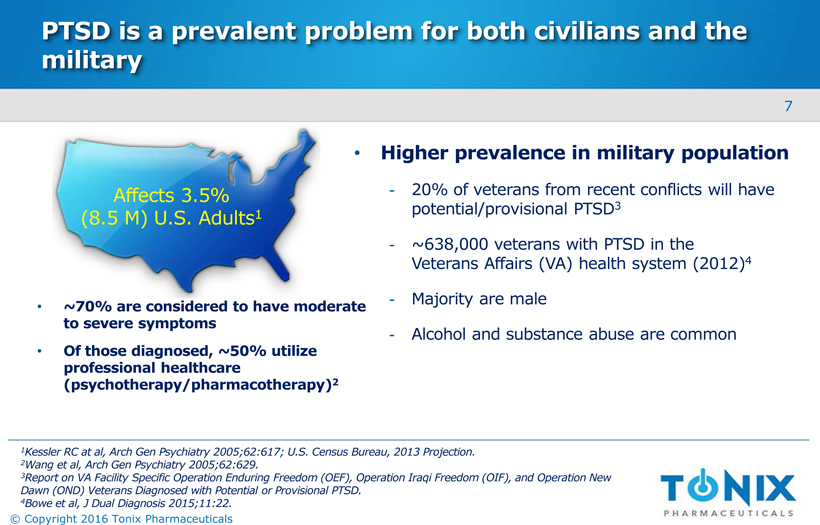
PTSD is a prevalent problem for both civilians and the military Affects 3.5% (8.5 M) U.S. Adults1 ~70% are considered to have moderate to severe symptoms Of those diagnosed, ~50% utilize professional healthcare (psychotherapy/pharmacotherapy)2 Higher prevalence in military population 20% of veterans from recent conflicts will have potential/provisional PTSD3 ~638,000 veterans with PTSD in the Veterans Affairs (VA) health system (2012)4 Majority are male Alcohol and substance abuse are common 1Kessler RC at al, Arch Gen Psychiatry 2005;62:617; U.S. Census Bureau, 2013 Projection. 2Wang et al, Arch Gen Psychiatry 2005;62:629. 3Report on VA Facility Specific Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) Veterans Diagnosed with Potential or Provisional PTSD. 4Bowe et al, J Dual Diagnosis 2015;11:22. 7 © Copyright 2016 Tonix Pharmaceuticals
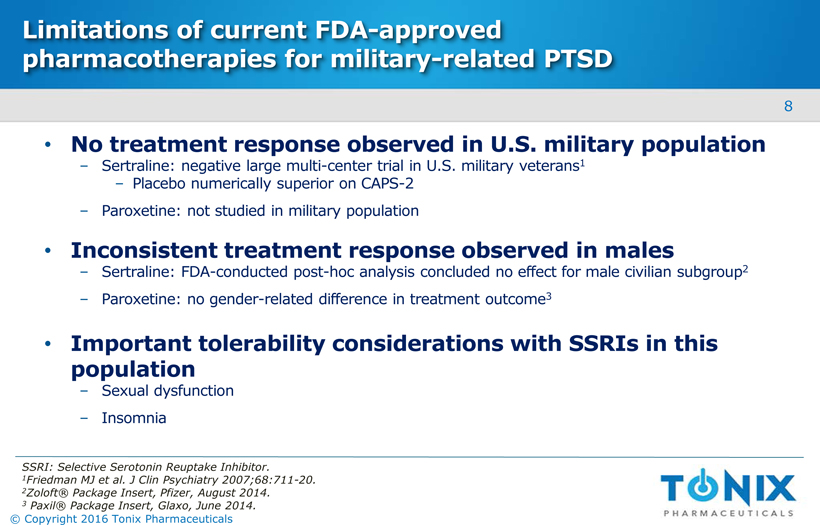
Limitations of current FDA-approved pharmacotherapies for military-related PTSD No treatment response observed in U.S. military populationSertraline: negative large multi-center trial in U.S. military veterans1Placebo numerically superior on CAPS-2 Paroxetine: not studied in military population Inconsistent treatment response observed in males Sertraline: FDA-conducted post-hoc analysis concluded no effect for male civilian subgroup2 Paroxetine: no gender-related difference in treatment outcome3 Important tolerability considerations with SSRIs in this populationSexual dysfunction Insomnia SSRI: Selective Serotonin Reuptake Inhibitor. 1Friedman MJ et al. J ClinPsychiatry 2007;68:711-20. 2Zoloft® Package Insert, Pfizer, August 2014. 3Paxil® Package Insert, Glaxo, June 2014. 8 © Copyright 2016 Tonix Pharmaceuticals
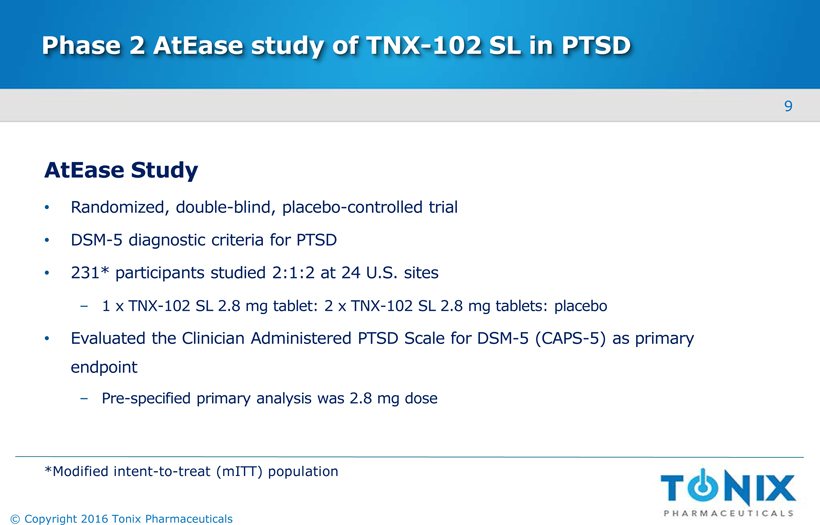
Phase 2 AtEase study of TNX-102 SL in PTSD AtEase Study Randomized, double-blind, placebo-controlled trial DSM-5 diagnostic criteria for PTSD 231* participants studied 2:1:2 at 24 U.S. sites1 x TNX-102 SL 2.8 mg tablet: 2 x TNX-102 SL 2.8 mg tablets: placebo Evaluated the Clinician Administered PTSD Scale for DSM-5 (CAPS-5) as primary endpointPre-specified primary analysis was 2.8 mg dose *Modified intent-to-treat (mITT) population 9 © Copyright 2016 Tonix Pharmaceuticals
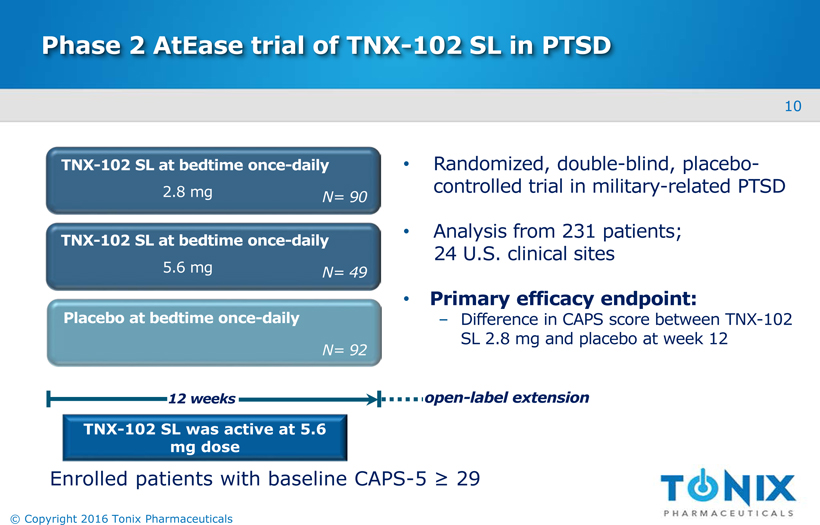
Phase 2 AtEase trial of TNX-102 SL in PTSD Randomized, double-blind, placebo-controlled trial in military-related PTSD Analysis from 231 patients; 24 U.S. clinical sites Primary efficacy endpoint:Difference in CAPS score between TNX-102 SL 2.8 mg and placebo at week 12 TNX-102 SL was active at 5.6 mg dose TNX-102 SL at bedtime once-daily Placebo at bedtime once-daily 12 weeks N= 90 TNX-102 SL at bedtime once-daily N= 92 N= 49 2.8 mg 5.6 mg open-label extension Enrolled patients with baseline CAPS-5 ≥ 29 10 © Copyright 2016 Tonix Pharmaceuticals
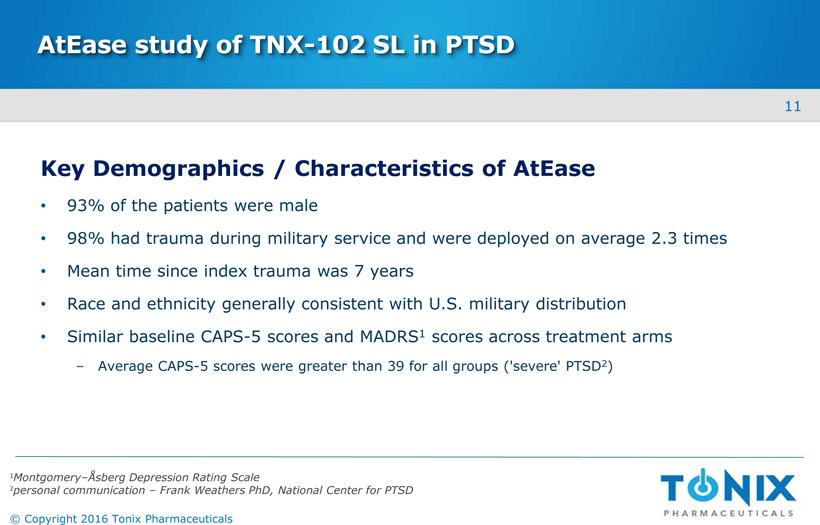
AtEasestudy of TNX-102 SL in PTSD Key Demographics / Characteristics of AtEase 93% of the patients were male 98% had trauma during military service and were deployed on average 2.3 times Mean time since index trauma was 7 years Race and ethnicity generally consistent with U.S. military distribution Similar baseline CAPS-5 scores and MADRS1scores across treatment armsAverage CAPS-5 scores were greater than 39 for all groups ('severe' PTSD2) 1Montgomery–ÅsbergDepression Rating Scale 2personal communication –Frank Weathers PhD, National Center for PTSD 11 © Copyright 2016 Tonix Pharmaceuticals
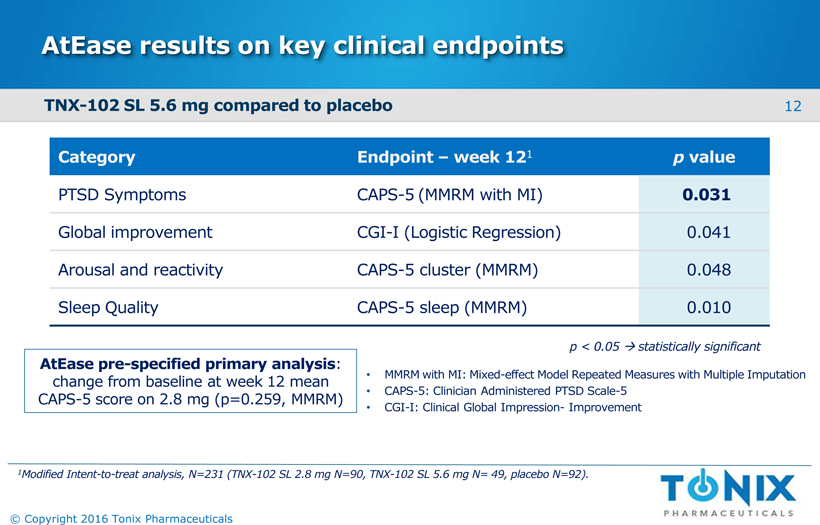
AtEaseresults on key clinical endpoints Category Endpoint –week 121 pvalue PTSD Symptoms CAPS-5(MMRM with MI) 0.031 Global improvement CGI-I (Logistic Regression) 0.041 Arousal and reactivity CAPS-5 cluster (MMRM) 0.048 Sleep Quality CAPS-5 sleep (MMRM) 0.010 12 © Copyright 2016 Tonix Pharmaceuticals
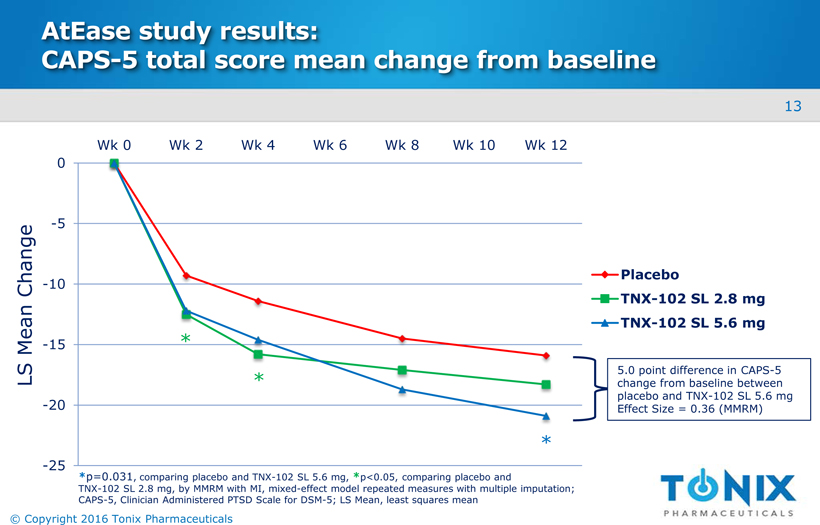
AtEasestudy results:CAPS-5 total score mean change from baseline -25 -20 -15 -10 -5 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * *p=0.031, comparing placebo and TNX-102 SL 5.6 mg,*p<0.05, comparing placebo and TNX-102 SL 2.8 mg, by MMRM with MI, mixed-effect model repeated measures with multiple imputation; CAPS-5, Clinician Administered PTSD Scale for DSM-5; LS Mean, least squares mean LS Mean Change * * 5.0 point difference in CAPS-5 change from baseline between placebo and TNX-102 SL 5.6 mg Effect Size = 0.36 (MMRM) 13 © Copyright 2016 Tonix Pharmaceuticals
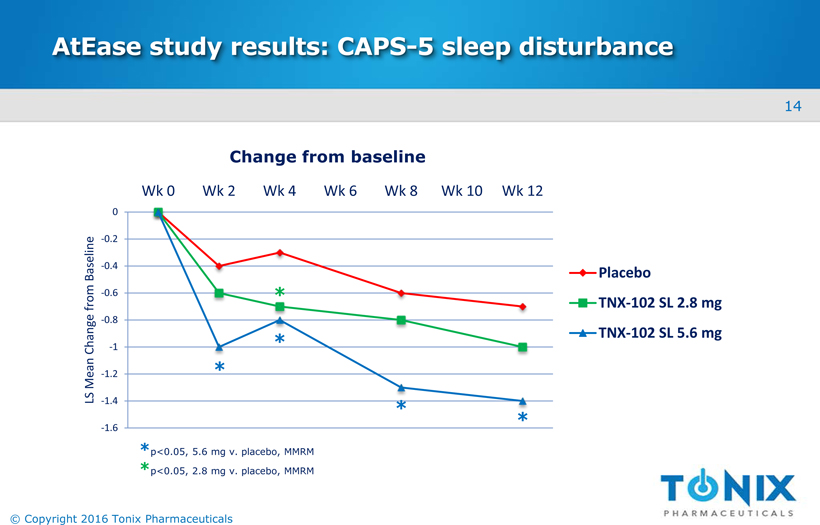
AtEasestudy results: CAPS-5 sleep disturbance -1.6 -1.4 -1.2 -1 -0.8 -0.6 -0.4 -0.2 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 LS Mean Change from Baseline Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg Change from baseline * * * * *p<0.05, 5.6 mg v. placebo, MMRM *p<0.05, 2.8 mg v. placebo, MMRM 14 © Copyright 2016 Tonix Pharmaceuticals
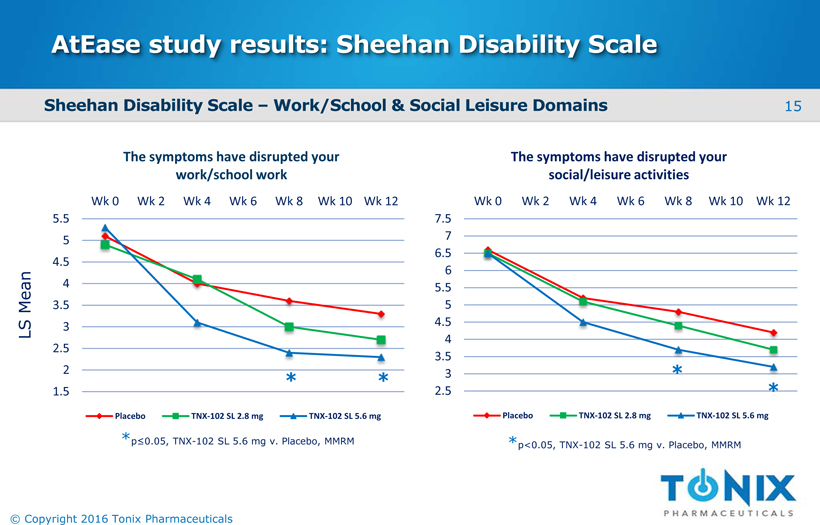
AtEasestudy results: Sheehan Disability Scale 1.5 2 2.5 3 3.5 4 4.5 5 5.5 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 The symptoms have disrupted your work/school work Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg 2.5 3 3.5 4 4.5 5 5.5 6 6.5 7 7.5 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 The symptoms have disrupted your social/leisure activities Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * * * *p≤0.05, TNX-102 SL 5.6 mg v. Placebo, MMRM *p<0.05, TNX-102 SL 5.6 mg v. Placebo, MMRM Sheehan Disability Scale –Work/School & Social Leisure Domains LS Mean 15 © Copyright 2016 Tonix Pharmaceuticals
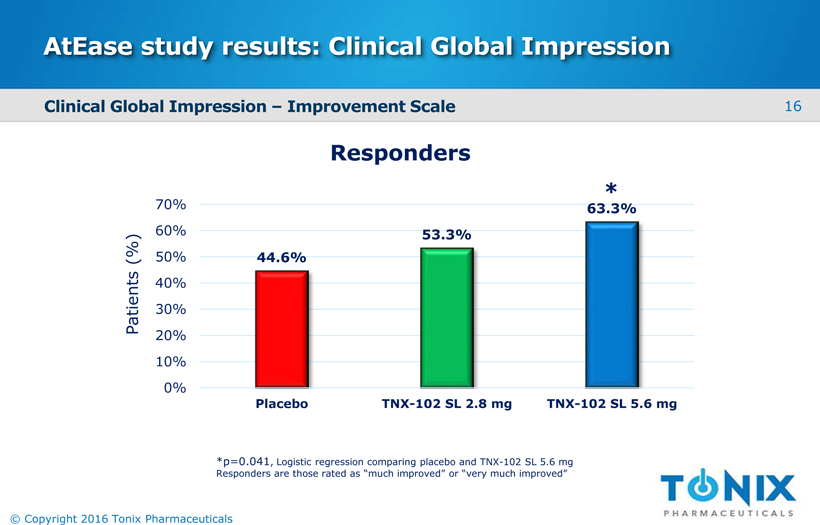
AtEasestudy results: Clinical Global Impression Clinical Global Impression –Improvement Scale Responders Patients (%) 44.6% 53.3% 63.3% 0% 10% 20% 30% 40% 50% 60% 70% Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg *p=0.041, Logistic regression comparing placebo and TNX-102 SL 5.6 mg Responders are those rated as “much improved” or “very much improved” 16 © Copyright 2016 Tonix Pharmaceuticals
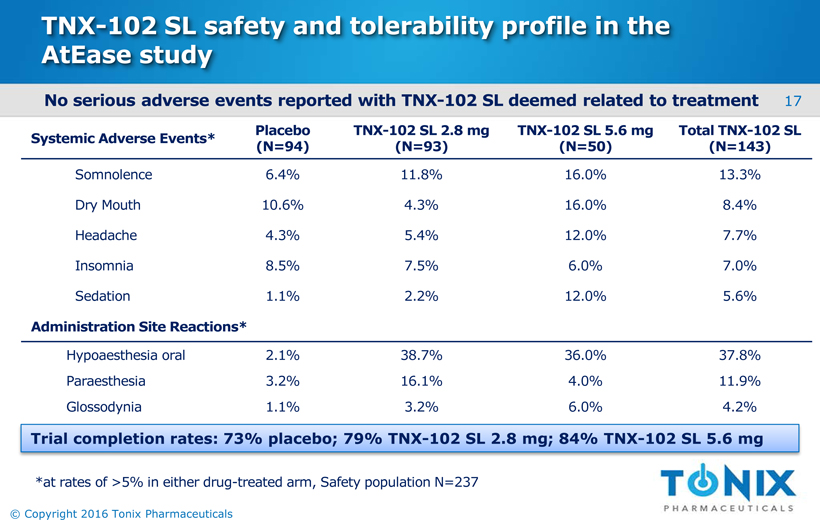
TNX-102 SL safety and tolerability profile in the AtEasestudy No serious adverse events reported with TNX-102 SL deemed related to treatment Systemic Adverse Events* Placebo(N=94) TNX-102 SL 2.8 mg(N=93) TNX-102 SL 5.6mg (N=50) Total TNX-102 SL(N=143) Somnolence 6.4% 11.8% 16.0% 13.3% DryMouth 10.6% 4.3% 16.0% 8.4% Headache 4.3% 5.4% 12.0% 7.7% Insomnia 8.5% 7.5% 6.0% 7.0% Sedation 1.1% 2.2% 12.0% 5.6% Administration Site Reactions* Hypoaesthesiaoral 2.1% 38.7% 36.0% 37.8% Paraesthesia 3.2% 16.1% 4.0% 11.9% Glossodynia 1.1% 3.2% 6.0% 4.2% 17 © Copyright 2016 Tonix Pharmaceuticals
Determining CAPS-5 severity criteria for entry in phase 3 trials CAPS-5hasnotbeenemployedpreviouslyinapharmacotherapytrial;entryseveritythresholdnotclear;≥29usedinthePhase2AtEasestudy PriorpharmacotherapytrialsinPTSDusingearlierversionsofCAPSwithdifferentscoringrangehavemostfrequentlyusedascoreof≥50forentry(similartoCAPS-5≥331) AnalysisofpatientswithCAPS-5≥33showedaneffectsizeof0.53onmeanchangefrombaselineatWeek12inTNX-102SL5.6mggroup(seefigureonnextslide) 1Sullivan, Gregory, et al. “The AtEaseStudy: Efficacy and Safety of a Low Dose, Bedtime, Sublingual Formulation of Cyclobenzaprine (TNX-102 SL) for the Treatment of Military-Related PTSD.” Poster session presented at: Military Health System Research Symposium (MHSRS) 2016 Annual Meeting; 2016 Aug 15-18; Kissimmee, FL. URL: http://bit.ly/2bFo4mx 18 © Copyright 2016 Tonix Pharmaceuticals
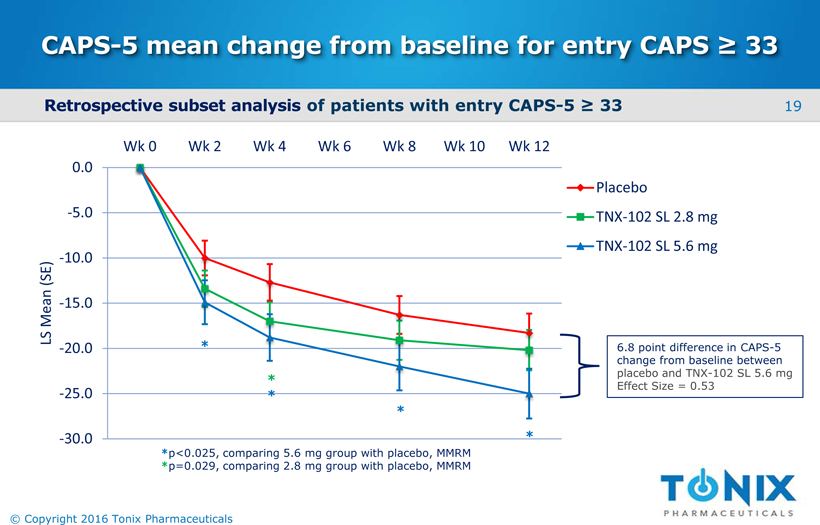
CAPS-5 mean change from baseline for entry CAPS ≥ 33 -30.0 -25.0 -20.0 -15.0 -10.0 -5.0 0.0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 LS Mean (SE) Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg 6.8 point difference in CAPS-5 change from baseline between placebo and TNX-102 SL 5.6 mg Effect Size = 0.53 * * * * * *p<0.025, comparing 5.6 mg group with placebo, MMRM *p=0.029, comparing 2.8 mg group with placebo, MMRM Retrospective subset analysisof patients with entry CAPS-5 ≥ 33 19 © Copyright 2016 Tonix Pharmaceuticals
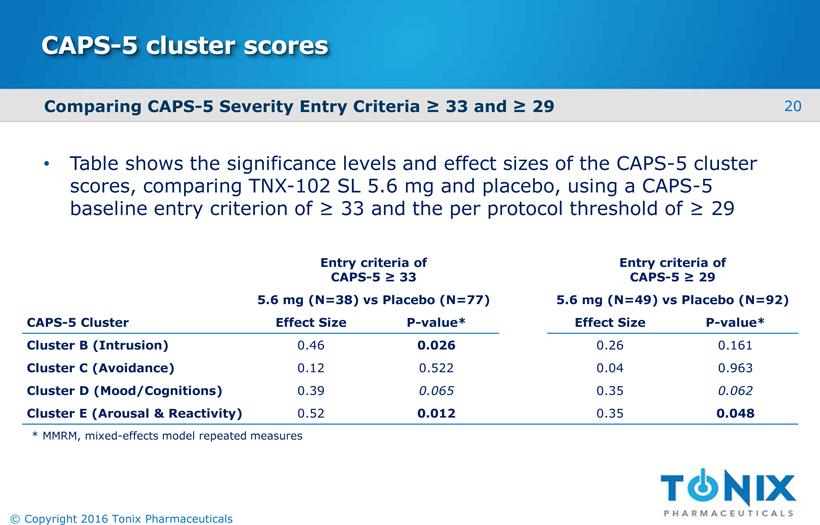
CAPS-5 cluster scores Table shows the significance levels and effect sizes of the CAPS-5 cluster scores, comparing TNX-102 SL 5.6 mg and placebo, using a CAPS-5 baseline entry criterion of ≥ 33 and the per protocol threshold of ≥ 29 Entry criteria of CAPS-5 ≥ 33 Entry criteria of CAPS-5 ≥ 29 5.6 mg (N=38) vs Placebo (N=77) 5.6 mg (N=49) vs Placebo (N=92) CAPS-5 Cluster Effect Size P-value* Effect Size P-value* Cluster B (Intrusion) 0.46 0.026 0.26 0.161 Cluster C (Avoidance) 0.12 0.522 0.04 0.963 Cluster D (Mood/Cognitions) 0.39 0.065 0.35 0.062 Cluster E (Arousal & Reactivity) 0.52 0.012 0.35 0.048 20 © Copyright 2016 Tonix Pharmaceuticals
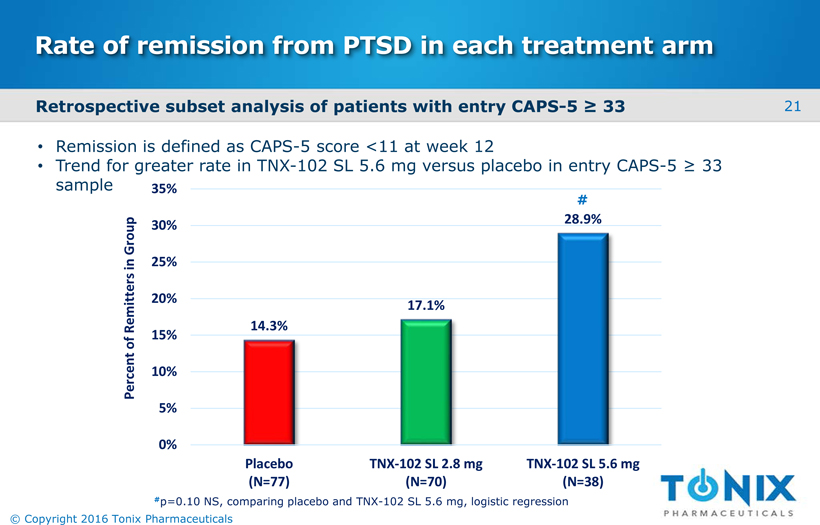
Rate of remission from PTSD in each treatment arm 14.3% 17.1% 28.9% 0% 5% 10% 15% 20% 25% 30% 35% Placebo(N=77) TNX-102 SL 2.8 mg(N=70) TNX-102 SL 5.6 mg(N=38) Percent of Remitters in Group #p=0.10 NS, comparing placebo and TNX-102 SL 5.6 mg, logistic regression # Remission is defined as CAPS-5 score <11 at week 12 Trend for greater rate in TNX-102 SL 5.6 mg versus placebo in entry CAPS-5 ≥ 33 sample Retrospective subset analysis of patients with entry CAPS-5 ≥ 33 21 © Copyright 2016 Tonix Pharmaceuticals
AtEasestudy conclusions This Phase 2 trial is the first large multi-center trial of an investigational new drug product that demonstrated efficacy in a population with military-related PTSDSymptom reduction (CAPS-5) Functional improvement (Sheehan Disability Scale domains) Global improvement (CGI-I) Early effects on sleep and hyperarousal are consistent with the mechanistic hypothesis of TNX-102 SL primary actions on sleep disturbance and autonomic balance Systemic side effects included include somnolence, dry mouth, headache and sedation; local reactions to sublingual TNX-102 SL administration were common (transient tongue numbness) A post-hoc analysis suggested that enrolling patients with a higher CAPS-5 score (≥ 33) would be similar to the entry criteria used in the registration studies supporting the approval of the marketed PTSD drug productsSame post-hoc analysis revealed a larger separation from placebo in the subset of patients with baseline CAPS-5 ≥ 33 at weeks 2, 4, 8 and 12 Phase 3 program will use CAPS-5 ≥ 33 as enrollment threshold 22 © Copyright 2016 Tonix Pharmaceuticals
Phase 3 program in PTSD being planned General Study Characteristics: Randomized, double-blind, placebo-controlled studies in PTSD N~400-500; approximately 35 U.S. clinical sites Primary Efficacy Endpoint: Mean change from baseline in total CAPS-5 at Week 12 compared between TNX-102 SL 5.6 mg and placebo Topline data for Phase 3 in Planning to confirm AtEasefinding in military-related PTSD and in predominantly civilian PTSD: •Larger studies •Targeting start in 2017 Placebo once-daily at bedtime 12 weeks TNX-102 SL once-daily at bedtime N ~ 200-250 N ~ 200-250 5.6 mg open-labelextension military-related PTSD study anticipated 1H 2018 23 © Copyright 2016 Tonix Pharmaceuticals
Intellectual property Composition-of-matter (eutectic)Patents filed Protection expected to 2034 Pharmacokinetics (PK)Patents filed Protection expected to 2033 Method-of-usePTSD: patents filed TNX-102 SL Wholly-owned by Tonixwith no obligations to others 24 © Copyright 2016 Tonix Pharmaceuticals
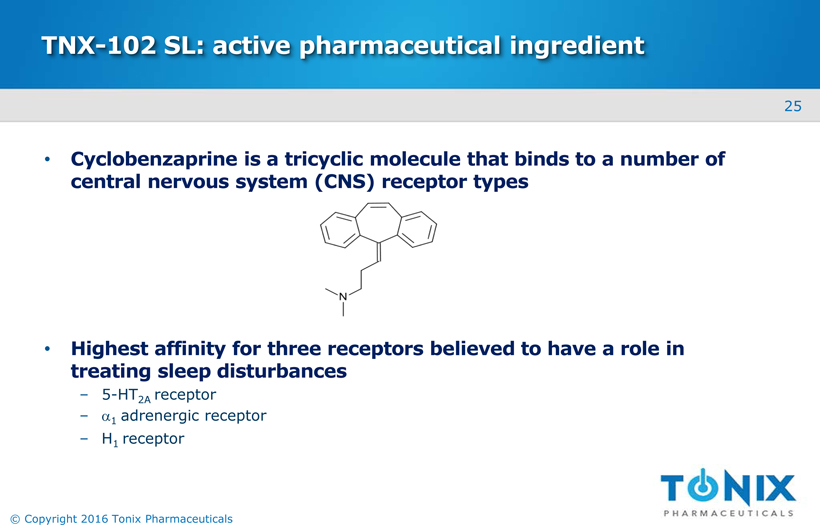
TNX-102 SL: active pharmaceutical ingredient
Cyclobenzaprine is a tricyclic molecule that binds to a number of central nervous system (CNS) receptor types
Highest affinity for three receptors believed to have a role in treating sleep disturbances5-HT2A receptor
α1 adrenergic receptor
H1 receptor
© Copyright 2016 Tonix Pharmaceuticals
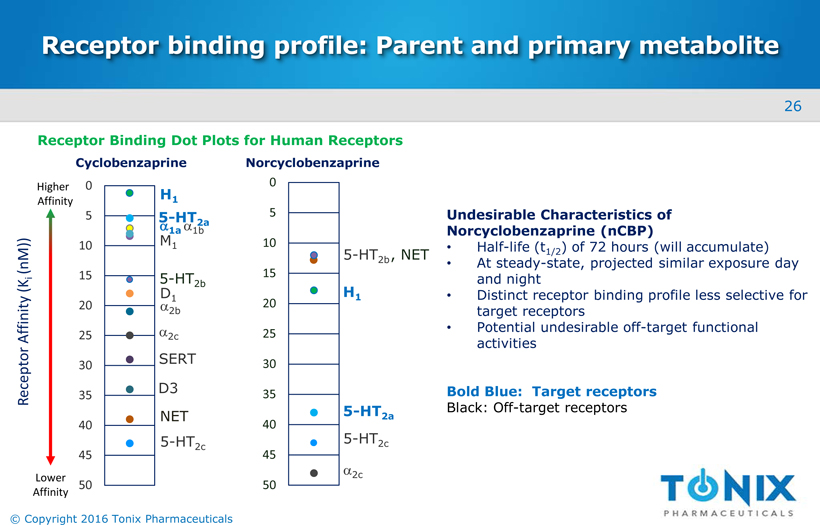
Receptor binding profile: Parent and primary metabolite 0 5 10 15 20 25 30 35 40 45 50 5-HT2c H1 5-HT2a α2c , NET 5-HT2b Undesirable Characteristics of Norcyclobenzaprine (nCBP) Half-life (t1/2) of 72 hours (will accumulate) At steady-state, projected similar exposure day and night Distinct receptor binding profile less selective for target receptors Potential undesirable off-target functional activities Bold Blue: Target receptors Black: Off-target receptors 0 5 10 15 20 25 30 35 40 45 50 Receptor Affinity (Ki (nM)) D1 α2b α2c SERT D3 NET 5-HT2c α1a M1 α1b H1 5-HT2a 5-HT2b Cyclobenzaprine Norcyclobenzaprine Lower Affinity Higher Affinity Receptor Binding Dot Plots for Human Receptors 26 © Copyright 2016 Tonix Pharmaceuticals
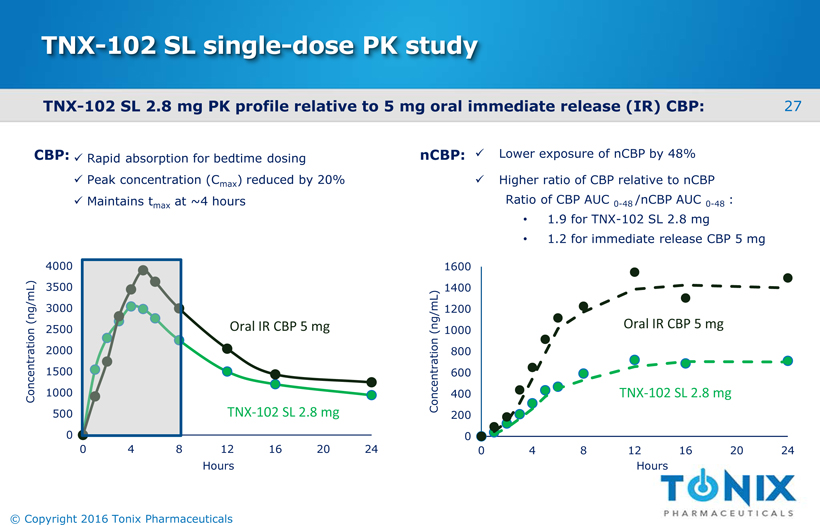
TNX-102 SL single-dose PK study TNX-102 SL 2.8 mg PK profile relative to 5 mg oral immediate release (IR) CBP: 0 500 1000 1500 2000 2500 3000 3500 4000 0 4 8 12 16 20 24 Concentration (ng/mL) TNX-102 SL 2.8 mg Oral IR CBP 5 mg xLower exposure of nCBP by 48% xHigher ratio of CBP relative to nCBP Ratio of CBP AUC 0-48 /nCBP AUC 0-48: •1.9 for TNX-102 SL 2.8 mg •1.2 for immediate release CBP 5 mg 0 200 400 600 800 1000 1200 1400 1600 0 4 8 12 16 20 24 Concentration (ng/mL) Oral IR CBP 5 mg TNX-102 SL 2.8 mg Hours Hours nCBP: xRapid absorption for bedtime dosing xPeak concentration (Cmax) reduced by 20% xMaintains tmaxat ~4 hours CBP: 27 © Copyright 2016 Tonix Pharmaceuticals
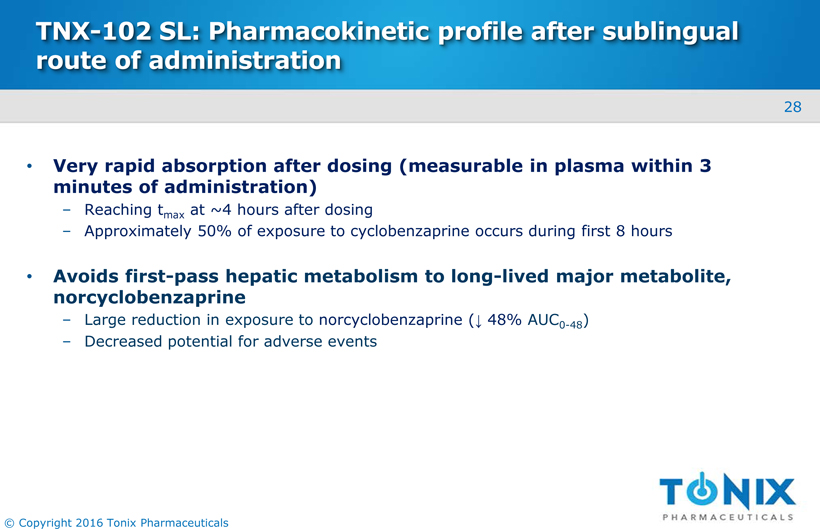
TNX-102 SL: Pharmacokinetic profile after sublingual route of administration Very rapid absorption after dosing (measurable in plasma within 3 minutes of administration)Reaching tmaxat ~4 hours after dosing Approximately 50% of exposure to cyclobenzaprine occurs during first 8 hours Avoids first-pass hepatic metabolism to long-lived major metabolite, norcyclobenzaprineLarge reduction in exposure to norcyclobenzaprine(↓48% AUC0-48) Decreased potential for adverse events 28 © Copyright 2016 Tonix Pharmaceuticals
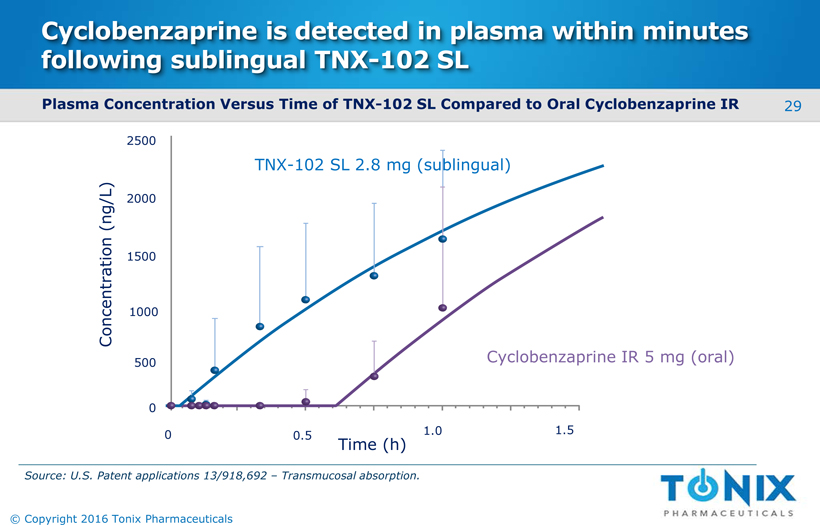
Cyclobenzaprine is detected in plasma within minutes following sublingual TNX-102 SL Plasma Concentration Versus Time of TNX-102 SL Compared to Oral Cyclobenzaprine IR 0 0.5 1.0 1.5 Concentration (ng/L) 2500 1500 1000 500 2000 0 TNX-102 SL 2.8 mg (sublingual) Cyclobenzaprine IR 5 mg (oral) Time (h) Source: U.S. Patent applications 13/918,692 –Transmucosalabsorption. 29 © Copyright 2016 Tonix Pharmaceuticals

Management team Seth Lederman, MD President & CEO Jessica Edgar Morris EVP, Operations Bruce Daugherty, PhD, MBA Chief Scientific Officer Ronald Notvest, PhD EVP, Commercial Planning & Development Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer 30 © Copyright 2016 Tonix Pharmaceuticals

Board of directors Seth Lederman, MD Chairman Ernest Mario, PhD ALZA, Glaxo, Reliant Pharma John Rhodes NYSERDA, NRDC, Booz Allen Hamilton Samuel Saks, MD Jazz Pharma, ALZA, Johnson & Johnson Charles Mather BTIG, Janney, Jefferies, Cowen, Smith Barney Stuart Davidson Labrador Ventures, Alkermes, Combion Patrick Grace Apollo Philanthropy, WR Grace, Chemed Donald Landry, MD, PhD Chair of Medicine, Columbia University 31 © Copyright 2016 Tonix Pharmaceuticals

Financial overview NASDAQ:TNXP Cash, cash equivalents, andmarketable securitiesreported at June 30, 2016 $ 31.2 million Net proceeds from overallotment in 3Q16 $1.4 million Sharesoutstanding(September 6, 2016) 25.9 million 32 © Copyright 2016 Tonix Pharmaceuticals

Milestones –recent and upcoming TNX-102 SL –Posttraumatic Stress Disorder December 2015Entered into Collaborative Research and Development Agreement (CRADA) with the United States Army Medical Materiel Development Activity (USAMMDA) May 2016Report results from AtEasestudy August 2016End of Phase 2 meeting with FDA
-Proposed Phase 3 and NDA plan accepted -Breakthrough Therapy Designation Request can be submitted for review Q1 2017 Target commencement of Phase 3 study in military-related PTSD Q2 2017 Target commencement of Phase 3 study in predominantly civilian PTSD 33 © Copyright 2016 Tonix Pharmaceuticals

NASDAQ: TNXP 509 Madison Avenue NewYork, NY 10022 (212) 980-9155 www.tonixpharma.com © Copyright 2016 Tonix Pharmaceuticals - Confidential - Do not duplicate or distribute