
Exhibit 99.01

© 2018 Tonix Pharmaceuticals Holding Corp. January 2018 Version P0094 1 - 19 - 18 (Doc 0313) Investor Presentation

© 2018 Tonix Pharmaceuticals Holding Corp. 2 Cautionary Note on Forward - Looking Statements Certain statements in this presentation regarding strategic plans, expectations and objectives for future operations or results are “forward - looking statements” as defined by the Private Securities Litigation Reform Act of 1995 . These statements may be identified by the use of forward - looking words such as “anticipate,” “believe,” “forecast,” “estimate” and “intend,” among others . These forward - looking statements are based on Tonix’s current expectations and actual results could differ materially . There are a number of factors that could cause actual events to differ materially from those indicated by such forward - looking statements . These factors include, but are not limited to, substantial competition ; our need for additional financing ; uncertainties of patent protection and litigation ; uncertainties of government or third party payor reimbursement ; limited research and development efforts and dependence upon third parties ; and risks related to failure to obtain U . S . Food and Drug Administration clearances or approvals and noncompliance with its regulations . As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commercialization of new products . The forward - looking statements in this presentation are made as of the date of this presentation, even if subsequently made available by Tonix on its website or otherwise . Tonix does not undertake an obligation to update or revise any forward - looking statement, except as required by law . Investors should read the risk factors set forth in the Annual Report on Form 10 - K for the year ended December 31 , 2016 , as filed with the Securities and Exchange Commission (the “SEC”) on April 13 , 2017 , and periodic reports filed with the SEC on or after the date thereof . All of Tonix's forward - looking statements are expressly qualified by all such risk factors and other cautionary statements .
© 2018 Tonix Pharmaceuticals Holding Corp. 3 Tonix is Developing a Portfolio of Potential Treatments for Important Public Health Challenges and Diseases with Significant Unmet Needs Tonmya ®1 (cyclobenzaprine HCl sublingual tablets) bedtime treatment for posttraumatric stress disorder (PTSD) • Phase 3 HONOR study of Tonmya in military - related PTSD enrolling • Breakthrough Therapy designation by the U.S. Food and Drug Administration (FDA) TNX - 102 SL (cyclobenzaprine HCl sublingual tablets) bedtime treatment for Agitation in Alzheimer’s (AAD) • Pre - IND meeting held Nov 2017 • IND to be submitted 1Q2018 TNX - 601 (tianeptine oxalate) daytime treatment for PTSD • Ongoing preclinical development • Novel salt and polymorph of an active ingredient marketed in Europe for depression with efficacy evidence in PTSD from published literature TNX - 801 (synthesized live horsepox virus) as a vaccine to prevent smallpox • Recent New England Journal of Medicine letter 2 found a 1902 U.S. smallpox vaccine to have a genomic core 99.7% similar to horsepox • Eligible for an FDA Priority Review Voucher (PRV) under the 21 st Century Cures Act 3 1 FDA has conditionally accepted Tonmya as the proposed proprietary name for cyclobenzaprine HCl sublingual tablets, or TNX - 102 SL, for PTSD, which is an investigational new drug and has not been approved for any indication. 2 Schrick , L. et al. , N Engl J Med 2017; 377:1491 - 1492, http://www.nejm.org/doi/full/10.1056/NEJMc1707600 3 PRV’s issued upon licensure if accepted as medical counter - measure.
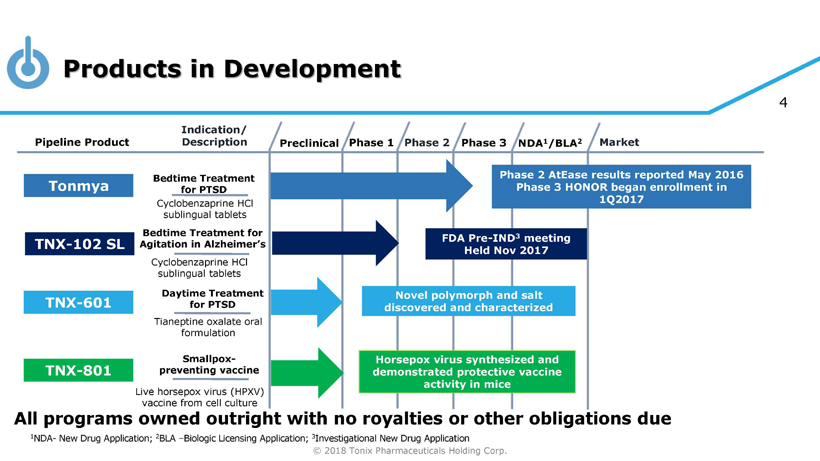
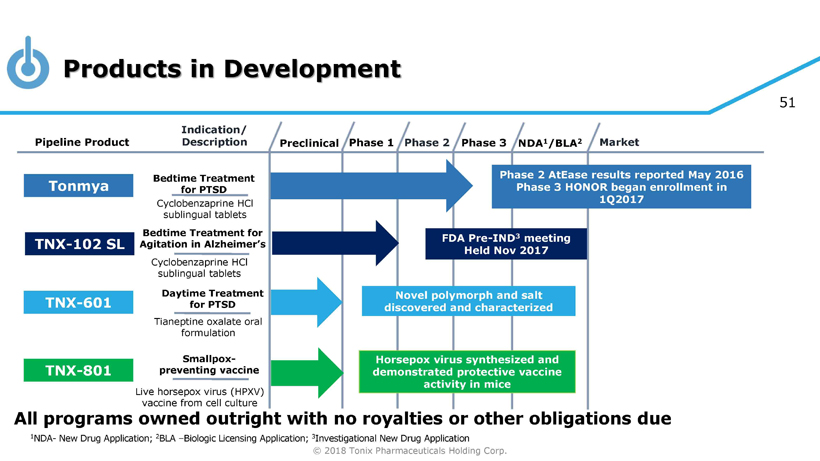
© 2018 Tonix Pharmaceuticals Holding Corp. 4 Products in Development Preclinical Phase 2 NDA 1 /BLA 2 Market Pipeline Product Indication/ Description Phase 3 Tonmya Bedtime Treatment for PTSD Daytime Treatment for PTSD TNX - 601 Novel polymorph and salt discovered and characterized TNX - 801 Horsepox virus synthesized and demonstrated protective vaccine activity in mice Smallpox - preventing vaccine Cyclobenzaprine HCl sublingual tablets Tianeptine oxalate oral formulation Live horsepox virus (HPXV) vaccine from cell culture Phase 1 Phase 2 AtEase results reported May 2016 Phase 3 HONOR began enrollment in 1Q2017 1 NDA - New Drug Application; 2 BLA – Biologic Licensing Application; 3 Investigational New Drug Application Bedtime Treatment for Agitation in Alzheimer’s Cyclobenzaprine HCl sublingual tablets FDA Pre - IND 3 meeting Held Nov 2017 TNX - 102 SL All programs owned outright with no royalties or other obligations due
© 2018 Tonix Pharmaceuticals Holding Corp. 5 Tonmya (Cyclobenzaprine HCl Sublingual Tablets) for PTSD Phase 3 HONOR study of Tonmya in military - related PTSD enrolling • Encouraging evidence of safety and efficacy was demonstrated in Phase 2 Breakthrough Therapy designation from the FDA • Expedited development and accelerated review are expected • Potential to file NDA 1 based on one Phase 3 study if data are statistically persuasive Proposed registration plan agreed to by the FDA • Additional nonclinical safety and clinical abuse potential studies are not required Patent protection through 2034 in U.S. 1 • Composition of matter patent for eutectic, required for transmucosal delivery of cyclobenzaprine Novel mechanism targets sleep quality • Memory processing during sleep is important to recovery 1 U.S. Patent No. 9,636,408 for eutectic proprietary Protectic ™ formulation
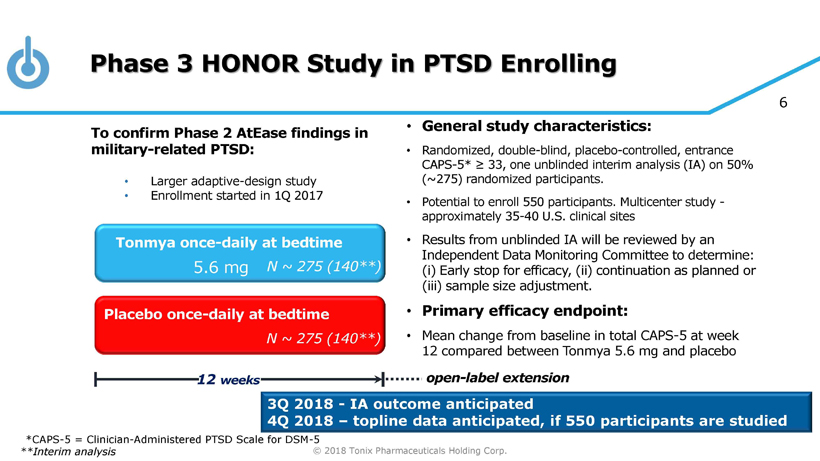
© 2018 Tonix Pharmaceuticals Holding Corp. 6 Phase 3 HONOR Study in PTSD Enrolling • General s tudy c haracteristics: • Randomized, double - blind, placebo - controlled, entrance CAPS - 5 * ≥ 33 , one unblinded interim analysis (IA) on 50% (~275) randomized participants. • P otential to enroll 550 participants. Multicenter study - approximately 35 - 40 U.S. clinical sites • Results from unblinded IA will be reviewed by an Independent Data Monitoring Committee to determine: ( i ) Early stop for efficacy, (ii) continuation as planned or (iii) sample size adjustment. • Primary e fficacy e ndpoint: • Mean change from baseline in total CAPS - 5 at w eek 12 compared between Tonmya 5.6 mg and placebo Placebo once - daily at bedtime 12 weeks Tonmya once - daily at bedtime N ~ 275 (140**) N ~ 275 (140**) 5.6 mg 3Q 2018 - IA outcome anticipated 4Q 2018 – topline data anticipated, if 550 participants are studied To confirm Phase 2 AtEase findings in military - related PTSD: • Larger adaptive - design study • Enrollment started in 1Q 2017 **Interim analysis open - label extension *CAPS - 5 = Clinician - Administered PTSD Scale for DSM - 5

© 2018 Tonix Pharmaceuticals Holding Corp. 7 Breakthrough Therapy Designation FDA granted Tonmya Breakthrough Therapy designation – reported December 19, 2016 • PTSD is a serious condition • Tonmya has potential advantages over existing therapies in military - related PTSD Benefits of Breakthrough Therapy designation • Eligibility for priority review of the NDA within 6 months instead of 10 months • Option to submit completed portions of the NDA for rolling review • An organizational commitment involving FDA's senior managers to accelerate the development and approval process, an opportunity to compress development time NDA filing based on HONOR study is possible if results are statistically persuasive • Discussed at March 9, 2017 Initial Cross - disciplinary Breakthrough Meeting with the FDA

© 2018 Tonix Pharmaceuticals Holding Corp. 8 No Recognized Abuse Potential in Clinical Studies Active ingredient is cyclobenzaprine, which is structurally related to tricyclic antidepressants • Cyclobenzaprine interacts with receptors that regulate sleep quality: 5 - HT 2A ; a 1 - adrenergic and histamine H 1 receptors • Cyclobenzaprine does NOT interact with the same receptors as traditional hypnotic sleep drugs, benzodiazepines or non - benzodiazepines that are associated with retrograde amnesia • Cyclobenzaprine - containing product was approved 40 years ago and current labeling (May 2016) indicates no abuse or dependence concern Tonmya NDA can be filed without abuse assessment studies • Discussed at March 9, 2017 Initial Cross - disciplinary Breakthrough Meeting with the FDA
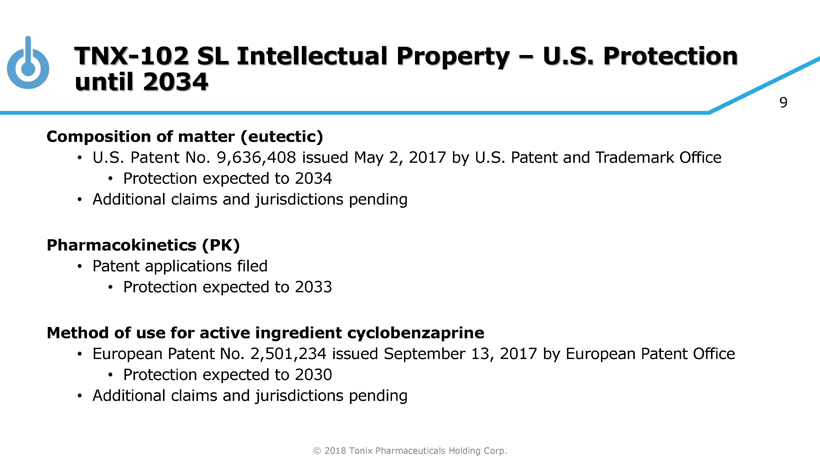
© 2018 Tonix Pharmaceuticals Holding Corp. 9 TNX - 102 SL Intellectual Property – U.S. Protection until 2034 Composition of matter (eutectic) • U.S. Patent No. 9,636,408 issued May 2, 2017 by U.S. Patent and Trademark Office • Protection expected to 2034 • Additional claims and jurisdictions pending Pharmacokinetics (PK) • Patent applications filed • Protection expected to 2033 Method of use for active ingredient cyclobenzaprine • European Patent No. 2,501,234 issued September 13, 2017 by European Patent Office • Protection expected to 2030 • Additional claims and jurisdictions pending
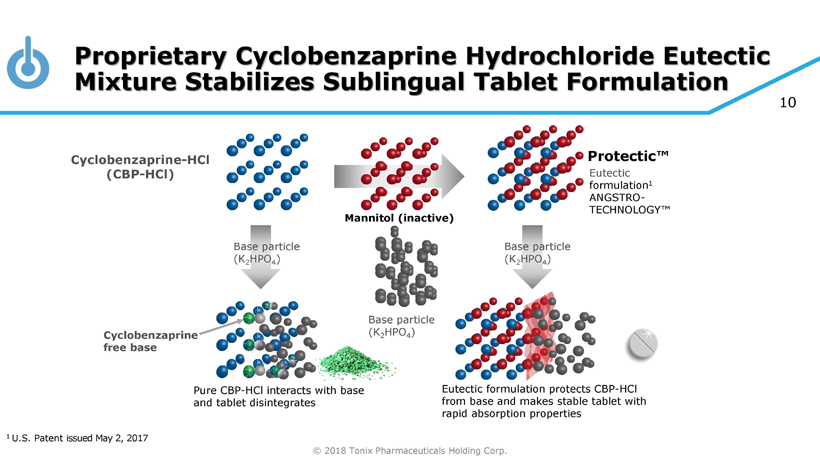
© 2018 Tonix Pharmaceuticals Holding Corp. 10 Proprietary Cyclobenzaprine Hydrochloride Eutectic Mixture Stabilizes Sublingual Tablet Formulation Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Base particle (K 2 HPO 4 ) Eutectic formulation 1 ANGSTRO - TECHNOLOGY™ Cyclobenzaprine - HCl (CBP - HCl) Eutectic formulation protects CBP - HCl from base and makes stable tablet with rapid absorption properties Pure CBP - HCl interacts with base and tablet disintegrates Cyclobenzaprine free base Protectic™ 1 U.S. Patent issued May 2, 2017 Mannitol (inactive)
© 2018 Tonix Pharmaceuticals Holding Corp. 11 TNX - 102 SL: Sublingual Formulation is Designed for Bedtime Administration TNX - 102 SL: Proprietary sublingual formulation of cyclobenzaprine (CBP) with transmucosal absorption • Innovation by design with patent protected CBP/mannitol eutectic • Rapid systemic exposure • Increases bioavailability during sleep • Avoids first - pass metabolism • Lowers exposure to long - lived active major metabolite, norcyclobenzaprine ( norCBP ) CBP undergoes extensive first - pass hepatic metabolism when orally ingested • Active major metabolite, norCBP 1 • Long half - life (~72 hours) • Less selective for target receptors ( 5 - HT 2A, a 1 - adrenergic, histamine H 1 ) • More selective for norepinephrine transporter 1 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015, Toronto Ont ario, Canada
© 2018 Tonix Pharmaceuticals Holding Corp. 12 Tonmya : Novel Mechanism Targets Sleep Quality for Recovery from PTSD PTSD is a disorder of recovery • Most people exposed to extreme trauma recover over a few weeks • In PTSD, recovery process impeded due to insufficient sleep - dependent memory processing Memory processing is essential to recovery • Vulnerability to memory intrusions and trauma triggers remains if no consolidation of new learning (extinction) Tonmya targets sleep quality 1 • Cyclobenzaprine interacts with receptors that regulate sleep quality: strongly binds and potently blocks 5 - HT 2A , a 1 - adrenergic and histamine H 1 receptors, permissive to sleep - dependent recovery processes 1 Daugherty et al., Abstract 728, Society of Biological Psychiatry 70th Annual Scientific Convention, May 14 - 16, 2015, Toronto Ont ario, Canada
© 2018 Tonix Pharmaceuticals Holding Corp. 13 What are the Symptoms of PTSD? Symptoms of PTSD fall into four clusters: 1. Intrusion (aversive memories, nightmares, flashbacks) 2. Avoidance (avoiding persons, places or situations) 3. Mood/cognitions (memory block, emotional numbing, detachment from others) 4. Hyperarousal (anxiety, agitation & sleep disturbance) CAPS - 5 is used to assess symptom severity and treatment effect • Recognized as the standard for rating PTSD severity in clinical trials • Takes into account all four symptom clusters
© 2018 Tonix Pharmaceuticals Holding Corp. 14 What are the Consequences of PTSD? Consequences: • Impaired daily function and substantial interference with work and social interactions • Reckless or destructive behavior • Increased health care utilization and greater medical morbidity PTSD as a risk factor for: • Depression • Alcohol or substance abuse • Absenteeism/unemployment • Homelessness • Violent acts • Suicidal thoughts and suicide
© 2018 Tonix Pharmaceuticals Holding Corp. 15 PTSD: U.S. Prevalence and Index Traumas A chronic response to traumatic event(s) • A majority of people will experience a traumatic event at some point in their lifetime 1 • 20% of women and 8% of men in the U.S. who experience significant trauma develop PTSD 1 • Lifetime prevalence: 6.8% 2 (~ 17.0 million adults in the U.S.) • Persistent - >1/3 fail to recover, even after several years following the trauma 2 • Twelve month prevalence: U.S. 3.5% (~ 8.6 million adults) 3 EU 2.3% (~10.0 million adults) 4 Most common forms of trauma 1 • Witnessing someone being badly injured or killed • Natural disaster • Life - threatening accident • Sexual or physical assault 1 Kessler et al., Arch Gen Psychiatry 1995; 52:1048 2 Kessler et al., Arch Gen Psychiatry 2005; 62:593 3 Kessler et al., Arch Gen Psychiatry 2005 ; 62:617 ; Prevalence rate of 3.5% applied to U.S. Census estimate of 247 million U.S. adult ( > 18) population in 2015 (www.census.gov/quickfacts/table/PST045215/00) 4 The European Union Market Potential for a New PTSD Drug. Prepared for Tonix Pharmaceuticals by Procela Consultants Ltd, Sept emb er 2016
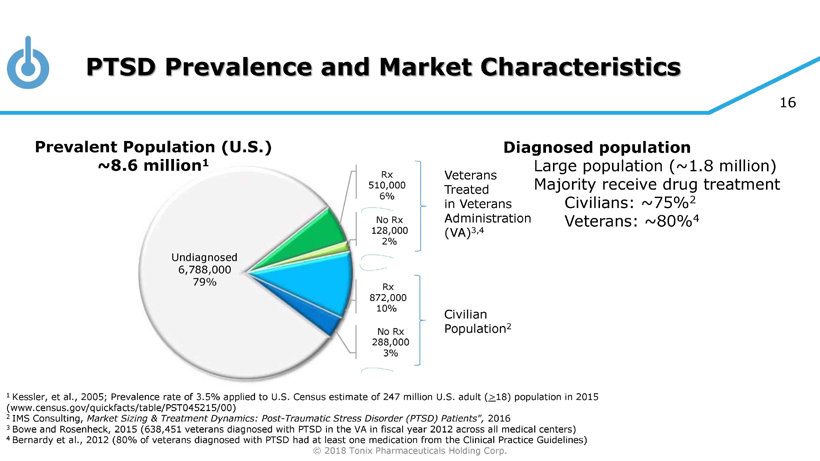
© 2018 Tonix Pharmaceuticals Holding Corp. 16 PTSD Prevalence and Market Characteristics 1 Kessler, et al., 2005; Prevalence rate of 3.5% applied to U.S. Census estimate of 247 million U.S. adult ( > 18) population in 2015 (www.census.gov/quickfacts/table/PST045215/00) 2 IMS Consulting, Market Sizing & Treatment Dynamics: Post - Traumatic Stress Disorder (PTSD) Patients", 2016 3 Bowe and Rosenheck , 2015 (638,451 veterans diagnosed with PTSD in the VA in fiscal year 2012 across all medical centers) 4 Bernardy et al., 2012 (80% of veterans diagnosed with PTSD had at least one medication from the Clinical Practice Guidelines) Veterans Treated in Veterans Administration (VA) 3,4 Civilian Population 2 Diagnosed population Large population (~1.8 million) Majority receive drug treatment Civilians: ~75% 2 Veterans: ~80% 4 Undiagnosed 6,788,000 79% Prevalent Population (U.S.) ~8.6 million 1 Rx 510,000 6% No Rx 128,000 2% Rx 872,000 10% No Rx 288,000 3%
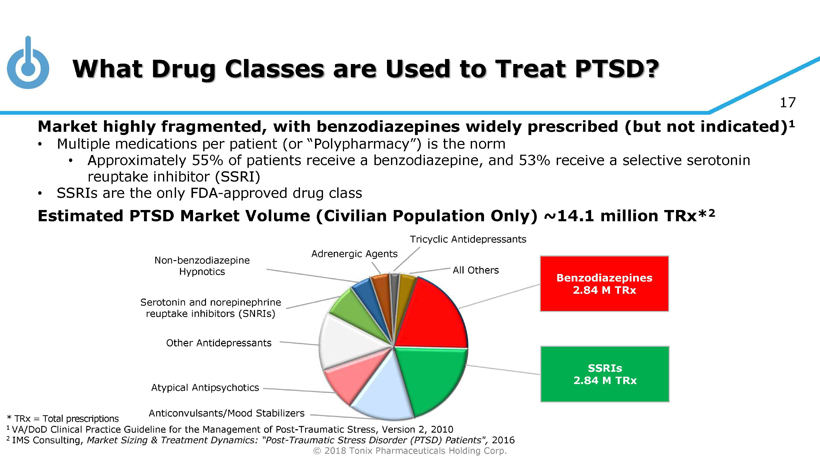
© 2018 Tonix Pharmaceuticals Holding Corp. 17 What Drug Classes are Used to Treat PTSD? * TRx = Total prescriptions 1 VA/DoD Clinical Practice Guideline for the Management of Post - Traumatic Stress, Version 2, 2010 2 IMS Consulting, Market Sizing & Treatment Dynamics: “Post - Traumatic Stress Disorder (PTSD) Patients", 2016 Benzodiazepines 2.84 M TRx SSRIs 2.84 M TRx Other Antidepressants Non - benzodiazepine Hypnotics Serotonin and norepinephrine reuptake inhibitors (SNRIs) Tricyclic Antidepressants Adrenergic Agents All Others Atypical Antipsychotics Anticonvulsants/Mood Stabilizers Market highly fragmented, with benzodiazepines widely prescribed (but not indicated) 1 • Multiple medications per patient (or “Polypharmacy”) is the norm • Approximately 55% of patients receive a benzodiazepine, and 53% receive a selective serotonin reuptake inhibitor (SSRI) • SSRIs are the only FDA - approved drug class Estimated PTSD Market Volume (Civilian Population Only) ~14.1 million TRx * 2

© 2018 Tonix Pharmaceuticals Holding Corp. 18 PTSD: Not Well - Served by Approved Treatments FDA - approved SSRIs, paroxetine and sertraline, have not shown efficacy in military - related PTSD Majority of patients unresponsive or intolerant to current treatments • Side effects relating to sexual dysfunction (particularly in males) and sleep are commonly reported Characteristics of drug therapy that would be compatible and complementary with behavioral therapy • Lack of retrograde amnesia (e.g., unlike off - label use of benzodiazepines and non - benzodiazepines) • Lack of interference on sleep (e.g., unlike approved SSRIs)
© 2018 Tonix Pharmaceuticals Holding Corp. 19 Why Initially Target Military - Related PTSD? 1 Friedman et al., J Clin Psychiatry 2007; 68:711 2 Zoloft Package Insert, August, 2014 3 Paxil Package Insert, June, 2014 4 Fava et al., Psychother Psychosom 84:72 - 81, 2015 Military - related PTSD not well - served by existing FDA - approved therapies • No clear treatment response observed in U.S. military population Sertraline: failed to show efficacy in a large multicenter trial in U.S. military (placebo numerically better) 1 Paroxetine: no large trials conducted with predominantly military trauma • Inconsistent treatment response observed in males Sertraline: FDA - conducted post - hoc analysis concluded no effect for male civilian subgroup 2 Paroxetine: no sex - related difference in treatment outcomes 3 • Important tolerability issues with SSRIs in this population Sexual dysfunction 2,3 Insomnia 2,3 SSRI withdrawal syndrome 4
© 2018 Tonix Pharmaceuticals Holding Corp. 20 High Prevalence of PTSD Among Combat Veterans 1 Kessler et al., Arch Gen Psych 2005; Prevalence rate of 3.5% applied to U.S. Census estimate of 247M U.S. adult ( > 18) population in 2015 ( www.census.gov/quickfacts/table/PST045215/00 ) ; 2 Norris, PTSD Res Quar . 2013; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; Among 1.9M separated OEF/OIF/OND veterans, 1.2M have obtained VA healthcare; 685k evaluated by VA with possible mental disorder, and 379k diagnosed with PTSD. >19% Operation Enduring Freedom (OEF; Afghanistan) / Operation Iraqi Freedom (OIF) veterans / Operation New Dawn (OND) 3 3 - 9% General population 1 19 - 31% Vietnam veterans 2 8.6 million American adults affected 1 Women more likely to develop than men 1 Susceptibility may run in families 1
© 2018 Tonix Pharmaceuticals Holding Corp. 21 Growing Economic and Social Burden to Care for Veterans with PTSD Health care costs associated with PTSD for OEF/OIF/OND veterans: Direct costs Indirect costs 1 CBO Report 2012; 2 Tanielan , Invisible Wounds of War . 2005; 3 Analysis of VA Health Care Utilization among Operation Enduring Freedom, Operation Iraqi Freedom, and Operation New Dawn Veterans, from 1st Qtr FY 2002 through 2nd Qtr FY 2015, Washington, DC ; OEF/OIF/OND, Operations Enduring Freedom, Iraqi Freedom and New Dawn. $3,000 - 5,000 per patient per year for OEF/OIF Veterans 1 ~ 1.9M Veterans out of 2.7M Service members deployed between 10/1/2001 and 3/31/2015 3 $2 - 3 billion estimated yearly cost to society 2 Families, social care agencies, schools, employers, welfare system 2
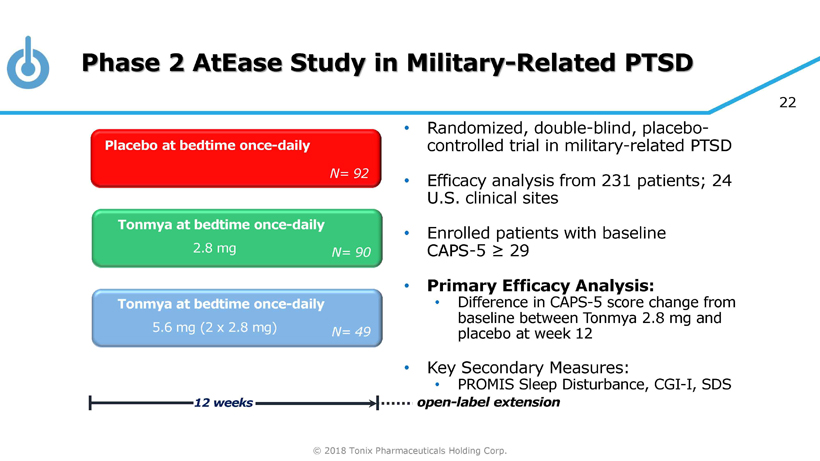
© 2018 Tonix Pharmaceuticals Holding Corp. 22 Phase 2 AtEase Study in Military - Related PTSD • Randomized, double - blind, placebo - controlled trial in military - related PTSD • Efficacy analysis from 231 patients; 24 U.S. clinical sites • Enrolled patients with baseline CAPS - 5 ≥ 29 • Primary Efficacy Analysis: • Difference in CAPS - 5 score change from baseline between Tonmya 2.8 mg and placebo at week 12 • Key Secondary Measures: • PROMIS Sleep Disturbance, CGI - I, SDS Tonmya at bedtime once - daily Placebo at bedtime once - daily 12 weeks N= 90 Tonmya at bedtime once - daily N= 92 N= 49 2.8 mg 5.6 mg (2 x 2.8 mg) open - label extension
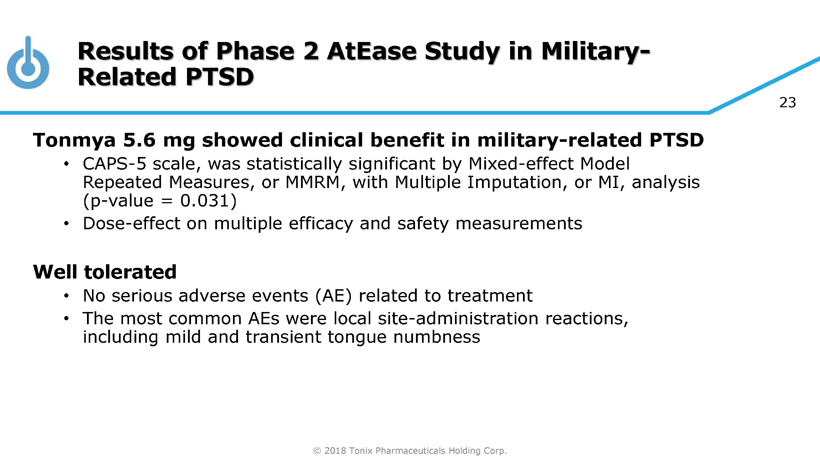
© 2018 Tonix Pharmaceuticals Holding Corp. 23 Results of Phase 2 AtEase Study in Military - Related PTSD Tonmya 5.6 mg showed clinical benefit in military - related PTSD • CAPS - 5 scale, was statistically significant by Mixed - effect Model Repeated Measures, or MMRM, with Multiple Imputation, or MI, analysis (p - value = 0.031) • Dose - effect on multiple efficacy and safety measurements Well tolerated • No serious adverse events (AE) related to treatment • The most common AEs were local site - administration reactions, including mild and transient tongue numbness
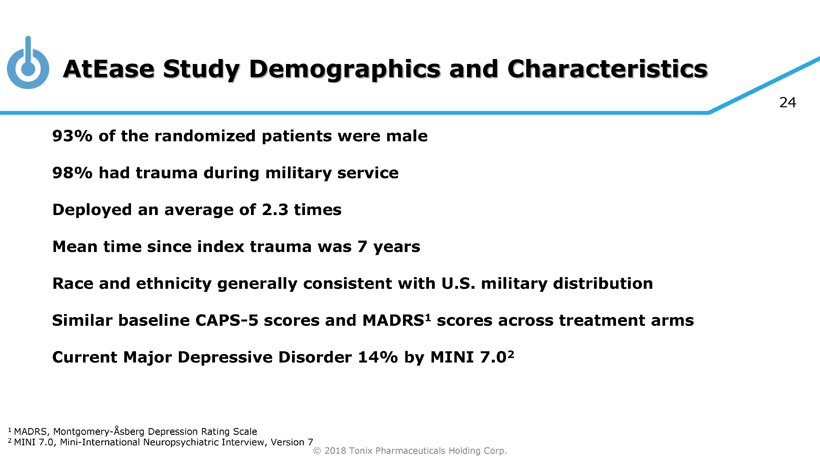
© 2018 Tonix Pharmaceuticals Holding Corp. 24 AtEase Study Demographics and Characteristics 93% of the randomized patients were male 98% had trauma during military service Deployed an average of 2.3 times Mean time since index trauma was 7 years Race and ethnicity generally consistent with U.S. military distribution Similar baseline CAPS - 5 scores and MADRS 1 scores across treatment arms Current Major Depressive Disorder 14% by MINI 7.0 2 1 MADRS, Montgomery - Åsberg Depression Rating Scale 2 MINI 7.0, Mini - International Neuropsychiatric Interview, Version 7
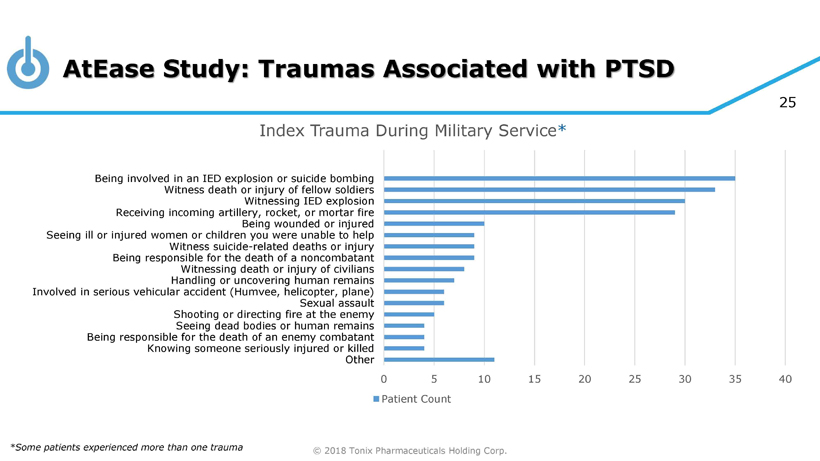
© 2018 Tonix Pharmaceuticals Holding Corp. 25 AtEase Study: Traumas Associated with PTSD *Some patients experienced more than one trauma 0 5 10 15 20 25 30 35 40 Other Knowing someone seriously injured or killed Being responsible for the death of an enemy combatant Seeing dead bodies or human remains Shooting or directing fire at the enemy Sexual assault Involved in serious vehicular accident (Humvee, helicopter, plane) Handling or uncovering human remains Witnessing death or injury of civilians Being responsible for the death of a noncombatant Witness suicide-related deaths or injury Seeing ill or injured women or children you were unable to help Being wounded or injured Receiving incoming artillery, rocket, or mortar fire Witnessing IED explosion Witness death or injury of fellow soldiers Being involved in an IED explosion or suicide bombing Index Trauma During Military Service * Patient Count
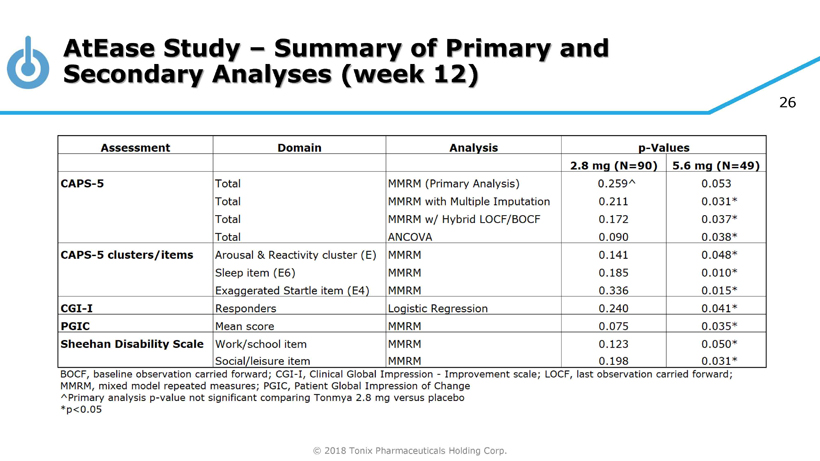
© 2018 Tonix Pharmaceuticals Holding Corp. 26 AtEase Study – Summary of Primary and Secondary Analyses (week 12)
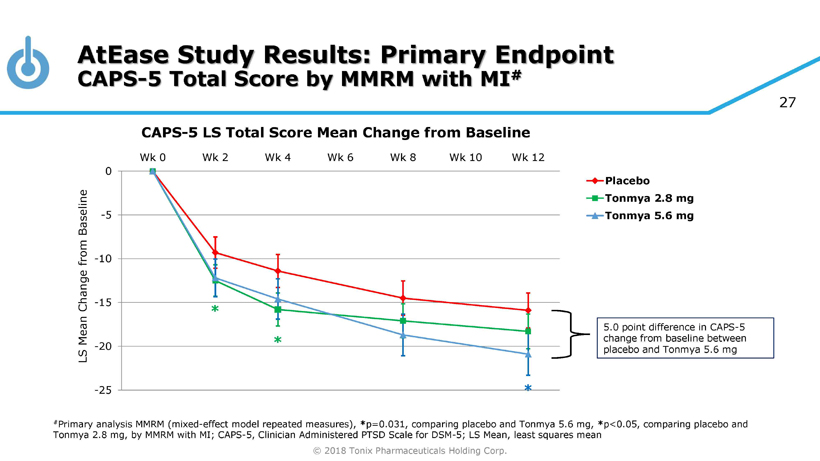
© 2018 Tonix Pharmaceuticals Holding Corp. 27 AtEase Study Results: Primary Endpoint CAPS - 5 Total Score by MMRM with MI # -25 -20 -15 -10 -5 0 Wk 0 Wk 2 Wk 4 Wk 6 Wk 8 Wk 10 Wk 12 Placebo Tonmya 2.8 mg Tonmya 5.6 mg * # Primary analysis MMRM (mixed - effect model repeated measures), * p=0.031, comparing placebo and Tonmya 5.6 mg, * p<0.05, comparing placebo and Tonmya 2.8 mg, by MMRM with MI; CAPS - 5, Clinician Administered PTSD Scale for DSM - 5; LS Mean, least squares mean * * CAPS - 5 LS Total Score Mean Change from Baseline 5.0 point difference in CAPS - 5 change from baseline between placebo and Tonmya 5.6 mg LS Mean Change from Baseline
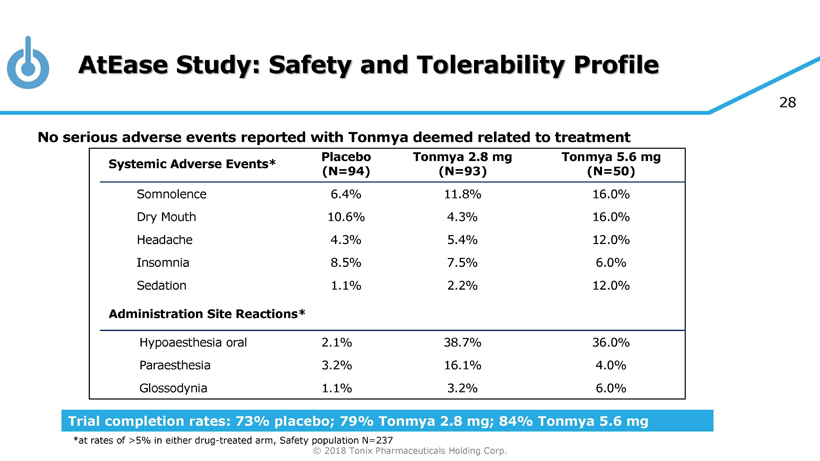
© 2018 Tonix Pharmaceuticals Holding Corp. 28 AtEase Study: Safety and Tolerability Profile No serious adverse events reported with Tonmya deemed related to treatment Systemic Adverse Events* Placebo (N=94) Tonmya 2.8 mg (N=93) Tonmya 5.6 mg (N=50) Somnolence 6.4% 11.8% 16.0% Dry Mouth 10.6% 4.3% 16.0% Headache 4.3% 5.4% 12.0% Insomnia 8.5% 7.5% 6.0% Sedation 1.1% 2.2% 12.0% Administration Site Reactions* Hypoaesthesia oral 2.1% 38.7% 36.0% Paraesthesia 3.2% 16.1% 4.0% Glossodynia 1.1% 3.2% 6.0% Trial completion rates: 73% placebo; 79% Tonmya 2.8 mg; 84% Tonmya 5.6 mg *at rates of >5% in either drug - treated arm, Safety population N=237
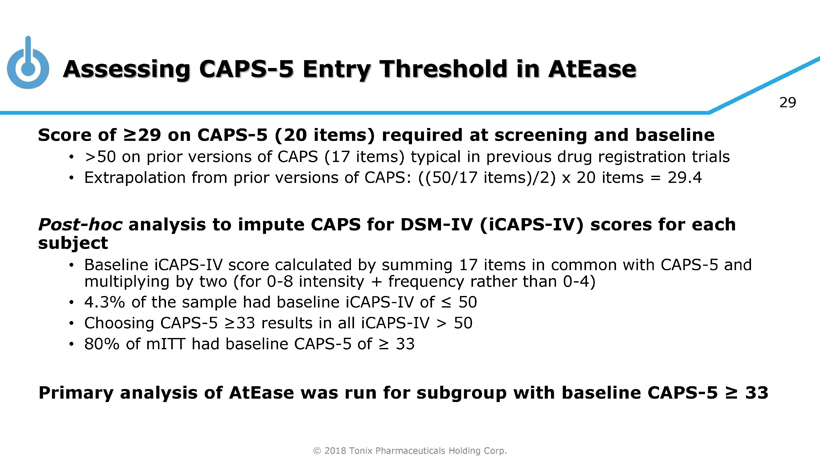
© 2018 Tonix Pharmaceuticals Holding Corp. 29 Assessing CAPS - 5 Entry Threshold in AtEase Score of ≥29 on CAPS - 5 (20 items) required at screening and baseline • >50 on prior versions of CAPS (17 items) typical in previous drug registration trials • Extrapolation from prior versions of CAPS: ((50/17 items)/2) x 20 items = 29.4 Post - hoc analysis to impute CAPS for DSM - IV ( iCAPS - IV) scores for each subject • Baseline iCAPS - IV score calculated by summing 17 items in common with CAPS - 5 and multiplying by two (for 0 - 8 intensity + frequency rather than 0 - 4) • 4.3% of the sample had baseline iCAPS - IV of ≤ 50 • Choosing CAPS - 5 ≥33 results in all iCAPS - IV > 50 • 80% of mITT had baseline CAPS - 5 of ≥ 33 Primary analysis of AtEase was run for subgroup with baseline CAPS - 5 ≥ 33
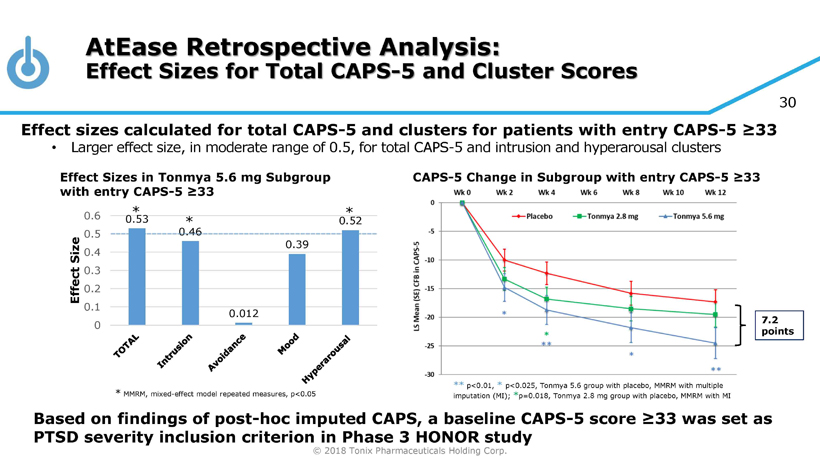
© 2018 Tonix Pharmaceuticals Holding Corp. 30 0.53 0.46 0.012 0.39 0.52 0 0.1 0.2 0.3 0.4 0.5 0.6 Effect Size AtEase Retrospective Analysis: Effect Sizes for Total CAPS - 5 and Cluster Scores * MMRM, mixed - effect model repeated measures, p<0.05 Effect sizes calculated for total CAPS - 5 and clusters for patients with entry CAPS - 5 ≥33 • Larger effect size, in moderate range of 0.5, for total CAPS - 5 and intrusion and hyperarousal clusters * * * Based on findings of post - hoc imputed CAPS, a baseline CAPS - 5 score ≥33 was set as PTSD severity inclusion criterion in Phase 3 HONOR study 7.2 points ** p<0.01, * p<0.025, Tonmya 5.6 group with placebo, MMRM with multiple imputation (MI); * p=0.018, Tonmya 2.8 mg group with placebo, MMRM with MI Effect Sizes in Tonmya 5.6 mg Subgroup with entry CAPS - 5 ≥33 CAPS - 5 Change in Subgroup with entry CAPS - 5 ≥33
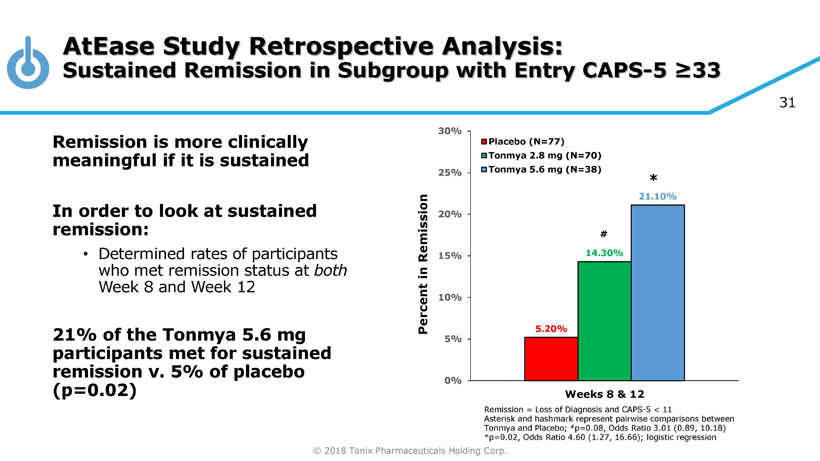
© 2018 Tonix Pharmaceuticals Holding Corp. 31 AtEase Study Retrospective Analysis: Sustained Remission in Subgroup with Entry CAPS - 5 ≥33 Remission = Loss of Diagnosis and CAPS - 5 < 11 Asterisk and hashmark represent pairwise comparisons between Tonmya and Placebo; # p=0.08, Odds Ratio 3.01 (0.89, 10.18) *p=0.02, Odds Ratio 4.60 (1.27, 16.66); logistic regression Remission is more clinically meaningful if it is sustained In order to look at sustained remission: • Determined rates of participants who met remission status at both Week 8 and Week 12 21% of the Tonmya 5.6 mg participants met for sustained remission v. 5% of placebo (p=0.02) 5.20% 14.30% 21.10% 0% 5% 10% 15% 20% 25% 30% Weeks 8 & 12 Percent in Remission Placebo (N=77) Tonmya 2.8 mg (N=70) Tonmya 5.6 mg (N=38) # *
© 2018 Tonix Pharmaceuticals Holding Corp. 32 TNX - 102 SL – Multi - Functional Mechanism Involves Antagonism at 3 Neuronal Receptors Active ingredient is cyclobenzaprine, interacts with 3 receptors • Antagonist at 5 - HT 2A receptors • Similar activity to trazodone and Nuplazid ® ( pimivanserin ) • Antagonist at a 1 - adrenergic receptor • Similar activity to prazosin • Antagonist at histamine H 1 receptors • Similar activity to Benadryl ® (diphenhydramine) and hydroxyzine Multi - functional activity suggests potential for other indications • TNX - 102 SL was developed for the management of fibromyalgia (Phase 3) • Sleep quality is a problem in other conditions
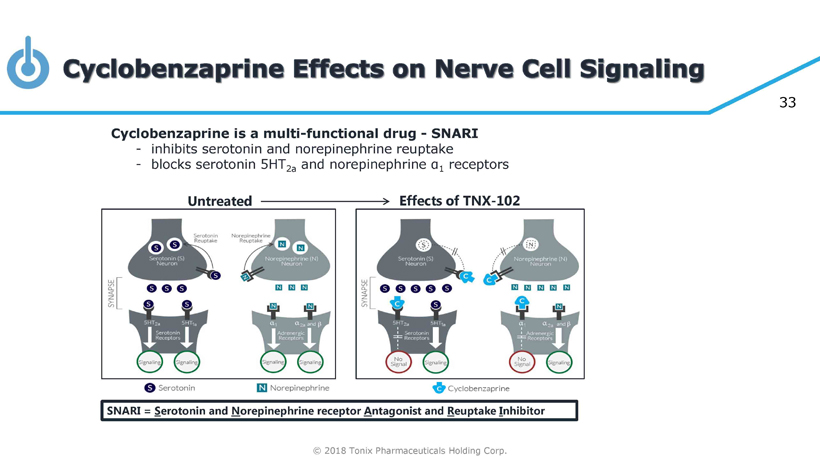
© 2018 Tonix Pharmaceuticals Holding Corp. 33 Untreated Effects of TNX - 102 Cyclobenzaprine is a multi - functional drug - SNARI - inhibits serotonin and norepinephrine reuptake - blocks serotonin 5HT 2a and norepinephrine α 1 receptors SNARI = S erotonin and N orepinephrine receptor A ntagonist and R euptake I nhibitor
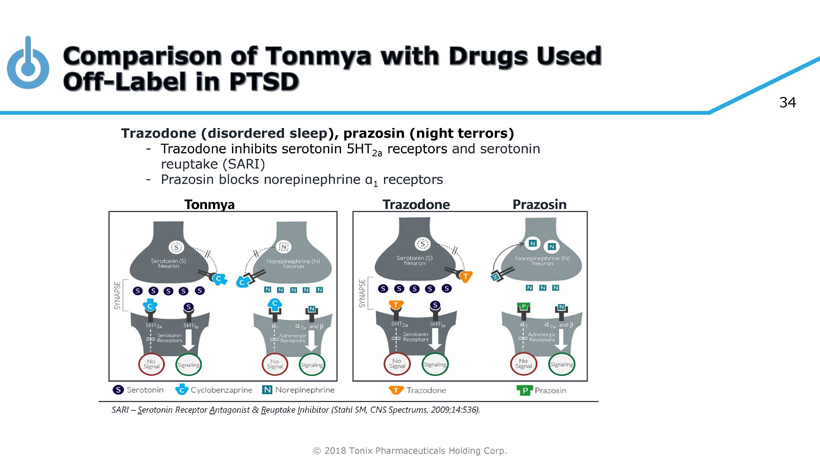
© 2018 Tonix Pharmaceuticals Holding Corp. 34 Tonmya Trazodone Trazodone (disordered sleep ), prazosin (night terrors) - Trazodone inhibits serotonin 5HT 2a receptors and serotonin reuptake (SARI) - Prazosin blocks norepinephrine α 1 receptors Prazosin SARI – S erotonin Receptor A ntagonist & R euptake I nhibitor (Stahl SM, CNS Spectrums, 2009;14:536).
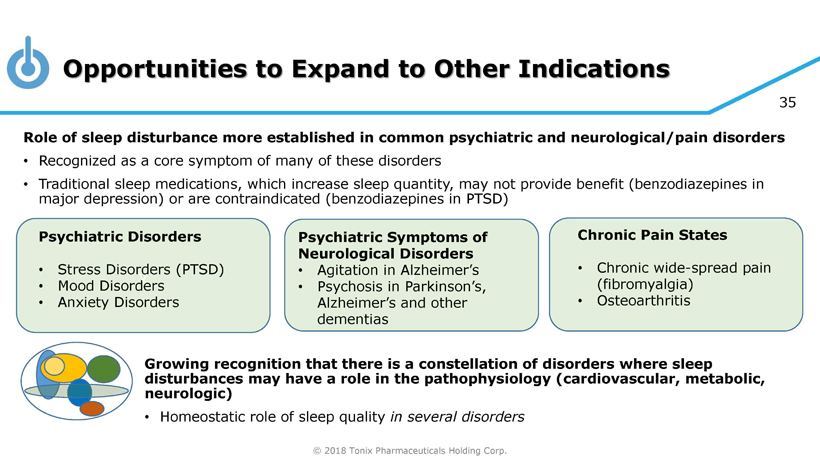
© 2018 Tonix Pharmaceuticals Holding Corp. 35 v Opportunities to Expand to Other Indications Growing recognition that there is a constellation of disorders where sleep disturbances may have a role in the pathophysiology (cardiovascular, metabolic, neurologic) • Homeostatic role of sleep quality in several disorders Psychiatric Disorders • Stress Disorders (PTSD) • Mood Disorders • Anxiety Disorders Chronic Pain States • Chronic wide - spread pain (fibromyalgia) • Osteoarthritis Role of sleep disturbance more established in common psychiatric and neurological/pain disorders • Recognized as a core symptom of many of these disorders • Traditional sleep medications, which increase sleep quantity, may not provide benefit (benzodiazepines in major depression) or are contraindicated (benzodiazepines in PTSD) v Psychiatric Symptoms of Neurological Disorders • Agitation in Alzheimer’s • Psychosis in Parkinson’s, Alzheimer’s and other dementias
© 2018 Tonix Pharmaceuticals Holding Corp. 36 TNX - 102 SL – Multiple Potential Indications Management of Fibromyalgia (FM) – chronic pain condition • TNX - 102 SL clinical development in FM was halted after near miss in Phase 3 at low dose (2.8 mg) – half the dose being developed for PTSD • Imbalance in “withdrawal of consent” led to statistical miss on responder analysis – a few TNX - 102 SL treated patients “moved out of state” • Average pain improvement (secondary endpoint) after 12 weeks of treatment showed statistical significance (P< 0.05) • TNX - 102 SL showed an improvement in sleep quality in Phase 2 and Phase 3 FM trials Agitation in Alzheimer’s Disease • FDA pre - IND meeting held November 2017 • FDA agrees Tonix has sufficient data to file an IND for a Phase 2/potential pivotal efficacy study

© 2018 Tonix Pharmaceuticals Holding Corp. 37 What is Agitation in Alzheimer’s Disease? Agitation is one of the most distressing and debilitating of the behavioral complications of Alzheimer’s disease • Includes emotional lability, restlessness, irritability and aggression 1 Link between disturbed sleep and agitation in Alzheimer’s 1 - 3 • Agitation is commonly diurnal (“ sundowning ”) Prevalence • Agitation is likely to affect more than half of the 5.3 million Americans who currently suffer from moderate to severe Alzheimer’s disease, and this number is expected to nearly triple by 2050 4 1 Rose, K.et al. (2015). American Journal of Alzheimer's Disease & Other Dementias , 30 :78 2 Shih, Y. H., et al. (2017). Journal of the American Medical Directors Association , 18 , 396. 3 Canevelli, M., et al. (2016). Frontiers in medicine , 3 . 4 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/

© 2018 Tonix Pharmaceuticals Holding Corp. 38 Consequences of Agitation in Alzheimer’s Disease Outcomes • Agitation is associated with significant poor outcomes for Alzheimer’s patients and challenges for their caregivers Common reason for institutionalization • Development of agitation, or its worsening, is one of the most common reasons for patients having to transition from lower - to higher levels of care (nursing homes and other long - term care settings) 1 Cost • The presence of agitation nearly doubles the cost of caring for patients with Alzheimer’s disease, and agitation is estimated to account for more than 12% of the healthcare and societal cost of Alzheimer’s disease, which is currently estimated to be $256 Billion for the year 2017 in the United States 1 1 The Alzheimer’s Association, 2017 Alzheimer’s Disease Facts and Figures: https://www.alz.org/facts/
© 2018 Tonix Pharmaceuticals Holding Corp. 39 Agitation in Alzheimer’s Disease – Potential New Indication for TNX - 102 SL Successful pre - IND meeting in November, 2017 • IND planned 1Q2018 to support a Phase 2 efficacy study Significant unmet need • No FDA approved drugs for the treatment of agitation in Alzheimer’s Mechanism of improving sleep quality • Sleep disturbance is a significant and common symptoms in Alzheimer’s Pharmacological advantages outweigh potential concerns of using TNX - 102 SL in treating agitation in Alzheimer’s disease • Blocks 3 receptors, not just one (e.g., 5 - HT 2A ) • Anti - muscarinic (M1) effect in patients on anticholinergics (e.g., donepezil and rivastigmine) possibly reduced with lower sublingual dose

© 2018 Tonix Pharmaceuticals Holding Corp. 40 TNX - 102 SL for Agitation in Alzheimer’s – Regulatory Status and Registration Strategy FDA confirmed no additional study is needed prior to IND submission • Pre - IND meeting established open dialogue with the FDA on pivotal clinical study design and efficacy endpoints to support product registration Planned IND submission in 1Q2018 • Proposed Phase 2 IND study can potentially serve as a pivotal efficacy study Approval of TNX - 102 SL in agitation in Alzheimer’s disease • Efficacy Supplement (sNDA 1 ) may be leveraged from the PTSD development program and supported by Initial NDA approval for PTSD 1 Supplemental New Drug Application
© 2018 Tonix Pharmaceuticals Holding Corp. 41 Scientific Rationale for Developing TNX - 102 SL for Agitation in Alzheimer’s Disease Connection between Sleep Disturbance and Agitation • Agitation in Alzheimer’s Disease is associated with sleep disturbance 1,2 Supported by Potential Mechanism of Action • TNX - 102 is a multifunctional agent including antagonism of 5 - HT 2A , a 1 adrenergic and histamine H 1 receptors • Certain 5 - HT 2A antagonists have shown clinical efficacy against agitation in dementia including trazodone 3,4 , and mirtazapine 5 • The a 1 adrenergic antagonist prazosin has shown efficacy in the treatment of agitation in dementia 5 • The H 1 antagonist hydroxyzine had historical use in treating agitation in dementia 1 Bachmen, D. and Rabins , P. Annu Rev Med. 2006;57:499. 2 Rose, K et al. Am J Alzheimers Dis Other Demen . 2015 30(1):78. 3 Lebert F. et al. Dement Geriatr Cogn Disord . 2004:17(4):355. 4 Sulzer DL et al. Am J Geriatr Psychiatry. 1997 5(1):60. 5 Cakir S. et el., Neuropsychiatr Dis Treat. 2008 4(5):963. 6 Wang, LY et al., Am J Geriatr Psychiatry. 2009 17(9):744
© 2018 Tonix Pharmaceuticals Holding Corp. 42 TNX - 102 SL Potentially Addresses Some of the Challenges in Treating Agitation in Alzheimer’s Sublingual route of administration (no swallowing) • Swallowing can be an issue for a significant number of Alzheimer’s patients Low dose taken daily at bedtime • Potentially minimize daytime anticholinergic side effects → improved tolerability Role of sleep in clearing debris from the brain • Animal studies have shown debris clearance from the brain during sleep including toxic proteins associated with Alzheimer’s progression 1 1 T Xie L, et al. Science. (2013);342(6156):373
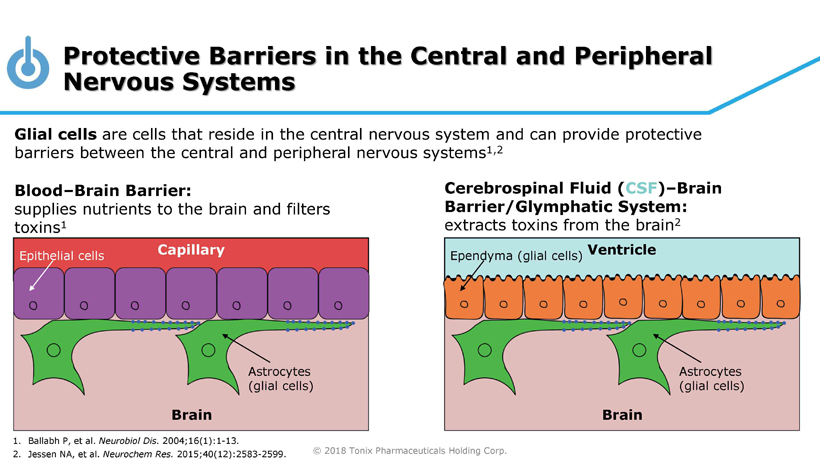
© 2018 Tonix Pharmaceuticals Holding Corp. Protective Barriers in the Central and Peripheral Nervous Systems Glial cells are cells that reside in the central nervous system and can provide protective barriers between the central and peripheral nervous systems 1,2 Blood – Brain Barrier: supplies nutrients to the brain and filters toxins 1 Cerebrospinal Fluid ( CSF ) – Brain Barrier/Glymphatic System: extracts toxins from the brain 2 Ventricle Brain Ependyma (glial cells) Astrocytes (glial cells) Capillary Brain Epithelial cells Astrocytes (glial cells) 1. Ballabh P, et al. Neurobiol Dis. 2004;16(1):1 - 13. 2. Jessen NA, et al. Neurochem Res. 2015;40(12):2583 - 2599.
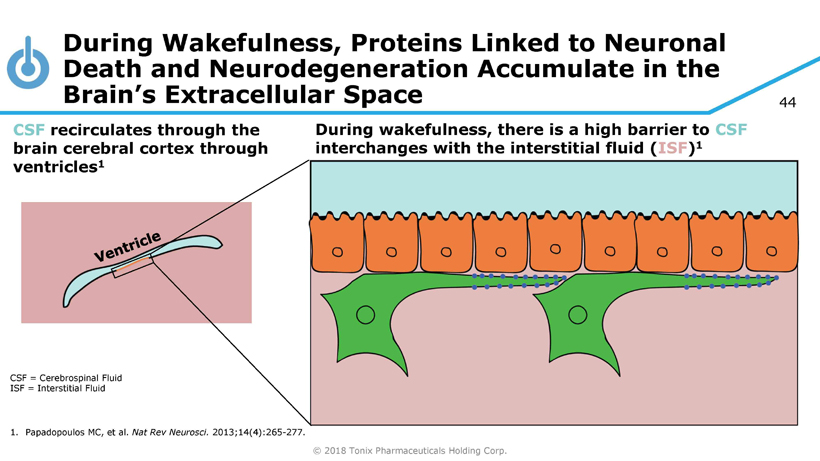
© 2018 Tonix Pharmaceuticals Holding Corp. 44 During Wakefulness, Proteins Linked to Neuronal Death and Neurodegeneration Accumulate in the Brain’s Extracellular Space CSF = Cerebrospinal Fluid ISF = Interstitial Fluid 1. Papadopoulos MC, et al. Nat Rev Neurosci . 2013;14(4):265 - 277. During wakefulness, there is a high barrier to CSF interchanges with the interstitial fluid ( ISF ) 1 CSF recirculates through the brain cerebral cortex through ventricles 1
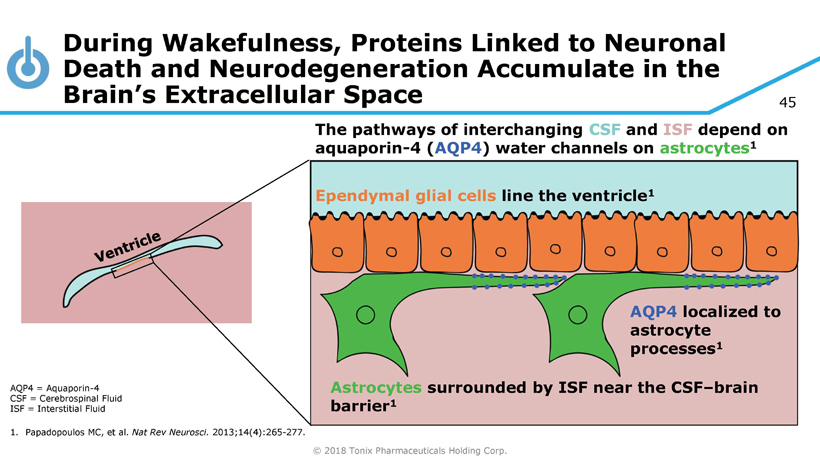
© 2018 Tonix Pharmaceuticals Holding Corp. 45 During Wakefulness, Proteins Linked to Neuronal Death and Neurodegeneration Accumulate in the Brain’s Extracellular Space AQP4 = A quaporin - 4 CSF = Cerebrospinal Fluid ISF = Interstitial Fluid 1. Papadopoulos MC, et al. Nat Rev Neurosci . 2013;14(4):265 - 277. Ependymal glial cells line the ventricle 1 AQP4 localized to astrocyte processes 1 Astrocytes surrounded by ISF near the CSF – brain barrier 1 The pathways of interchanging CSF and ISF depend on aquaporin - 4 ( AQP4 ) water channels on astrocytes 1
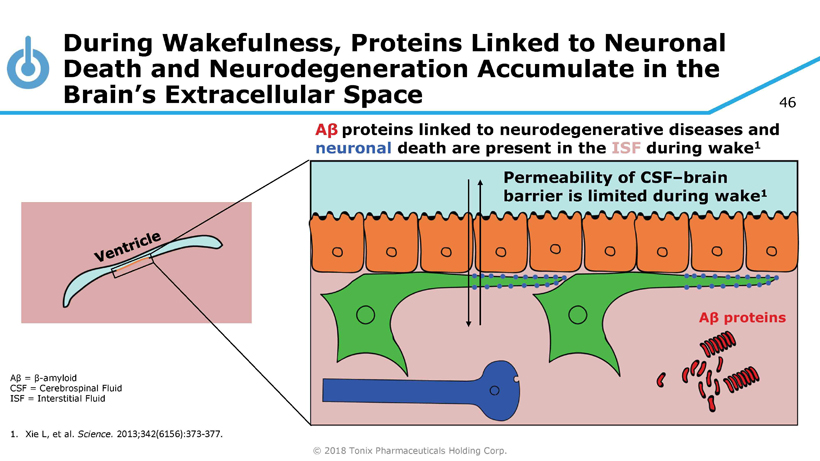
© 2018 Tonix Pharmaceuticals Holding Corp. 46 During Wakefulness, Proteins Linked to Neuronal Death and Neurodegeneration Accumulate in the Brain’s Extracellular Space Aβ = β - amyloid CSF = Cerebrospinal Fluid ISF = Interstitial Fluid 1. Xie L, et al. Science. 2013;342(6156):373 - 377. Permeability of CSF – brain barrier is limited during wake 1 A β proteins linked to neurodegenerative diseases and neuronal death are present in the ISF during wake 1
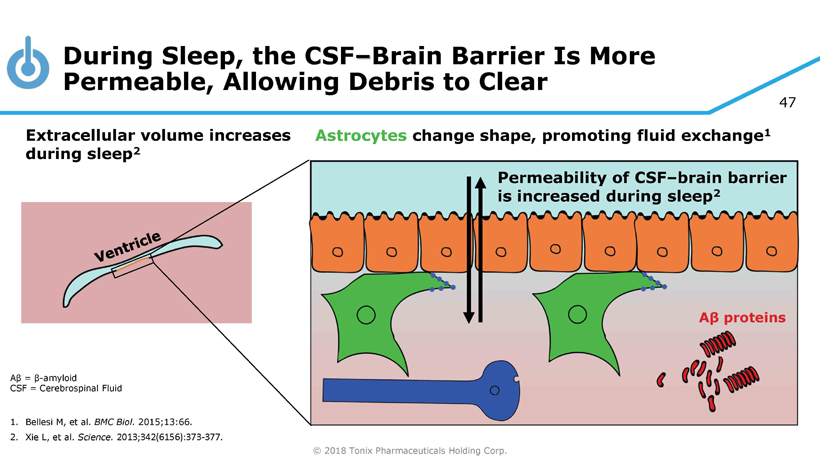
© 2018 Tonix Pharmaceuticals Holding Corp. 47 During Sleep, the CSF – Brain Barrier Is More Permeable, Allowing Debris to Clear Aβ = β - amyloid CSF = Cerebrospinal Fluid 1. Bellesi M, et al. BMC Biol. 2015;13:66. 2. Xie L, et al. Science. 2013;342(6156):373 - 377. Permeability of CSF – brain barrier is increased during sleep 2 Astrocytes change shape, promoting fluid exchange 1 Extracellular volume increases during sleep 2
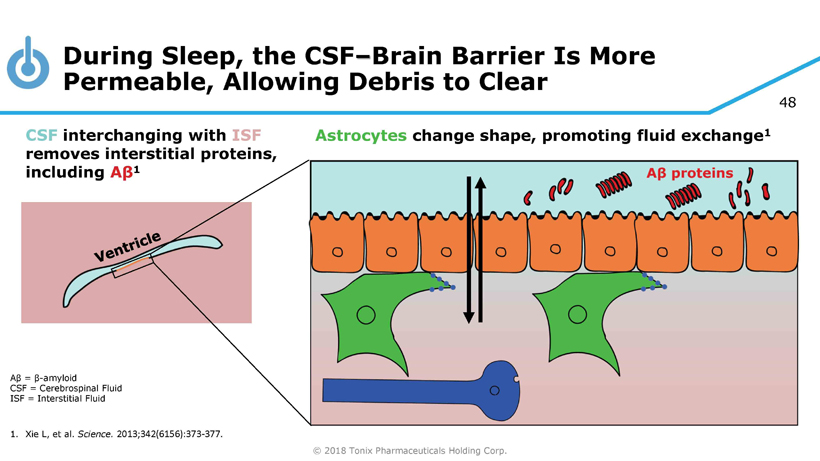
© 2018 Tonix Pharmaceuticals Holding Corp. 48 Aβ = β - amyloid CSF = Cerebrospinal Fluid ISF = Interstitial Fluid 1. Xie L, et al. Science. 2013;342(6156):373 - 377. During Sleep, the CSF – Brain Barrier Is More Permeable, Allowing Debris to Clear CSF interchanging with ISF removes interstitial proteins, including A β 1 Astrocytes change shape, promoting fluid exchange 1
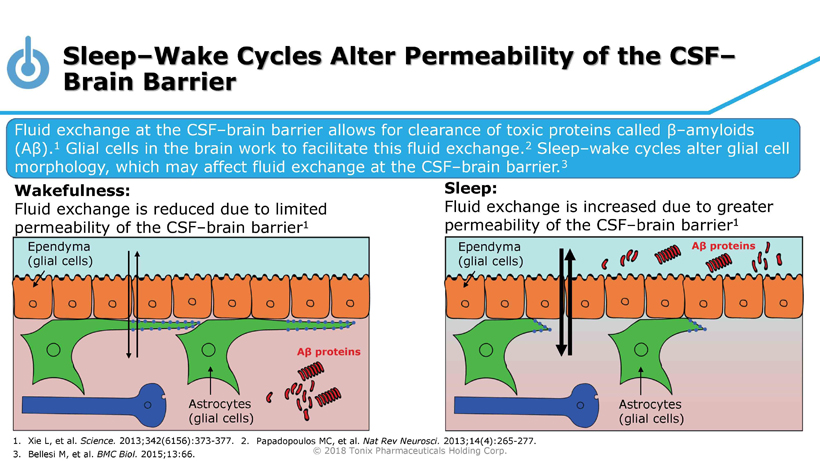
© 2018 Tonix Pharmaceuticals Holding Corp. Sleep – Wake Cycles Alter Permeability of the CSF – Brain Barrier Fluid exchange at the CSF – brain barrier allows for clearance of toxic proteins called β – amyloids (A β ). 1 Glial cells in the brain work to facilitate this fluid exchange. 2 Sleep – wake cycles alter glial cell morphology, which may affect fluid exchange at the CSF – brain barrier. 3 Wakefulness: Fluid exchange is reduced due to limited permeability of the CSF – brain barrier 1 Sleep: Fluid exchange is increased due to greater permeability of the CSF – brain barrier 1 1. Xie L, et al. Science. 2013;342(6156):373 - 377. 3. Bellesi M, et al. BMC Biol. 2015;13:66. 2. Papadopoulos MC, et al. Nat Rev Neurosci. 2013;14(4):265 - 277. Ependyma (glial cells) Astrocytes (glial cells) Ependyma (glial cells) Astrocytes (glial cells)

© 2018 Tonix Pharmaceuticals Holding Corp. 50 Agitation in Alzheimer’s – Competitive Landscape of Select Drugs in Development Competitive landscape • 5HT2A Antagonists/inverse agonists • Nelotanserin ( Axovant ) • Atypical Antip sychotics (also have 5HT2A antagonism) • Rexulti ® brexpiprazole (Otsuka/ Lundbeck ) • Lumateperone ( InterCellular ) • Dextromethorphans – believed to act as SSRI, glutamate/NMDA and sigma - 1 receptor modulators • Deudextromethorphan ( Avenir /Otsuka) - deuterated version of Nuedexta ® • Dextromethorphan/ buproprion ( Axesome ) TNX - 102 SL uniquely designed for bedtime dosing and transmucosal absorption • Maximize drug exposure during sleep → improving sleep quality • Other 5 - HT 2A antagonists not designed for bedtime sublingual dosing
© 2018 Tonix Pharmaceuticals Holding Corp. 51 Products in Development Preclinical Phase 2 NDA 1 /BLA 2 Market Pipeline Product Indication/ Description Phase 3 Tonmya Bedtime Treatment for PTSD Daytime Treatment for PTSD TNX - 601 Novel polymorph and salt discovered and characterized TNX - 801 Horsepox virus synthesized and demonstrated protective vaccine activity in mice Smallpox - preventing vaccine Cyclobenzaprine HCl sublingual tablets Tianeptine oxalate oral formulation Live horsepox virus (HPXV) vaccine from cell culture Phase 1 Phase 2 AtEase results reported May 2016 Phase 3 HONOR began enrollment in 1Q2017 1 NDA - New Drug Application; 2 BLA – Biologic Licensing Application; 3 Investigational New Drug Application Bedtime Treatment for Agitation in Alzheimer’s Cyclobenzaprine HCl sublingual tablets FDA Pre - IND 3 meeting Held Nov 2017 TNX - 102 SL All programs owned outright with no royalties or other obligations due
© 2018 Tonix Pharmaceuticals Holding Corp. 52 TNX - 601 ( Tianeptine Oxalate): A Potential Clinical Candidate for PTSD Pre - IND Candidate • Targeted as a 1 st line monotherapy for PTSD : oral formulation for daytime dosing x Leverages expertise in PTSD (clinical and regulatory experience, market analysis, etc.) x Mechanism of Action (MOA) is different from Tonmya • Tianeptine sodium (amorphous) has been approved in EU, Russia, Asia and Latin America for depression since 1987 with established post - marketing experience • Identified new oxalate salt polymorph with improved pharmaceutical properties ideal for reformulation • Filed patent application on novel salt polymorph • Issued patent on steroid - induced cognitive impairment and memory loss issues Targeting a Public Health Challenge • Clinical evidence for PTSD • Several studies have shown tianeptine to be active in the treatment of PTSD 1 - 4 1 Frančišković T, et al. Psychiatr Danub . 2011 Sep;23(3):257 - 63. PMID: 21963693 2 Rumyantseva GM and, Stepanov AL. Neurosci Behav Physiol. 2008 Jan;38(1):55 - 61. PMID: 18097761 3 Aleksandrovskiĭ IA, et al. Zh Nevrol Psikhiatr Im S S Korsakova . 2005;105(11):24 - 9. PMID: 16329631 [Russian] 4 Onder E, et al. Eur Psychiatry. 2006 (3):174 - 9. PMID: 15964747
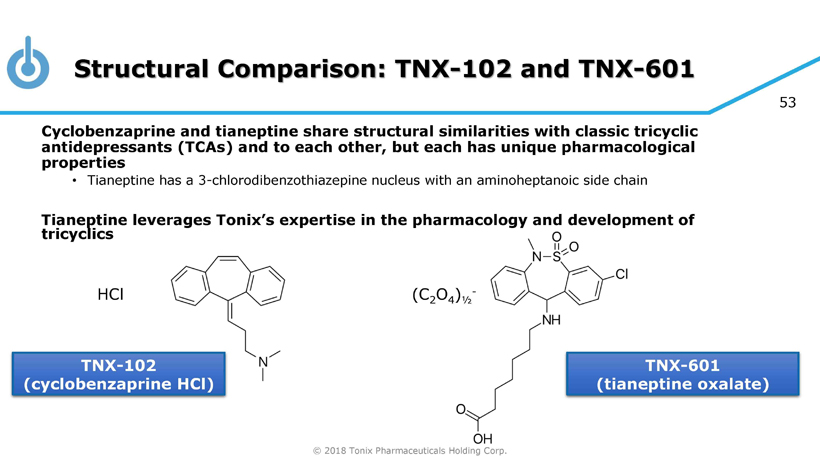
© 2018 Tonix Pharmaceuticals Holding Corp. 53 Structural Comparison: TNX - 102 and TNX - 601 Cyclobenzaprine and tianeptine share structural similarities with classic tricyclic antidepressants (TCAs) and to each other, but each has unique pharmacological properties • Tianeptine has a 3 - chlorodibenzothiazepine nucleus with an aminoheptanoic side chain Tianeptine leverages Tonix’s expertise in the pharmacology and development of tricyclics TNX - 102 (cyclobenzaprine HCl) TNX - 601 (tianeptine oxalate) HCl (C 2 O 4 ) ½ -

© 2018 Tonix Pharmaceuticals Holding Corp. 54 TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate Pre - IND Stage Potential improvement over current biodefense tools against smallpox ✓ Leverages Tonix’s government affairs effort ✓ Collaboration with Professor David Evans and Dr. Ryan Noyce at University of Alberta ✓ Demonstrated protective vaccine activity in mice ✓ Patent application on novel vaccine submitted Regulatory strategy • FDA’s “Animal Rule” can be applied to establish human efficacy • Good Manufacturing Practice (GMP) viral production process in development for human safety study Targeting a Public Health Issue Material threat medical countermeasure under 21 st Century Cures Act • Qualifies for Priority Review Voucher* (PRV) upon licensure ✓ PRVs have no expiration date, are transferrable and have sold for ~$125 M 1 PRV can be applied to any BLA/NDA for priority 6 - month review

© 2018 Tonix Pharmaceuticals Holding Corp. 55 TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate Synthesis from sequence of a 1976 Mongolian isolate 1 In mice, TNX - 801 behaved like attenuated vaccinia virus • Vaccinia is the term used to classify the live poxviruses that are used as smallpox vaccines, including ACAM2000, which is the latest smallpox vaccine licensed in the U.S. How is HPXV related to modern vaccines? • Multiple sources 2 - 4 indicate that the smallpox vaccine discovered by Dr. Edward Jenner in the early 19 th century was either HPXV or a very similar virus and that vaccinia vaccines are derived from this ancestral strain • A 1902 U.S. smallpox vaccine was found to be highly similar (99.7% similarity in core genome 5 ) to HPXV sequence from the 1976 Mongolian isolate • Horsepox is now believed to be extinct 4 1 Tulman et al., Journal of Virology, 2006; 80(18): 9244 - 9258 2 Qin et al., Journal of Virology, 2011; 85(24):13049 - 13060 3 Medaglia et al., Journal of Virology, 2015; 89(23):11909 - 11925 4 Esparza J. Veterinary Record. 2013; 173: 272 - 273 5 Schrick, L. et al. , N Engl J Med 2017; 377:1491 - 1492, http://www.nejm.org/doi/full/10.1056/NEJMc1707600

© 2018 Tonix Pharmaceuticals Holding Corp. 56 ACAM2000 is sold to the U.S. Strategic National Stockpiles 1 • Sold by Emergent BioSolutions • Sanofi divested ACAM2000 to Emergent BioSolutions in 2017 for $97.5 M upfront plus milestones • ACAM2000 was developed by Acambis which was acquired by Sanofi in 2008 for $513 M Vaccinia (VACV) strains have demonstrated potential for zoonotic infections and re - infection of humans 2 - 5 • No known evidence for zoonosis of ACAM2000, but it has not been widely administered Modern VACV smallpox vaccines are associated with cardiotoxicity 6 The Currently Licensed Smallpox Vaccine ACAM2000 is a Live Vaccinia Virus (VACV) Vaccine 1 Nalca, A et al. Drug design, development and Therapy. (2010) 4:71 - 79 2 Medaglia MLG, et al. J Virol. (2015) 89:11909 – 11925. doi:10.1128/JVI.01833 - 15. 3 Trindade,GS. et al. Clinical Infectious Diseases. (2009) 48:e37 – 40 4 Leite,JA, et al. Emerging Infectious Diseases. (2005) www.cdc.gov/eid • Vol. 11, No. 12 5 Medaglia MLG, et al. Emerging Infectious Diseases (2009) www.cdc.gov/eid • Vol. 15, No. 7 6 Engler RJM et al., PloS ONE (2015) 10(3): e0118283. doi:10.1371/journal.pone.0118283
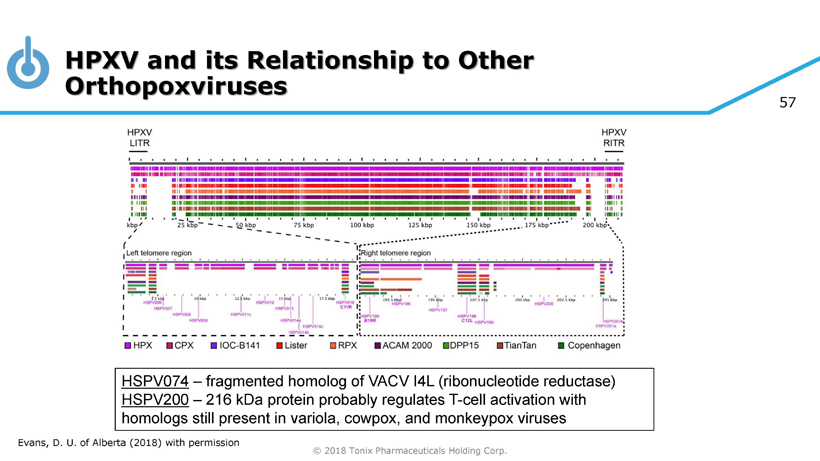
57 © 2018 Tonix Pharmaceuticals Holding Corp. HSPV074 – fragmented homolog of VACV I4L (ribonucleotide reductase) HSPV200 – 216 kDa protein probably regulates T - cell activation with homologs still present in variola , cowpox, and monkeypox viruses HPXV and its Relationship to Other Orthopoxviruses Evans, D. U. of Alberta (2018) with permission
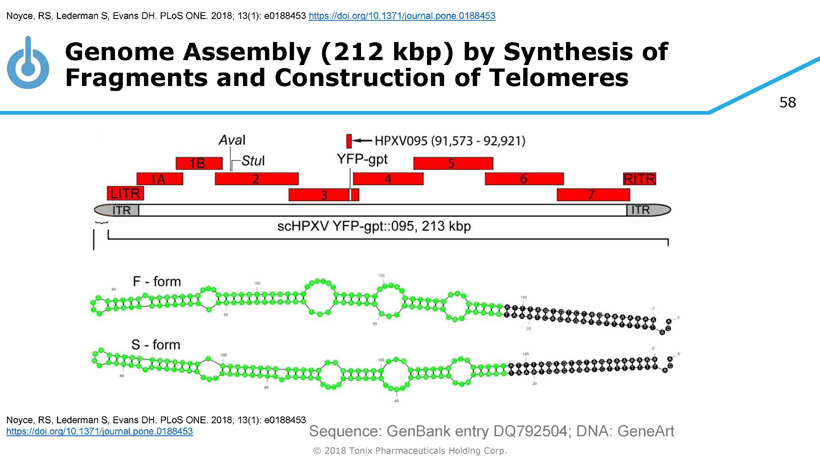
58 © 2018 Tonix Pharmaceuticals Holding Corp. Sequence: GenBank entry DQ792504; DNA: GeneArt Genome Assembly (212 kbp ) by Synthesis of Fragments and Construction of Telomeres Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453 Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
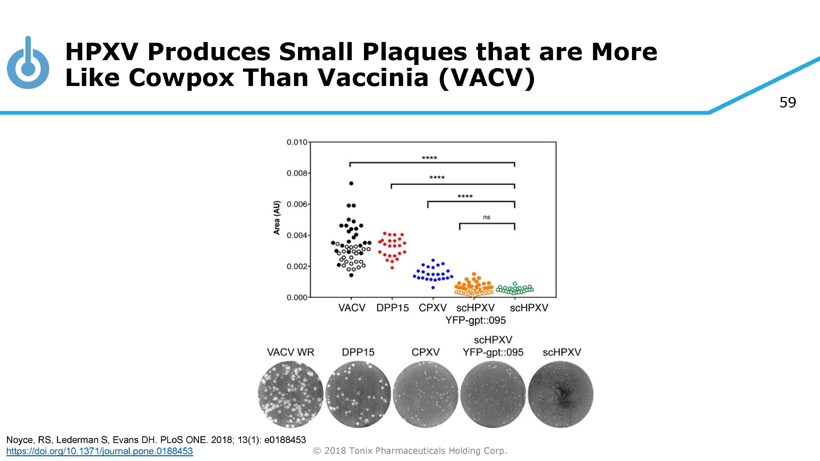
59 © 2018 Tonix Pharmaceuticals Holding Corp. HPXV Produces Small Plaques that are More Like Cowpox Than Vaccinia (VACV) Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
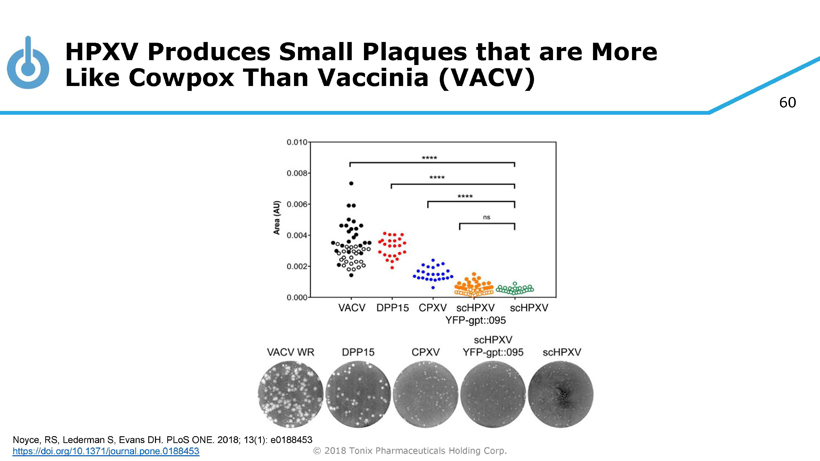
60 © 2018 Tonix Pharmaceuticals Holding Corp. HPXV Produces Small Plaques that are More Like Cowpox Than Vaccinia (VACV) Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
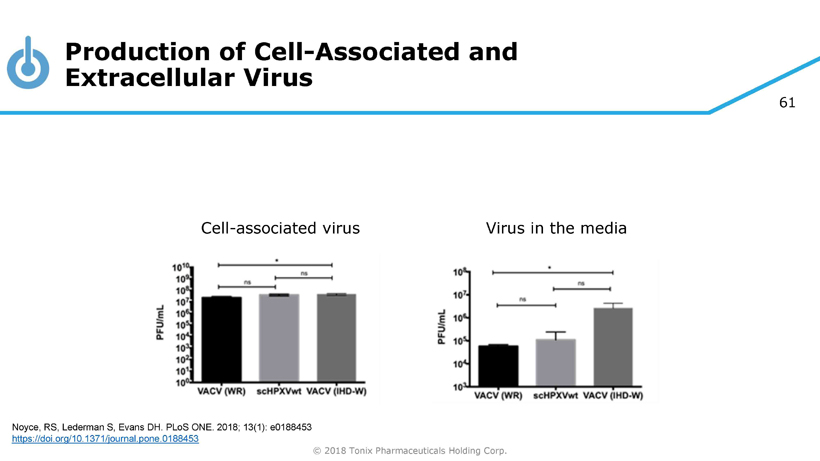
61 © 2018 Tonix Pharmaceuticals Holding Corp. Cell - associated virus Virus in the media Production of Cell - Associated and Extracellular Virus Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
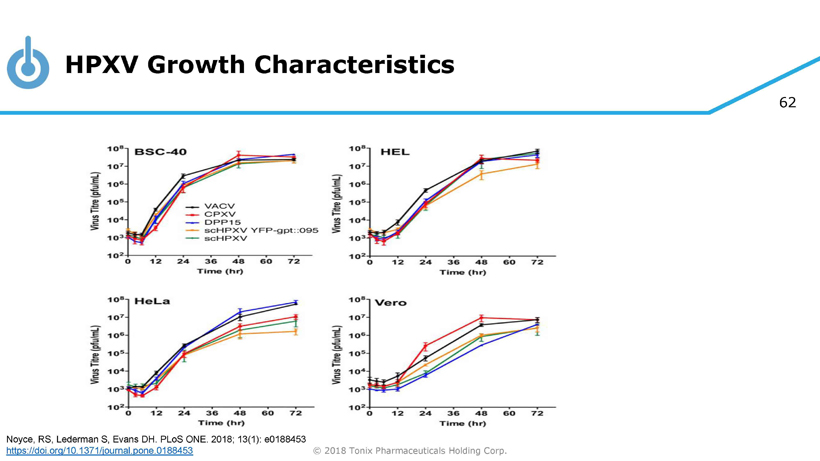
62 © 2018 Tonix Pharmaceuticals Holding Corp. HPXV Growth Characteristics Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
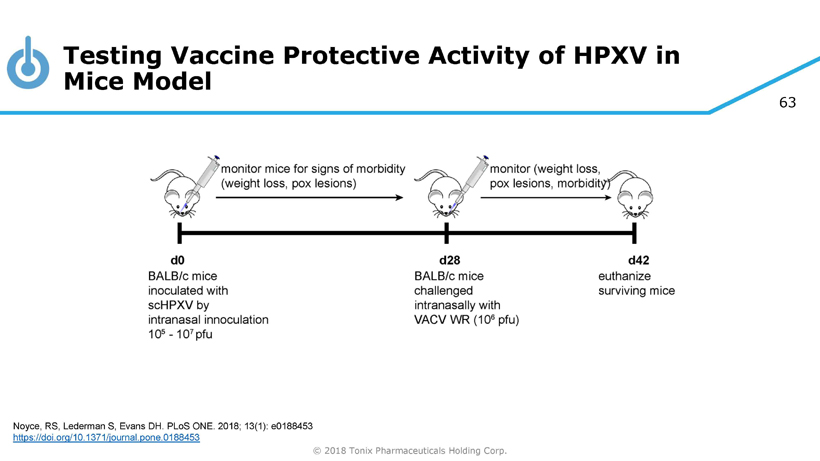
63 © 2018 Tonix Pharmaceuticals Holding Corp. Testing Vaccine Protective Activity of HPXV in Mice Model Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
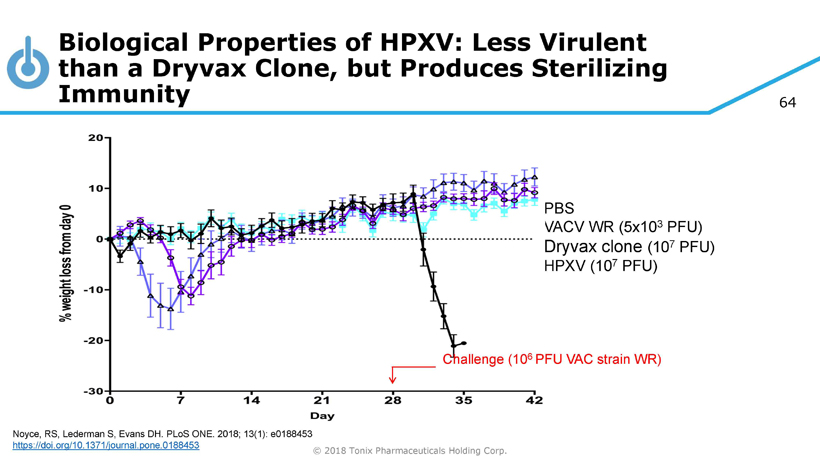
64 © 2018 Tonix Pharmaceuticals Holding Corp. PBS VACV WR (5x10 3 PFU) Dryvax clone (10 7 PFU) HPXV (10 7 PFU) Challenge (10 6 PFU VAC strain WR) Biological Properties of HPXV: Less Virulent than a Dryvax Clone, but Produces Sterilizing Immunity Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
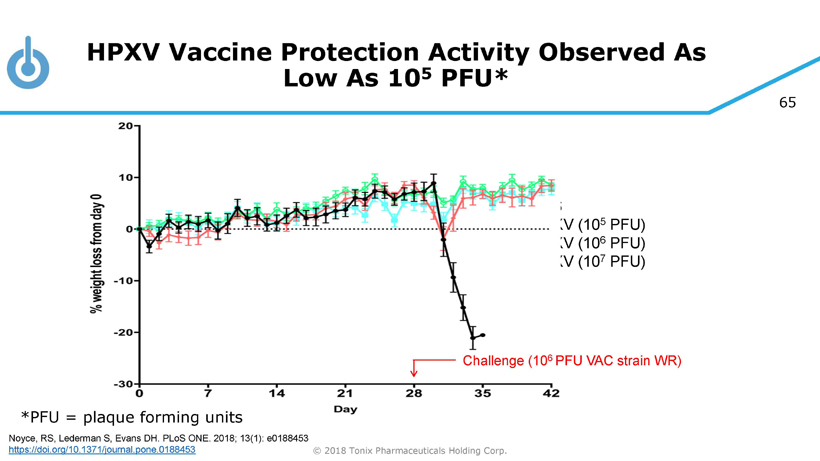
65 © 2018 Tonix Pharmaceuticals Holding Corp. PBS HPXV (10 5 PFU) HPXV (10 6 PFU) HPXV (10 7 PFU) Challenge (10 6 PFU VAC strain WR) HPXV Vaccine Protection Activity Observed As Low As 10 5 PFU* *PFU = plaque forming units Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
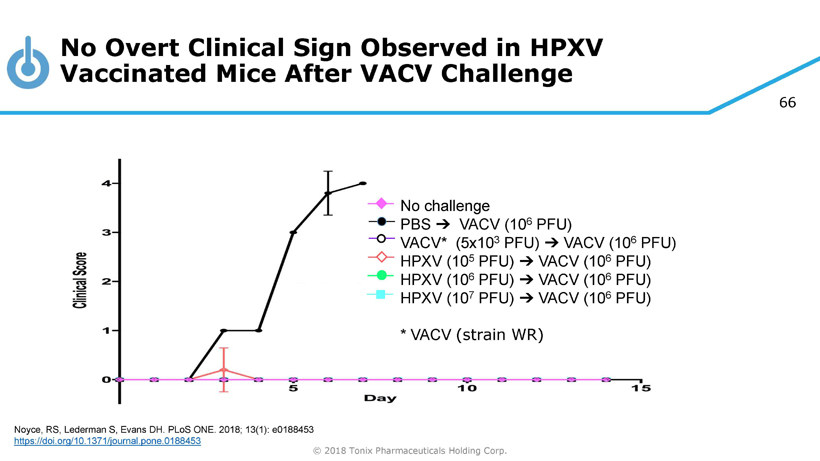
66 © 2018 Tonix Pharmaceuticals Holding Corp. No challenge PBS ➔ VACV (10 6 PFU) VACV* (5x10 3 PFU) ➔ VACV (10 6 PFU) HPXV (10 5 PFU) ➔ VACV (10 6 PFU) HPXV (10 6 PFU) ➔ VACV (10 6 PFU) HPXV (10 7 PFU) ➔ VACV (10 6 PFU) * VACV (strain WR) No Overt Clinical Sign Observed in HPXV Vaccinated Mice After VACV Challenge Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453
© 2018 Tonix Pharmaceuticals Holding Corp. 67 HPXV or TNX - 801 – May Have an Improved Safety Profile as a Smallpox Preventing Vaccine Horsepox is caused by HPXV and is characterized by mouth and skin eruptions HXPV isolate from the 1976 outbreak later sequenced Modern smallpox vaccines are associated with cardiotoxicity 1 HPXV has potential for slower proliferation leading to possibly decreased toxicity 2 1 Engler RJM et al., PloS ONE 10(3): e0118283. doi:10.1371/journal.pone.0118283 (2015) 2 Noyce, RS, Lederman S, Evans DH. PLoS ONE. 2018; 13(1): e0188453 https://doi.org/10.1371/journal.pone.0188453

© 2018 Tonix Pharmaceuticals Holding Corp. 68 An Improved Smallpox - Preventing Vaccine is Important and Necessary for a Potential Public Health Issue Smallpox was eradicated as a result of global public health campaigns No cases of naturally - occurring smallpox have been reported since 1977 Accidental or intentional transmission of smallpox does not require a natural reservoir Stockpiles of smallpox - preventing vaccines are currently maintained and refreshed in case of need

© 2018 Tonix Pharmaceuticals Holding Corp. 69 Ongoing vaccination of U.S. troops • Troops in the Global Response Force Threat of smallpox re - introduction • Strategic National Stockpile & public health policy Re - emergence of monkey pox 1 • Believed to resurgent because of vaccinia - naïve populations in Africa • Multiple U.S. military operations ongoing in Africa Current Needs to Vaccinate Against Smallpox 1 Nda - Isaiah, J. Nigeria: Monkey Pox Scourge Spreads to Seven States. All Africa. 12 OCTOBER 2017, HTTP://ALLAFRICA.COM/STORIES/201710120177.HTML

© 2018 Tonix Pharmaceuticals Holding Corp. 70 TNX - 801: A Potential Medical Countermeasure 21st Century Cures Act (2016), Section 3086 • Encouraging treatments for agents that present a national security threat Medical countermeasures are drugs, biologics (vaccines) or devices intended to treat: • Biological, chemical, radiological, or nuclear agents that present a national security threat • Public health issues stemming from a naturally occurring emerging disease or a natural disaster New Priority Review Voucher program for “Material Threat Medical Countermeasures” • Priority Review Voucher may be transferred or sold
© 2018 Tonix Pharmaceuticals Holding Corp. 71 Live virus vaccines stimulate cross - reactive immunity • Protects from possible infection with smallpox virus • Renders recipient “immune” • Provides indirect protection to non - immunized population “herd immunity” Potential safety improvement over existing vaccines • Cardiotoxicity limits widespread smallpox vaccination in at - risk population Exclusivity • Patent application filed on novel virus composition • 12 years exclusivity can be anticipated Eligibility for Priority Review Voucher upon licensure Mechanism of Action Possible advantages of TNX - 801 TNX - 801 (HPVX) • Synthesized live horsepox virus • Shares structural characteristics with vaccinia - based smallpox vaccines • Unique properties that suggest lower toxicity TNX - 801 (Synthesized Live Horsepox Virus): A Smallpox - Preventing Vaccine Candidate

© 2018 Tonix Pharmaceuticals Holding Corp. 72 Given that smallpox is eradicated the only evidence of effectiveness for modern vaccines is from historical use when smallpox was endemic • Stimulates interest in the evolution of vaccinia Vaccinia stocks around the world diverged from Jenner’s 1798 vaccine • Evolutionary argument that common progenitor was horsepox or a similar virus U.S. vaccine from 1902 was found to be 99.7% similar to horsepox in core viral sequence 1 • Strong evidence linking a horsepox - like virus as progenitor to modern vaccinia • Effectiveness of older vaccines support belief that HPXV will be protective against smallpox Evidence of Effectiveness for Smallpox Vaccine 1 Schrick, L. et al (2017) An Early American Smallpox Vaccine Based on Horsepox N Engl J Med 2017; 377:1491

© 2018 Tonix Pharmaceuticals Holding Corp. 73 Single clone picked from “swarm” of Dryvax ® 1 • Some rationale for selection 2 Growth in serum free Vero cells • Eliminates risk of Bovine Spongiform Encephalopathy (BSE)/prion contamination – safety concerns in Wyeth’s Dryvax (grown in calf lymph) In 2000, the evolutionary connection between vaccinia and horsepox was not understood • Tulman’s sequence of horsepox was published in 2006 3 ACAM2000 1 – Best Technology of its Time 1 US licensed smallpox preventing vaccine – ACAM2000 is currently marketed, Dryvax has been withdrawn from marketing 2 Monath, TP et al. Int. J. of Inf. Dis. (2004) 8S2:S31 3 Tulman, ER. Genome of Horsepox Virus J. Virol. (2006) 80(18) 9244
© 2018 Tonix Pharmaceuticals Holding Corp. 74 Toxicity concern of modern vaccinia (VACV) vaccines limit wildly administration • Not recommended for use, even in first responders • U.S. soldiers in the Global Response Force are immunized Modern VACV vaccination safety studied in 1081 VACV ( Dryvax [62.5%] and ACAM2000 [37.5%]) vaccinees 1 • New onset chest pain, dyspnea and/or palpitations 10.6% of VACV - vaccinees and 2.6% of control immunized (TIV) 2 • Clinical: 4 probable myo - and 1 suspected peri - carditis (5 cases out of 1081 VACV vaccinees – 0.5%) • Cardiac specific troponin T ( cTnT ) elevation in 31 VACV vaccinees (3%) Rationale for Developing a Potentially Improved New Smallpox Vaccine 1 Engler RJM,, et al. (2015) A Prospective Study of the Incidence of Myocarditis/Pericarditis and New Onset Cardiac Symptoms following Smallpox and Influenza Vaccination. PLoS ONE 10(3) 2 TIV = trivalent influenza vaccine - control vaccinees
© 2018 Tonix Pharmaceuticals Holding Corp. 75 Postulated Divergence of Historical Strains of Vaccinia Proposed Evolution of Vaccinia Vaccines NYCBH/European precursor stocks Horsepox - like ancestral strain Lister/Evans/USSR Dryvax ® IHD - J WR Copenhagen Tashkent RPXV TianTan Ankara LIVP IHD - J=International Health Department - Japan LIVP=Lister Vaccine Strain NYCBH=New York City Board of Health RPXV=Rabbitpox Virus WR=Western Reserve Figure Adapted from Qin et al. Journal of Virology. 2015;89(3):1809 - 1824.
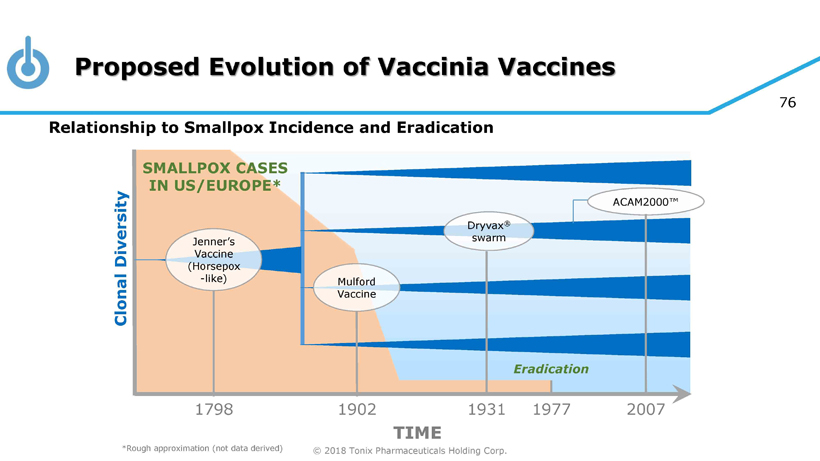
© 2018 Tonix Pharmaceuticals Holding Corp. 76 Relationship to Smallpox Incidence and Eradication Proposed Evolution of Vaccinia Vaccines TIME *Rough approximation (not data derived) 1977 Eradication SMALLPOX CASES IN US/EUROPE* ACAM2000™ 1798 1902 1931 2007 Dryvax ® swarm Mulford Vaccine Jenner’s Vaccine (Horsepox - like) Clonal Diversity

© 2018 Tonix Pharmaceuticals Holding Corp. 77 Theoretical effectiveness of modern vaccinia vaccines are based on extrapolation from older vaccines • Newer/modern vaccines were not widely used when smallpox was endemic MVA ( Modified Virus Ankara) which has large deletions also produces different T cell responses • In non - human primates, MVA is less effective than ACAM2000 in protecting against monkeypox 1 • MVA has fewer epitopes, and elicits different responses to existing epitopes 2 • MVA effectiveness argument is based on the immune response to intracellular mature virus (IMV) • Immunity to the other form of virus, extracellular enveloped virus (EEV), is weak because the immunodominant B5 gene is heavily mutated and deleted in MVA What’s the Evidence of Effectiveness of Smallpox Vaccines for Preventing Smallpox? 1 Golden JW, et al. (2012). PLoS ONE 7(7): e42353. doi:10.1371/journal.pone.0042353 2 Tscharke, DC et al., J. Exp. Med. 2005 201(1):95

© 2018 Tonix Pharmaceuticals Holding Corp. 78 Preventing Vaccine • Jenner’s vaccine, HPXV (upon licensure), Vaccinia Post - exposure vaccination 1 • Jenner’s vaccine Priming of the immune system • Imvamune ® (MVA) and DNA vaccines 2 Pharmacotherapy for infected or exposed individuals • Arestvyr ® ( tecovirimat , formerly ST - 246) Treatment of disseminated viremia in immunocompromised 3 • Arestvyr ® , Brincidofovir and vaccinia immune globulin Possible Smallpox Prevention and Treatment Strategies 1 Described by Jenner as one of his major discoveries 2 Hooper, JW et al. Smallpox DNA Vaccine Protects Nonhuman Primates Against Lethal Monkeypox . J. Virol . 2004. 78 (9) 4433 3 Lederman, ER et al, Progressive Vaccinia: Case Description and Laboratory - Guided Therapy With Vaccinia Immune Globulin, ST - 246, and CMX001 JID 2012. 206:1372
© 2018 Tonix Pharmaceuticals Holding Corp. 79 Pox vaccines with low or no replication appear safer than vaccines replicate fast in human cells • Canarypox and Imvamune ® (Modified Virus Ankara/MVA) appear to have good tolerability • Relatively safe in immunocompromised hosts • Rapidly replicating modern vaccinia vaccines ( Dryvax ® and ACAM2000®) are associated with myocarditis Replication correlates positively with immunogenicity • Jenner’s vaccine and modern vaccinia engender strong immunity • Canarypox and MVA appear to be weak immunogens, suitable for priming of the immune system in healthy human being and potentially safe enough to use in immunocompromised people Viral Replication Proficiency is Critical to Human Immunogenicity but May Compromise Safety

© 2018 Tonix Pharmaceuticals Holding Corp. 80 TNX - 801 (HPXV) is expected to have similar scalability for mass production as ACAM2000 • TNX - 801 grows well in cell lines – immunity is expected after single administration (immunization) • Only a small dose (replicating live virus) is required for immunization MVA is hard to scale up for commercial production • Requires high dose* to engender an immune response (non - replicating virus) • Cumbersome immunization schedule – two doses, 4 weeks apart, are used typically to prime the immune system (slow growth) Antivirals • Relatively expensive to manufacture – requires repeated dosing • May provide logistical challenges to at risk population over the at risk period Manufacturing and Dosing Requirements

© 2018 Tonix Pharmaceuticals Holding Corp. 81 Vaccination protects against smallpox – both individuals and populations at risk • Use of Jenner’s vaccine resulted in eradication of smallpox Vaccination can protect AFTER smallpox infection • Vaccinia can be administered 1 - 3 days after infection Vaccination indirectly protects non - immunized people in a population • “Wetting the forest” or “herd immunity” Vaccination can be cost effective with safe/low - risk vaccines • Replication - efficient live virus vaccines can be manufactured and administered for broader use “The Time is Right” New synthetic biology technology and new understanding of vaccinia evolution provide an opportunity for a potentially safer vaccine using HPXV Rationale for Developing a Potentially Improved New Smallpox Vaccine Based on Jenner’s Vaccine
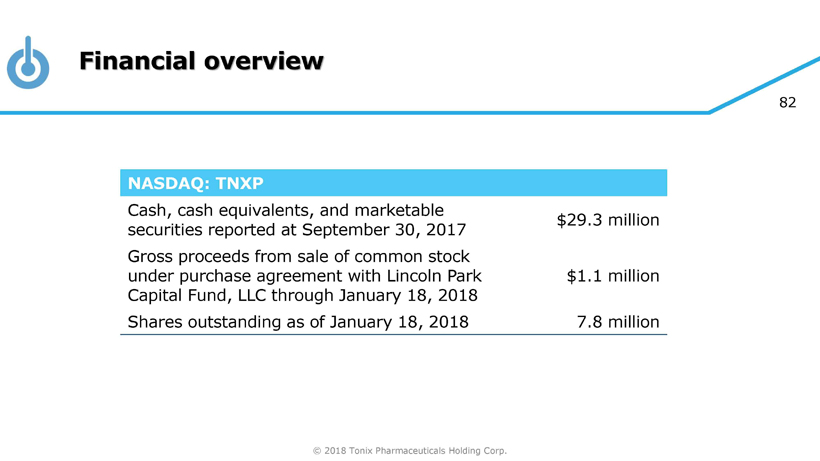
© 2018 Tonix Pharmaceuticals Holding Corp. 82 Financial overview NASDAQ: TNXP Cash, cash equivalents, and marketable securities reported at September 30, 2017 $29.3 million Gross proceeds from sale of common stock under purchase agreement with Lincoln Park Capital Fund, LLC through January 18, 2018 $1.1 million Shares outstanding as of January 18, 2018 7.8 million
© 2018 Tonix Pharmaceuticals Holding Corp. 83 Management Team Seth Lederman, MD President & CEO Jessica Morris Chief Operating Officer Gregory Sullivan, MD Chief Medical Officer Bradley Saenger, CPA Chief Financial Officer
© 2018 Tonix Pharmaceuticals Holding Corp. 84 Board of Directors Seth Lederman, MD Chairman Ernest Mario, PhD ALZA, Glaxo, Reliant Pharma John Rhodes NYSERDA, NRDC, Booz Allen Hamilton Samuel Saks, MD Jazz Pharma, ALZA, Johnson & Johnson Charles Mather BTIG, Janney, Jefferies, Cowen, Smith Barney Stuart Davidson Labrador Ventures, Alkermes , Combion Patrick Grace Apollo Philanthropy, WR Grace, Chemed Donald Landry, MD, PhD Chair of Medicine, Columbia University Margaret Smith Bell Standard Life Investments, Putnam Investments, State Street Research
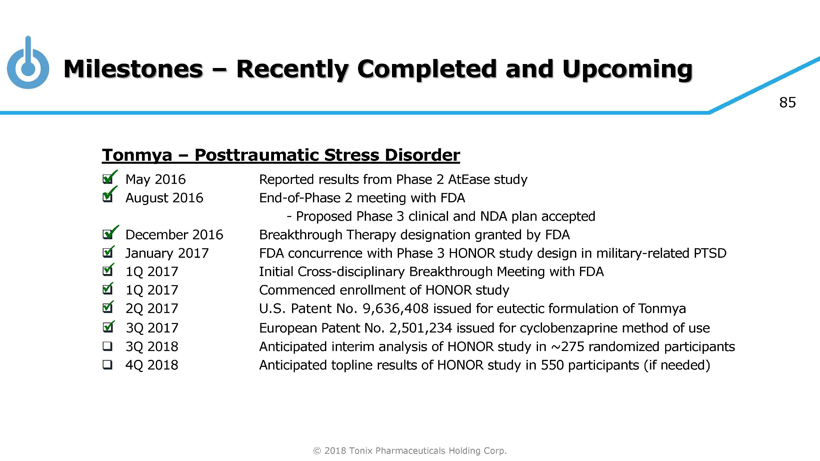
© 2018 Tonix Pharmaceuticals Holding Corp. 85 Milestones – Recently Completed and Upcoming T onmya – Posttraumatic Stress Disorder □ May 2016 Report ed results from Phase 2 AtEase study □ August 2016 End - of - Phase 2 meeting with FDA - Proposed Phase 3 clinical and NDA plan accepted □ December 201 6 Breakthrough Therapy designation granted by FDA □ January 201 7 FDA concurrence with Phase 3 HONOR study design in military - related PTSD □ 1Q 2017 Initial Cross - disciplinary Breakthrough Meeting with FDA □ 1Q 2017 Commenced enrollment of HONOR study □ 2 Q 2017 U.S. Patent No. 9,636,408 issued for eutectic formulation of Tonmya □ 3 Q 2017 European Patent No. 2,501,234 issued for cyclobenzaprine method of use □ 3Q 201 8 Anticipated interim analysis of HONOR study in ~ 275 randomized participants □ 4Q 201 8 Anticipated topline results of HONOR study in 550 participants (if needed) x x x x x x x x
© 2018 Tonix Pharmaceuticals Holding Corp. 86 Summary Strong position for value growth with Phase 3 development in a major medical indication: PTSD including military - related PTSD • PTSD is an important public health issue Tonmya for PTSD is designated as a Breakthrough Therapy by FDA • Accelerated development and approval process is expected Phase 3 HONOR study in military - related PTSD began enrollment in 1Q 2017 • Outcome of the unblinded interim analysis on ~275 randomized participants (50%) expected to be available 3Q 201 8 • Completion of the 550 - participant trial, if needed, and announcement of topline results expected in 4Q 2018 • NDA approval can be solely based on HONOR if the data are statistically persuasive

© 2018 Tonix Pharmaceuticals Holding Corp. Thank you ! NASDAQ: TNXP