
Exhibit 99.01

© 2017 Tonix Pharmaceuticals Holding Corp. Presented by Gregory Sullivan, MD at American Society of Clinical Psychopharmacology Annual Meeting, Miami, FL May 30, 2017 Bedtime Sublingual Transmucosal Cyclobenzaprine (TNX - 102 SL) for the Treatment of Military - Related PTSD: Retrospective Analyses of the Mediators and Moderators of Treatment Response

© 2017 Tonix Pharmaceuticals Holding Corp. 2 What is Military - Related PTSD and Why Study It? » Proposed indication for TNX - 102 SL* is for the treatment of posttraumatic stress disorder (PTSD): » Affects 8.6 million U.S. adults 1 » Definition of military - related PTSD: » Any PTSD that has developed in response to any DSM - 5 PTSD Criterion A - qualifying trauma(s) that occurred during military service – includes combat and non - combat traumas » Why target military - related PTSD? » No treatment response observed in U.S. military population with the two FDA - approved selective serotonin reuptake inhibitors (SSRIs) for PTSD 2,3,4 » No other type of pharmacological treatment had been shown to be effective in any large multicenter clinical trial in a U.S. military population 1 Kessler et al., Arch Gen Psych 2005; Prevalence rate of 3.5% applied to U.S. Census estimate of 247M U.S. adult ( > 18) population in 2015; 2 Friedman MJ et al. J Clin Psychiatry 2007;68:711 - 20. 3 Zoloft® Package Insert, Pfizer, NY, NY; August 2014. 4 Paxil® Package Insert, Glaxo, June 2014; ( www.census.gov/quickfacts/table/PST045215/00 ) ; *T NX - 102 SL (cyclobenzaprine HCl sublingual tablets) is an investigational new drug and is not approved for any indication.
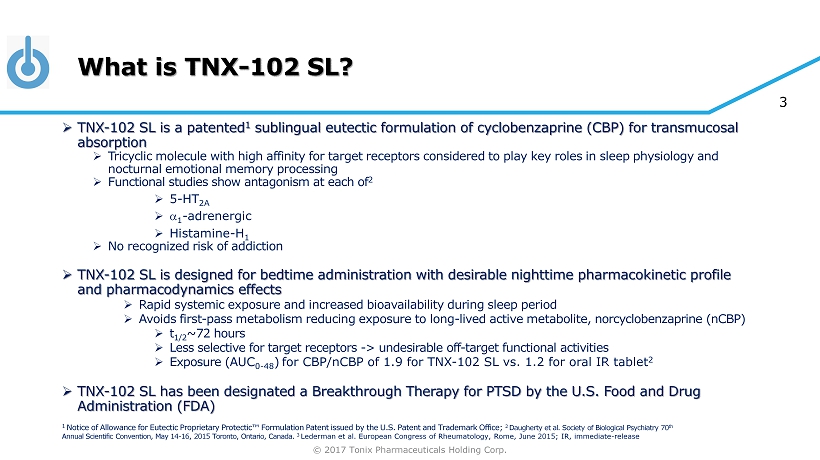
© 2017 Tonix Pharmaceuticals Holding Corp. 3 What is TNX - 102 SL? » TNX - 102 SL is a patented 1 sublingual eutectic formulation of cyclobenzaprine (CBP) for transmucosal absorption » Tricyclic molecule with high affinity for target receptors considered to play key roles in sleep physiology and nocturnal emotional memory processing » F unctional studies show antagonism at each of 2 » 5 - HT 2A » a 1 - adrenergic » Histamine - H 1 » No recognized risk of addiction » TNX - 102 SL is designed for bedtime administration with desirable nighttime pharmacokinetic profile and pharmacodynamics effects » Rapid systemic exposure and increased bioavailability during sleep period » Avoids first - pass metabolism reducing exposure to long - lived active metabolite, norcyclobenzaprine ( nCBP ) » t 1/2 ~72 hours » Less selective for target receptors - > undesirable off - target functional activities » Exposure ( AUC 0 - 48 ) for CBP/ nCBP of 1.9 for TNX - 102 SL vs. 1.2 for oral IR tablet 2 » TNX - 102 SL has been designated a Breakthrough Therapy for PTSD by the U.S. Food and Drug Administration (FDA) 1 Notice of Allowance for Eutectic Proprietary Protectic ™ Formulation Patent issued by the U.S. Patent and Trademark Office; 2 Daugherty et al. Society of Biological Psychiatry 70 th Annual Scientific Convention, May 14 - 16, 2015 Toronto, Ontario, Canada. 3 Lederman et al. European Congress of Rheumatology, Rome, June 2015; IR, immediate - release

© 2017 Tonix Pharmaceuticals Holding Corp. 4 Rationale for Targeting of Sleep for Treatment of PTSD » PTSD is a disorder of recovery » Most people exposed to an extreme trauma recover in a few weeks » New learning, e.g. extinction, and memory processing are essential to recovery » In PTSD, memory processing, e.g. extinction consolidation, 1,2 may be impeded due to insufficient sleep quality » TNX - 102 SL targets sleep quality » Potent binding and antagonism at receptors that regulate sleep quality 3 , e.g. 5 - HT 2A , a 1 - adrenergic, and histamine H 1 receptors, during the sleep period is hypothesized to be permissive to sleep quality - dependent recovery processes from trauma and PTSD 1 Pace - Schott et al. Biol Mood Anxiety Disord 2015;5:3. 2 Menz et al. J Neurosci 2016;36(7):2148. 3 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015,Toronto Ontario, Canada
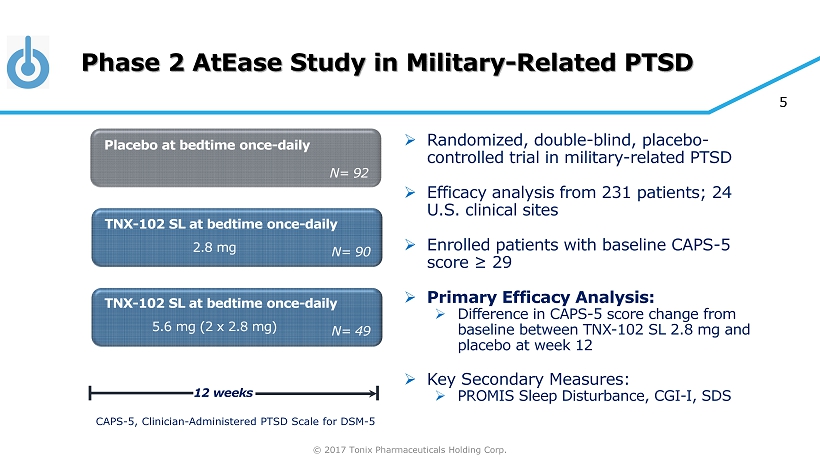
© 2017 Tonix Pharmaceuticals Holding Corp. 5 Phase 2 AtEase Study in Military - Related PTSD » Randomized, double - blind, placebo - controlled trial in military - related PTSD » Efficacy analysis from 231 patients; 24 U.S. clinical sites » Enrolled patients with baseline CAPS - 5 score ≥ 29 » Primary Efficacy Analysis: » Difference in CAPS - 5 score change from baseline between TNX - 102 SL 2.8 mg and placebo at week 12 » Key Secondary Measures: » PROMIS Sleep Disturbance, CGI - I, SDS TNX - 102 SL at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 90 TNX - 102 SL at bedtime once - daily N= 92 N= 49 2.8 mg 5.6 mg (2 x 2.8 mg) CAPS - 5, Clinician - Administered PTSD Scale for DSM - 5
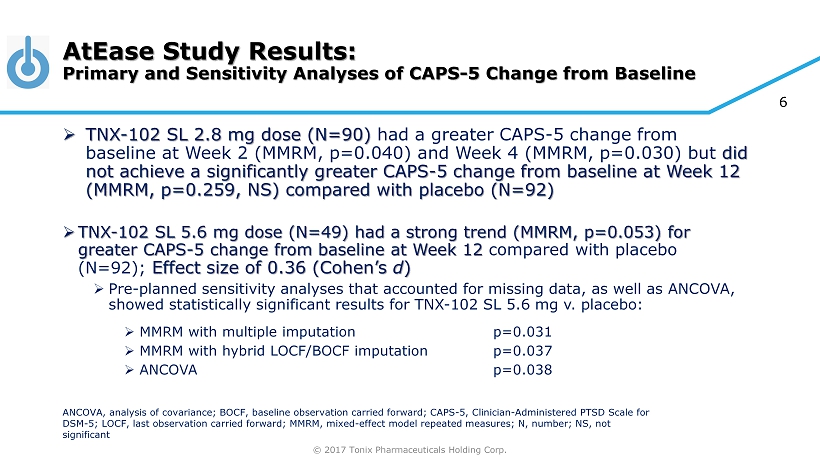
© 2017 Tonix Pharmaceuticals Holding Corp. 6 AtEase Study Results: Primary and Sensitivity Analyses of CAPS - 5 Change from Baseline » TNX - 102 SL 2.8 mg dose (N=90) had a greater CAPS - 5 change from baseline at Week 2 (MMRM, p=0.040) and Week 4 (MMRM, p=0.030) but did not achieve a significantly greater CAPS - 5 change from baseline at Week 12 (MMRM, p=0.259, NS) compared with placebo (N=92) » TNX - 102 SL 5.6 mg dose (N=49) had a strong trend (MMRM, p=0.053) for greater CAPS - 5 change from baseline at Week 12 compared with placebo (N=92); Effect size of 0.36 (Cohen’s d ) » Pre - planned sensitivity analyses that accounted for missing data, as well as ANCOVA, showed statistically significant results for TNX - 102 SL 5.6 mg v. placebo: » MMRM with multiple imputation p=0.031 » MMRM with hybrid LOCF/BOCF imputation p=0.037 » ANCOVA p=0.038 ANCOVA, analysis of covariance; BOCF, baseline observation carried forward; CAPS - 5, Clinician - Administered PTSD Scale for DSM - 5; LOCF, last observation carried forward; MMRM, mixed - effect model repeated measures; N, number; NS, not significant
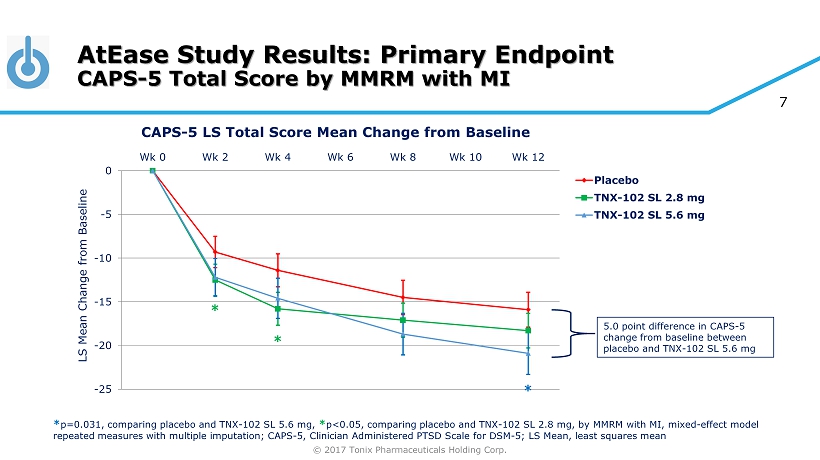
© 2017 Tonix Pharmaceuticals Holding Corp. 7 AtEase Study Results: Primary Endpoint CAPS - 5 Total Score by MMRM with MI -25 -20 -15 -10 -5 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo TNX-102 SL 2.8 mg TNX-102 SL 5.6 mg * * p=0.031, comparing placebo and TNX - 102 SL 5.6 mg, * p<0.05, comparing placebo and TNX - 102 SL 2.8 mg, by MMRM with MI, mixed - effect model repeated measures with multiple imputation; CAPS - 5, Clinician Administered PTSD Scale for DSM - 5; LS Mean, least squares mean * * CAPS - 5 LS Total Score Mean Change from Baseline 5.0 point difference in CAPS - 5 change from baseline between placebo and TNX - 102 SL 5.6 mg LS Mean Change from Baseline
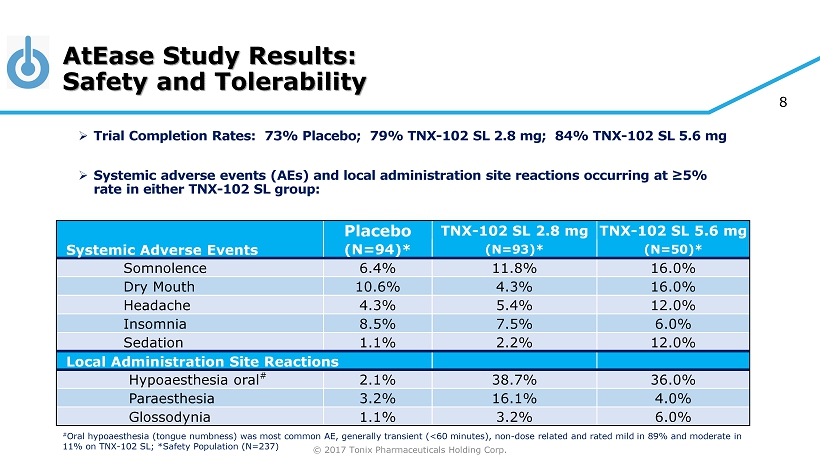
© 2017 Tonix Pharmaceuticals Holding Corp. 8 AtEase Study Results: Safety and Tolerability » Trial Completion Rates: 73% Placebo; 79% TNX - 102 SL 2.8 mg; 84% TNX - 102 SL 5.6 mg » Systemic adverse events (AEs) and local administration site reactions occurring at ≥5% rate in either TNX - 102 SL group: Placebo TNX - 102 SL 2.8 mg TNX - 102 SL 5.6 mg Systemic Adverse Events (N=94)* (N=93)* (N=50)* Somnolence 6.4% 11.8% 16.0% Dry Mouth 10.6% 4.3% 16.0% Headache 4.3% 5.4% 12.0% Insomnia 8.5% 7.5% 6.0% Sedation 1.1% 2.2% 12.0% Local Administration Site Reactions Hypoaesthesia oral # 2.1% 38.7% 36.0% Paraesthesia 3.2% 16.1% 4.0% Glossodynia 1.1% 3.2% 6.0% # Oral hypoaesthesia (tongue numbness) was most common AE, generally transient (<60 minutes), non - dose related and rated mild in 89% and moderate in 11% on TNX - 102 SL; *Safety Population (N=237)
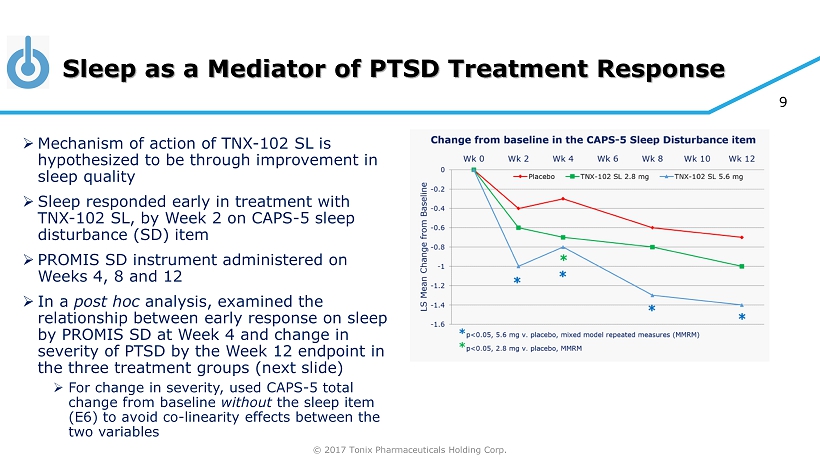
© 2017 Tonix Pharmaceuticals Holding Corp. 9 Sleep as a Mediator of PTSD Treatment Response » Mechanism of action of TNX - 102 SL is hypothesized to be through improvement in sleep quality » Sleep responded early in treatment with TNX - 102 SL, by Week 2 on CAPS - 5 sleep disturbance (SD) item » PROMIS SD instrument administered on Weeks 4, 8 and 12 » In a post hoc analysis, examined the relationship between early response on sleep by PROMIS SD at Week 4 and change in severity of PTSD by the Week 12 endpoint in the three treatment groups (next slide) » For change in severity, used CAPS - 5 total change from baseline without the sleep item (E6) to avoid co - linearity effects between the two variables
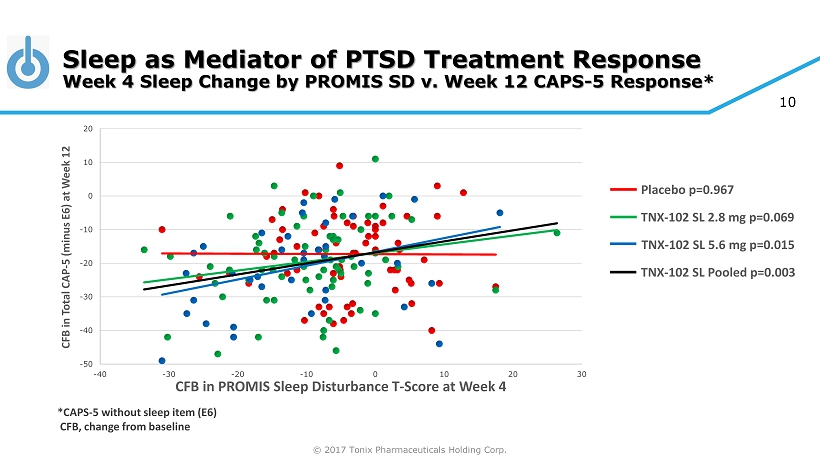
© 2017 Tonix Pharmaceuticals Holding Corp. 10 Sleep as Mediator of PTSD Treatment Response Week 4 Sleep Change by PROMIS SD v. Week 12 CAPS - 5 Response* -50 -40 -30 -20 -10 0 10 20 -40 -30 -20 -10 0 10 20 30 CFB in Total CAP - 5 (minus E6) at Week 12 CFB in PROMIS Sleep Disturbance T - Score at Week 4 Placebo p=0.967 TNX - 102 SL 2.8 mg p=0.069 TNX - 102 SL 5.6 mg p=0.015 TNX - 102 SL Pooled p=0.003 *CAPS - 5 without sleep item (E6) CFB, change from baseline
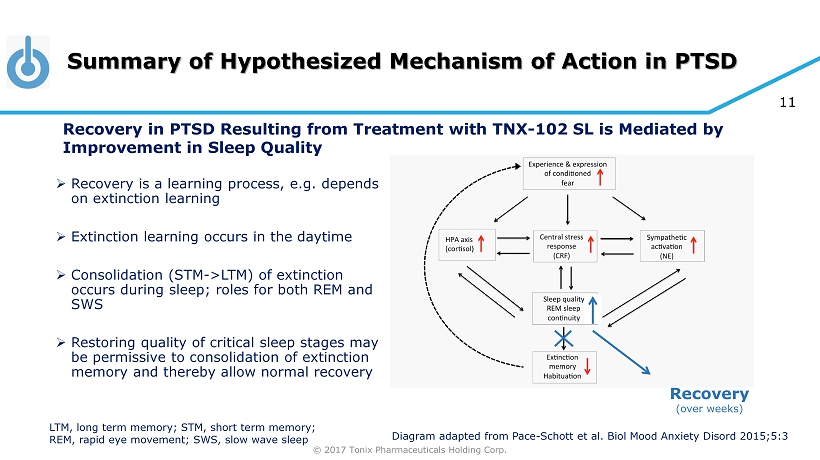
© 2017 Tonix Pharmaceuticals Holding Corp. 11 Summary of Hypothesized Mechanism of Action in PTSD Recovery (over weeks) Diagram adapted from Pace - Schott et al. Biol Mood Anxiety Disord 2015;5:3 » Recovery is a learning process, e.g. depends on extinction learning » Extinction learning occurs in the daytime » Consolidation (STM - >LTM) of extinction occurs during sleep; roles for both REM and SWS » Restoring quality of critical sleep stages may be permissive to consolidation of extinction memory and thereby allow normal recovery LTM, long term memory; STM, short term memory; REM, rapid eye movement; SWS, slow wave sleep Recovery in PTSD Resulting from Treatment with TNX - 102 SL is Mediated by Improvement in Sleep Quality
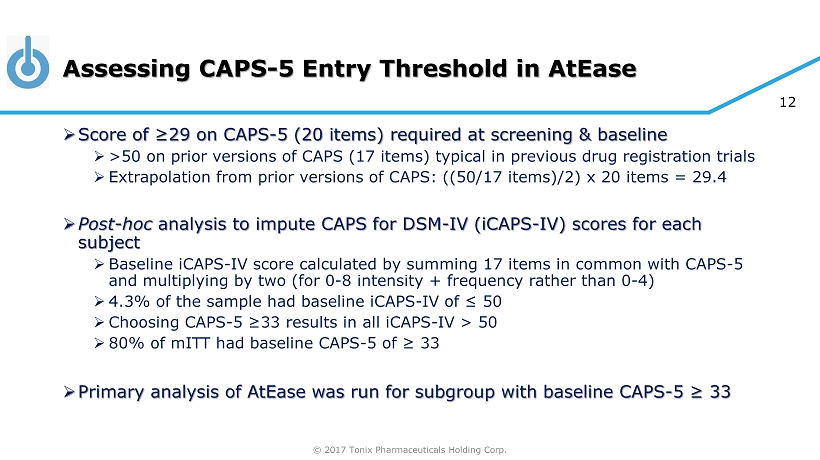
© 2017 Tonix Pharmaceuticals Holding Corp. 12 Assessing CAPS - 5 Entry Threshold in AtEase » Score of ≥29 on CAPS - 5 (20 items) required at screening & baseline » >50 on prior versions of CAPS (17 items) typical in previous drug registration trials » Extrapolation from prior versions of CAPS: ((50/17 items)/2) x 20 items = 29.4 » Post - hoc analysis to impute CAPS for DSM - IV ( iCAPS - IV) scores for each subject » Baseline iCAPS - IV score calculated by summing 17 items in common with CAPS - 5 and multiplying by two (for 0 - 8 intensity + frequency rather than 0 - 4) » 4.3% of the sample had baseline iCAPS - IV of ≤ 50 » Choosing CAPS - 5 ≥33 results in all iCAPS - IV > 50 » 80% of mITT had baseline CAPS - 5 of ≥ 33 » Primary analysis of AtEase was run for subgroup with baseline CAPS - 5 ≥ 33
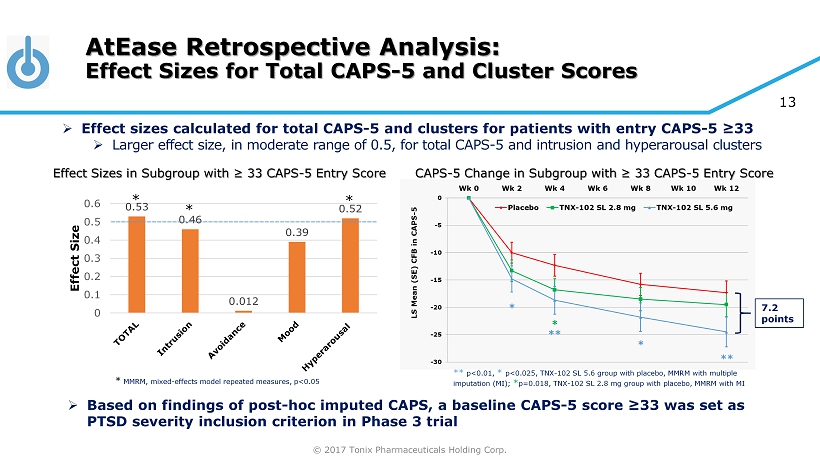
© 2017 Tonix Pharmaceuticals Holding Corp. 13 0.53 0.46 0.012 0.39 0.52 0 0.1 0.2 0.3 0.4 0.5 0.6 Effect Size AtEase Retrospective Analysis: Effect Sizes for Total CAPS - 5 and Cluster Scores * MMRM, mixed - effects model repeated measures, p<0.05 » Effect sizes calculated for total CAPS - 5 and clusters for patients with entry CAPS - 5 ≥33 » Larger effect size, in moderate range of 0.5, for total CAPS - 5 and intrusion and hyperarousal clusters * * * » Based on findings of post - hoc imputed CAPS, a baseline CAPS - 5 score ≥33 was set as PTSD severity inclusion criterion in Phase 3 trial 7.2 points ** p<0.01, * p<0.025, TNX - 102 SL 5.6 group with placebo, MMRM with multiple imputation (MI); * p=0.018, TNX - 102 SL 2.8 mg group with placebo, MMRM with MI Effect Sizes in Subgroup with ≥ 33 CAPS - 5 Entry Score CAPS - 5 Change in Subgroup with ≥ 33 CAPS - 5 Entry Score
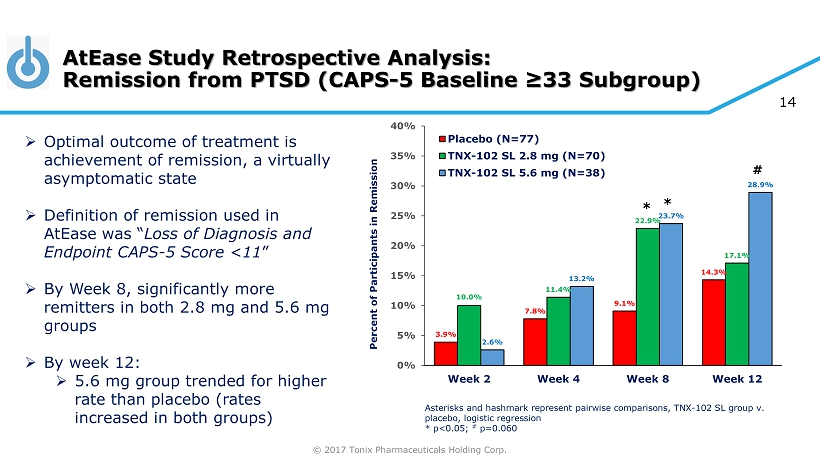
© 2017 Tonix Pharmaceuticals Holding Corp. 14 AtEase Study Retrospective Analysis: Remission from PTSD (CAPS - 5 Baseline ≥33 Subgroup) Asterisks and hashmark represent pairwise comparisons, TNX - 102 SL group v. placebo, logistic regression * p<0.05; # p=0.060 » Optimal outcome of treatment is achievement of remission, a virtually asymptomatic state » Definition of remission used in AtEase was “ Loss of Diagnosis and Endpoint CAPS - 5 Score <11 ” » By Week 8, significantly more remitters in both 2.8 mg and 5.6 mg groups » By week 12: » 5.6 mg group trended for higher rate than placebo (rates increased in both groups) 3.9% 7.8% 9.1% 14.3% 10.0% 11.4% 22.9% 17.1% 2.6% 13.2% 23.7% 28.9% 0% 5% 10% 15% 20% 25% 30% 35% 40% Week 2 Week 4 Week 8 Week 12 Percent of Participants in Remission Placebo (N=77) TNX-102 SL 2.8 mg (N=70) TNX-102 SL 5.6 mg (N=38) # * *
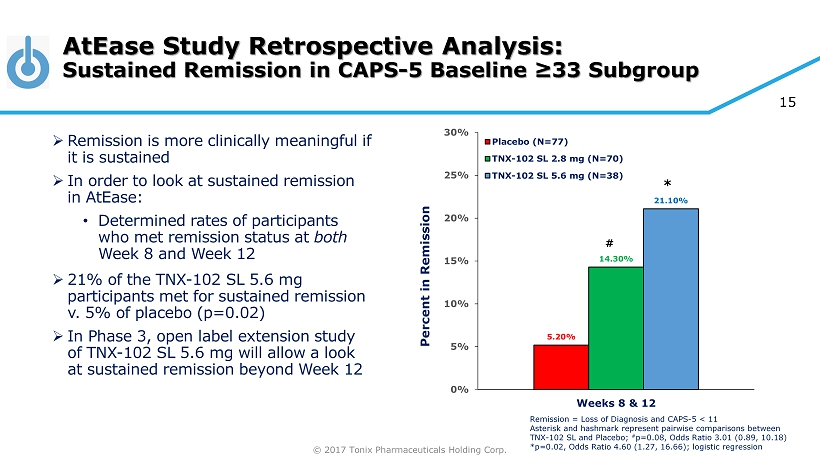
© 2017 Tonix Pharmaceuticals Holding Corp. 15 AtEase Study Retrospective Analysis: Sustained Remission in CAPS - 5 Baseline ≥33 Subgroup 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) TNX-102 SL 2.8 mg (N=70) TNX-102 SL 5.6 mg (N=38) # * Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between TNX - 102 SL and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression » Remission is more clinically meaningful if it is sustained » In order to look at sustained remission in AtEase : • Determined rates of participants who met remission status at both Week 8 and Week 12 » 21% of the TNX - 102 SL 5.6 mg participants met for sustained remission v. 5% of placebo (p=0.02) » In Phase 3, open label extension study of TNX - 102 SL 5.6 mg will allow a look at sustained remission beyond Week 12
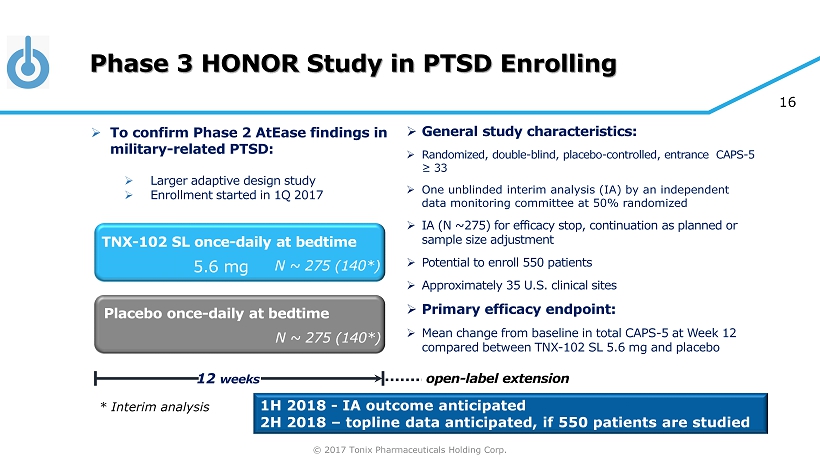
© 2017 Tonix Pharmaceuticals Holding Corp. 16 Phase 3 HONOR Study in PTSD Enrolling » General s tudy c haracteristics: » Randomized, double - blind, placebo - controlled, entrance CAPS - 5 ≥ 33 » One unblinded interim analys i s (IA) by an independent data monitoring committee at 50% randomized » IA (N ~ 275 ) for efficacy stop , continuation as planned or sample size adjustment » Potential to enroll 550 patients » Approximately 3 5 U.S. clinical sites » Primary e fficacy e ndpoint: » Mean change from baseline in total CAPS - 5 at Week 12 compared between TNX - 102 SL 5.6 mg and placebo Placebo once - daily at bedtime 12 weeks TNX - 102 SL once - daily at bedtime N ~ 275 (140*) N ~ 275 (140*) 5.6 mg 1H 2018 - IA outcome anticipated 2H 2018 – topline data anticipated, if 550 patients are studied » To confirm Phase 2 AtEase findings in military - related PTSD: » Larger adaptive design study » Enrollment started in 1Q 2017 * Interim analysis open - label extension
© 2017 Tonix Pharmaceuticals Holding Corp. 17 Conclusions » Phase 2 clinical investigation established that TNX - 102 SL 5.6 mg is the potential efficacious and safe dose to treat PTSD in a military - related PTSD population (TNX - 102 SL 5.6 mg, N=49 v. placebo, N=92) » Established CAPS - 5 ≥33 as entry threshold for Phase 3 studies to confirm AtEase findings » Relationship between early sleep improvement and Week 12 PTSD recovery supports mechanistic hypothesis that improved sleep quality is a mediator of TNX - 102 SL treatment response » TNX - 102 SL 5.6 mg treatment resulted in sustained remission between Weeks 8 and 12 in 21% of participants that was statistically significant relative to placebo and approximately 4X the rate in placebo in the CAPS - 5 ≥33 subgroup (TNX - 102 SL, N=38 v. placebo, N=77) » Phase 3 clinical investigation of TNX - 102 SL 5.6 mg in military - related PTSD is ongoing

© 2017 Tonix Pharmaceuticals Holding Corp. Thank you ! NASDAQ: TNXP